Abstract
Purpose:
Evaluate whether combining a humanized anti-disialoganglioside monoclonal antibody (hu14.18K322A) with induction chemotherapy improves early responses and outcomes in children with newly diagnosed high-risk neuroblastoma.
Patients and Methods:
We conducted a prospective nonrandomized, single-arm, two-stage, phase II clinical trial. Six courses of induction chemotherapy were coadministered with hu14.18K322A and followed with granulocyte/macrophage colony-stimulating factor (GM-CSF) and low-dose interleukin-2 (IL-2). Consolidation was performed with a busulfan/melphalan preparative regimen. An additional course of hu14.18K322A was administered with parent-derived natural killer cells, when available, during consolidation. Hu14.18K322A, GM-CSF, IL-2, and isotretinoin were then administered. Secondary outcomes included reduced tumor volume and semiquantitative 123I-metaiodobenzylguanidine scoring [i.e., Curie scores (CS)] at the end of induction.
Results:
Forty-two patients received hu14.18K322A and induction chemotherapy. This regimen was well tolerated, with continuous-infusion narcotics adjusted to patient tolerance. Partial responses (PR) or better after the first two chemoimmunotherapy courses occurred in 32 patients [76.2%; 95% confidence interval (CI), 60.6–88.0]. This was accompanied by primary tumor volume reductions (median, –76%; range, –100% to 5%). Of 35 patients with stage 4 disease who completed induction, 31 had end-of-induction CSs of 2 or less. No patients experienced progression during induction. Two-year event-free survival (EFS) was 85.7% (95% CI, 70.9–93.3).
Conclusions:
Adding hu14.18K322A to induction chemotherapy produced early PR or better in most patients, reduced tumor volumes, improved CSs at the end of induction, and yielded an encouraging 2-year EFS. These results, if validated in a larger study, may change the standard of care for children with high-risk neuroblastoma.
Introduction
The current standard treatment for high-risk neuroblastoma includes high-dose induction chemotherapy, surgery, and consolidation with myeloablative chemotherapy, autologous hematopoietic cell transplant (AHCT), radiotherapy, and treatment of minimal residual disease (MRD) with a monoclonal antibody (mAb) that targets the disialoganglioside GD2 on neuroblasts. A chimeric anti-GD2 antibody (dinutuximab) in combination with granulocyte-macrophage–colony-stimulating factor (GM-CSF), interleukin-2 (IL-2), and isotretinoin administered at the end of therapy, in the context of MRD, significantly improves 2-year event-free survival (EFS) (66% vs. 46%; P = 0.01) (1). Despite this aggressive regimen, nearly half of all patients still experience relapse and succumb to disease.
Dinutuximab was administered at the end of therapy to avoid chemotherapy-induced immunosuppression, which is thought to adversely affect antibody-dependent cell-mediated cytotoxicity (ADCC). However, preclinical studies in neuroblastoma models and clinical studies of adult cancers demonstrated that concurrent chemotherapy with various monoclonal antibodies provides additive/synergistic benefits (2–9). We postulated that the addition of an anti-GD2 antibody to induction chemotherapy for neuroblastoma would further improve outcomes. We initially tested the tolerability of a unique anti-GD2 antibody, hu14.18K322A, administered with chemotherapy in a small group of patients with relapsed disease. When we observed excellent responses (10), we immediately proceeded to evaluate this approach in children with newly diagnosed disease. Hu14.18K322A retains the binding specificity of dinutuximab, is 98% human to reduce allergic reactions, has a single point mutation to reduce complement-associated pain, and is produced in an YB2/0 rat myeloma cell line to reduce fucosylation and enhance ADCC (11).
Children’s Oncology Group (COG) investigators reported the addition of cyclophosphamide and topotecan to an intense induction regimen in a pilot trial (12). This induction regimen was used for children with newly diagnosed high-risk neuroblastoma in the recently completed ANBL0532 protocol. We used the identical induction regimen as the chemotherapy backbone for a prospective nonrandomized, single-arm, two-stage, phase II clinical trial in which hu14.18K322A was added to induction chemotherapy for children with newly diagnosed high-risk neuroblastoma. Primary outcomes were early responses (after two courses of induction chemoimmunotherapy) and 2-year EFS. Secondary outcomes included reduced tumor volume and semiquantitative 123I-metaiodobenzylguanidine (MIBG) scoring [i.e., Curie scores (CS)] at the end of induction.
Methods
Patient Selection
Children (< 19 years) with newly diagnosed high-risk neuroblastoma were eligible for enrollment. Patients had either histologically verified neuroblastoma or clumps of tumor in bone marrow with increased urinary catecholamine metabolites. Diagnosis, staging, and response assessments were performed according to the International Neuroblastoma Staging System (INSS) criteria (13), and high-risk neuroblastoma was defined by the criteria used by the COG (14). Both assessments were identical to those used by Park et al. (12), which included the historical control group for our study.
This prospective pilot phase II trial () was approved by our institutional review board in accordance with the Belmont Report and the U.S. Common Rule. The trial opened in May 2013 and enrollment continues. Written informed consent was obtained from all participants in accordance with institutional guidelines. All patients were treated at St. Jude Children’s Research Hospital.
Hu14.18K322A
The hu14.18K322A production cell line was provided by Merck Serono (Darmstadt, Germany) and manufactured for clinical use by the Children’s GMP, LLC (Memphis, TN). On day 1 of each course, serum hu14.18 K322A levels were measured at 1 hour after antibody infusion by ELISA, as previously described (10, 15).
Treatment
The schedule and dosages of the induction chemotherapy agents cyclophosphamide, topotecan, cisplatin, etoposide, doxorubicin, and vincristine were identical to those reported by Park et al. (12). Four daily doses of hu14.18K322A (days 2–5) were added to each course of induction chemotherapy. Each dose was planned to be administered over 4 hours. This was successful in approximately half of the 256 courses of antibody/chemotherapy administered. According to patient tolerance and at the discretion of the treating physician, antibody infusions were extended to 8 or 16 hours for some patients (Supplementary Table 1). Because hu14.18K322A is known to cause dose-dependent toxicity and patients with newly diagnosed neuroblastoma are often seriously ill with some organ system compromise at diagnosis, we selected a mAb dose that would be tolerated in combination with induction chemotherapy and would not require delays in subsequent chemotherapy or mAb treatment due to mAb-related dose-limiting toxicity. Therefore, the hu14.18K322A dosage was fixed at 40 mg/m2 per dose, which is two dose levels below the single-agent maximum-tolerated dose (11) and known to be tolerable as single-agent therapy and in combination with multiagent chemotherapy in children with recurrent/refractory disease (10). The selected dose is approximately twice that of the current dose of FDA-approved dinutuximab (ch14.18) when used with IL-2 and GM-CSF as maintenance treatment. All patients were treated with continuous infusions of narcotics (morphine, hydromorphone, or fentanyl) at standard dosages approximately 30 minutes before starting antibody infusions, and adjusted as needed. Systemic steroids were not allowed, per protocol study procedures. Each course was followed by daily subcutaneous GM-CSF (250 μg/m2 per day) through the nadir until absolute neutrophil count ≥ 2000/mm3 and IL-2 (106 units/m2), which was continued every other day for six doses. Hematopoietic progenitor cells were collected after induction cycle 2 or 4. Primary tumors were resected or debulked during induction.
Consolidation therapy included AHCT with a busulfan/melphalan (Bu/Mel) conditioning regimen (16) and, for consenting patients, experimental therapy with an additional course of daily hu14.18K322A (40 mg/m2 per day for 4 days) administration, beginning 2 days (days 2–5) after AHCT (given on day 0), and parental natural killer (NK) cell infusions (when available), derived as previously described (16). If both parents were suitable donors, the donor with greater KIR mismatch was chosen. KIR mismatch was defined as the presence of an inhibitory KIR gene in the donor coupled with the absence of the KIR ligand (HLA) gene in the recipient. The NK cells were infused 4 days after AHCT. After recovering from AHCT (typically within 43 days of stem cell reinfusion), patients received intensity-modulated radiation therapy or scanned proton beam radiation therapy (2340 cGy in 180 cGy fractions). Those with macroscopic residual disease after induction chemotherapy received an additional 720 cGy to those sites.
Radiation therapy was delivered to the presurgical gross tumor volume and sites of residual disease on the basis of computed tomography, MIBG nuclear imaging, and/or magnetic resonance imaging scans performed at the end of induction. Every effort was made to begin therapy for MRD with hu14.18K322A, GM-CSF, IL-2, and isotretinoin with doses and schedules identical to those reported by Yu et al. (1) by 100 days after AHCT, with a substitution of hu14.18K322A (40 mg/m2 per day for 4 days) for dinutuximab (1). Adverse events were assessed by the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). If narcotic infusions required increased dosages, pain was designated grade 3. Response to chemotherapy was assessed by one study radiologist (MBM), as described by Park and colleagues (12) (i.e., according to INSS criteria) (13). Tumor volume was also assessed. Semiquantitative MIBG scoring [i.e., Curie score (CS)] was assessed by one nuclear medicine physician (BS) at diagnosis and after courses 2 and 6 of induction chemotherapy, as described by Yanik et al. (17).
Study Design
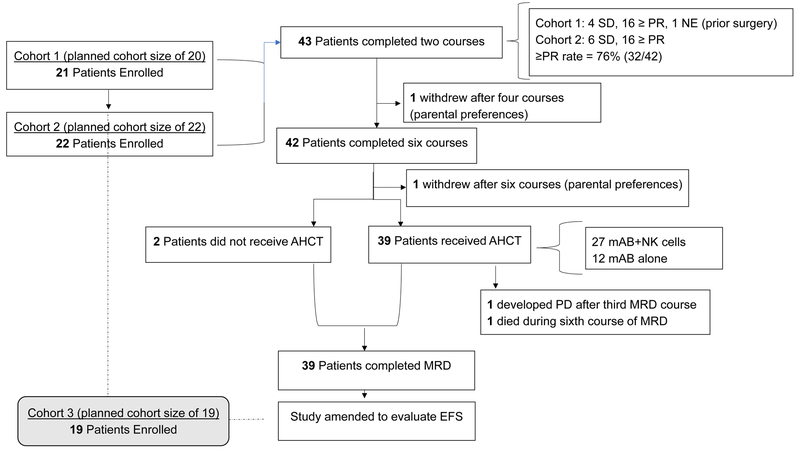
We designed our study to evaluate whether the overall response rate [i.e., partial response (PR) or better] after the first two courses of chemoimmunotherapy exceeded 40%. We performed a sample size calculation for the study by using an alternative response rate of 60% (i.e., response considered worthy of further evaluation of the new therapy), 80% power, and 5% type I error. A sample size of 42 evaluable patients was required to satisfy these parameters. A two-stage group sequential design (18) was used to allow early stopping for lack of sufficient antitumor activity. Stage 1 and 2 were planned for 20 and 22 evaluable patients, respectively. All patients who began course 1 with evaluable disease were considered evaluable for response. The study was amended to add an EFS primary objective, and the two-stage design was extended to a three-stage group sequential design (18). The sample size was recalculated by using 90% power, and the sample size was updated to 61 evaluable patients (stage 3, 19 additional patients). Accrual for this objective is ongoing. We generated a study flow diagram depicting how the trial was conducted (Fig. 1). As surrogates of overall survival (OS), we also evaluated early primary tumor volume changes (19) and MIBG scoring (i.e., CSs) (17), which in other reports have been shown to be prognostic indicators of outcomes in patients with high-risk neuroblastoma.
Figure 1.
Study flowchart.
Statistical Analysis
Patient characteristics were summarized by descriptive statistics and compared with those of a previous study (12) from which the historical control rate was established using Fisher exact tests (Table 1). The response rate after the first two courses and its exact 95% confidence interval (CI) were estimated. To evaluate the treatment effect, the response rate after the first two courses was compared to the estimate from the historical trial (12) using Fisher’s exact tests. The EFS and OS point estimates with 95% CIs were estimated with the Kaplan–Meier method. EFS was defined as the time from enrollment to the first occurrence of local failure, relapse, progressive disease, secondary neoplasm, or death from any cause. OS was defined as the time from enrollment to the time of death from any cause. Patients without an event were censored at the time of last follow-up or withdrawal when evaluating EFS and OS.
Table 1.
Patient characteristics at diagnosis
| Characteristic | No. (%) | No. (%) | P |
|---|---|---|---|
| NB2012 (n = 43) | ANBL02P1a (n = 31) | ||
| Age (months) | NS | ||
| < 18 | 3 (7) | 5 (16) | |
| ≥ 18 | 40 (93) | 26 (84) | |
| Sex | 0.045 | ||
| Female | 19 (44) | 6 (19) | |
| Male | 24 (56) | 25 (81) | |
| Race | NS | ||
| White | 29 (67) | 23 (88) | |
| Black | 11 (26) | 2 (8) | |
| Other | 3 (7) | 1 (4) | |
| Unreported | - | 5 | |
| INSS stage | NS | ||
| 2B | 1 (2) | 4 (13 combined) | |
| 3 | 6 (14) | ||
| 4 | 36 (84) | 27 (87) | |
| MYCN status | NS | ||
| Not amplified | 24 (56) | 15 (60) | |
| Amplified | 19 (44) | 10 (40) | |
| Unreported | - | 6 | |
| Shimada histology | NS | ||
| Favorable | 2 (6) | 1 (5) | |
| Unfavorable | 30 (94) | 21 (95) | |
| Not performedb/Unreported | 11 | 9 |
Abbreviations: INSS, International Neuroblastoma Staging System; NS, not significant (P > 0.05).
Park et al., J Clin Oncol 2011.
Diagnosis by urine catecholamine and/or positive bone marrow for NB2012.
The Cox proportional hazards model was used to evaluate the relationship of age (months), sex, amplified MYCN, pretherapy tumor volume, INSS stage, race, pretherapy CS, course 2 CS, course 6 CS, course 2 response, and course 6 response on OS. Both univariable and multivariable analyses were performed. Multivariable Cox proportional hazards models were used to evaluate the association of early response on OS, adjusting for age, sex, amplified MYCN, and INSS stage. A similar analysis was performed for EFS.
Statistical analyses were conducted with SAS software, version 9.4 (SAS Institute, Cary, NC). A two-sided significance level of P < 0.05 was considered statistically significant.
Results
We report our findings from 43 patients enrolled during the first two stages of accrual. Forty-two of the 43 patients had measurable or evaluable disease. One patient whose INSS stage 2b adrenal tumor was resected before induction chemotherapy and contained high-level MYCN amplification was not evaluable for early response. Patient characteristics are described and compared with those of the historical control group in Table 1. Most patients were male (n = 24), white (n = 29), at least 18 months old at diagnosis (n = 40), and a median age of 2.8 years (range, 0.5–15.2 years), and most had INSS stage 4 disease (n = 36). These characteristics were similar to those of the historical comparison group, except for sex (P = 0.045; Table 1).
The addition of hu14.18K322A to induction chemotherapy was well tolerated, with continuous-infusion narcotics carefully adjusted to patient tolerance. All therapy-related grade 3/4 toxicities during induction chemotherapy–hu14.18K322A coadministration are described in Table 2. Toxicities attributed to antibody infusion included pain (17%; 43 patient episodes over 256 courses), hypotension (2%; 6/256), cough (1%; 3/256), and hypoxia (4%; 11/256) (1,20).
Table 2.
Treatment-related grade 3/4 toxicities during the first six courses of induction therapy
| Toxicity | Course 1 (n = 43) | Course 2 (n = 43) | Course 3 (n = 43) | Course 4 (n = 43) | Course 5 (n = 42)a | Course 6 (n = 42)a |
|---|---|---|---|---|---|---|
| Abdominal distension | 1 | |||||
| Abdominal pain | 3 | 2 | 1 | 2 | 2 | 5 |
| Acidosis | 2 | 4 | 5 | 5 | 2 | 1 |
| Acute kidney injury | 1 | |||||
| Agitation | 1 | |||||
| Alanine aminotransferase increased | 3 | 3 | 4 | 1 | 1 | 1 |
| Alkalosis | 1 | 1 | ||||
| Allergic reaction | 1 | 2 | ||||
| Anaphylaxis | 1 | |||||
| Anemia | 41 | 40 | 35 | 40 | 38 | 42 |
| Anorexia | 1 | 1 | 1 | 2 | ||
| Apnea | 1 | |||||
| Aspartate aminotransferase increased | 4 | 1 | 1 | 1 | ||
| Asystole | 1 | |||||
| Atelectasis | 1 | |||||
| Back pain | 1 | 1 | ||||
| Bladder infection | 1 | 2 | ||||
| Blood antidiuretic hormone abnormal | 1 | |||||
| Blood bilirubin increased | 4 | 1 | 2 | 1 | 4 | |
| Bone pain | 1 | 1 | ||||
| Bronchospasm | 2 | |||||
| Buttock pain | 1 | |||||
| Catheter related infection | 1 | 2 | 1 | 2 | 1 | 6 |
| Chest pain, cardiac | 1 | |||||
| Colitis | 1 | 1 | 2 | 1 | 1 | |
| Constipation | 1 | |||||
| Cough | 1 | 1 | 1 | |||
| Cystitis noninfective | 2 | 1 | 1 | 1 | ||
| Dehydration | 1 | 1 | 1 | 1 | ||
| Device related infection | 1 | 2 | ||||
| Diarrhea | 1 | 1 | 2 | 1 | 3 | 1 |
| Enterocolitis | 4 | 2 | 5 | 9 | 5 | 7 |
| Epistaxis | 2 | 2 | 3 | 2 | ||
| Erythema multiforme | 1 | |||||
| Esophagitis | 1 | 1 | 6 | |||
| Febrile neutropenia | 19 | 9 | 2 | 23 | 8 | 20 |
| Fever | 11 | 2 | 3 | 3 | 6 | |
| GGT increased | 5 | 5 | 1 | 1 | 5 | |
| Gum infection | 1 | |||||
| Headache | 1 | 1 | ||||
| Hearing impaired | 2 | 2 | 1 | 5 | ||
| Hematuria | 1 | |||||
| Hypercalcemia | 1 | |||||
| Hyperglycemia | 2 | 4 | 6 | 5 | 4 | 2 |
| Hyperkalemia | 1 | 1 | 2 | |||
| Hypermagnesemia | 1 | |||||
| Hypertension | 1 | 1 | 1 | 1 | ||
| Hypoalbuminemia | 2 | 1 | 1 | 2 | 1 | |
| Hypocalcemia | 5 | 3 | 1 | 3 | 3 | |
| Hypoglycemia | 2 | 1 | ||||
| Hypokalemia | 13 | 3 | 16 | 8 | 15 | 10 |
| Hyponatremia | 6 | 1 | 11 | 6 | 4 | 5 |
| Hypophosphatemia | 4 | 16 | 1 | 16 | 7 | |
| Hypotension | 1 | 1 | 1 | 1 | 2 | |
| Hypoxia | 8 | 2 | 1 | |||
| Ileus | 1 | |||||
| Infections and infestations | 1 | 1 | ||||
| Infusion related reaction | 3 | 2 | ||||
| Injection site reaction | 1 | |||||
| Left ventricular systolic dysfunction | 1 | |||||
| Lung infection | 1 | |||||
| Lymphocyte count decreased | 38 | 41 | 42 | 43 | 41 | 42 |
| Lymphocyte count increased | 3 | 3 | 1 | 3 | 4 | 6 |
| Malabsorption | 1 | |||||
| Metabolism and nutrition disorders | 1 | |||||
| Mucositis, oral | 1 | 17 | 1 | 18 | ||
| Nausea | 1 | 3 | 3 | 1 | ||
| Neutrophil count decreased | 43 | 42 | 35 | 43 | 41 | 42 |
| Pain | 9 | 3 | 4 | 3 | 1 | 1 |
| Photophobia | 1 | |||||
| Platelet count decreased | 34 | 37 | 41 | 43 | 41 | 42 |
| Pleural effusion | 4 | 1 | ||||
| Pneumonitis | 1 | |||||
| Pruritus | 1 | |||||
| Rash acneiform | 2 | |||||
| Rash, maculo-papular | 2 | |||||
| Respiratory failure | 1 | |||||
| Scrotal infection | 1 | |||||
| Sepsis | 1 | 3 | ||||
| Sinus tachycardia | 1 | |||||
| Skin infection | 1 | 2 | 2 | |||
| Sore throat | 1 | |||||
| Stridor | 1 | |||||
| Syncope | 1 | |||||
| Thromboembolic event | 1 | 1 | ||||
| Tracheitis | 1 | |||||
| Tumor lysis syndrome | 1 | |||||
| Typhlitis | 1 | |||||
| Upper gastrointestinal hemorrhage | 1 | |||||
| Upper respiratory infection | 7 | 9 | 3 | 7 | 6 | 15 |
| Urethral infection | 1 | |||||
| Urinary retention | 1 | |||||
| Urinary tract infection | 3 | 4 | 5 | 2 | 4 | |
| Urostomy obstruction | 1 | |||||
| Vascular access complication | 1 | |||||
| Vomiting | 4 | 5 | 1 | 3 | ||
| Vulval infection | 1 | |||||
| Weight loss | 1 | |||||
| White blood cell decreased | 43 | 39 | 31 | 43 | 41 | 42 |
| Wound infection | 1 |
Abbreviation: GGT, gamma-glutamyltransferase.
Entries in the table represent patient numbers. If a patient experienced multiple episodes of a particular toxicity during a course of therapy, only the highest observed grade of a toxicity was reported.
One patient withdrew after course 4 of therapy (parental preference).
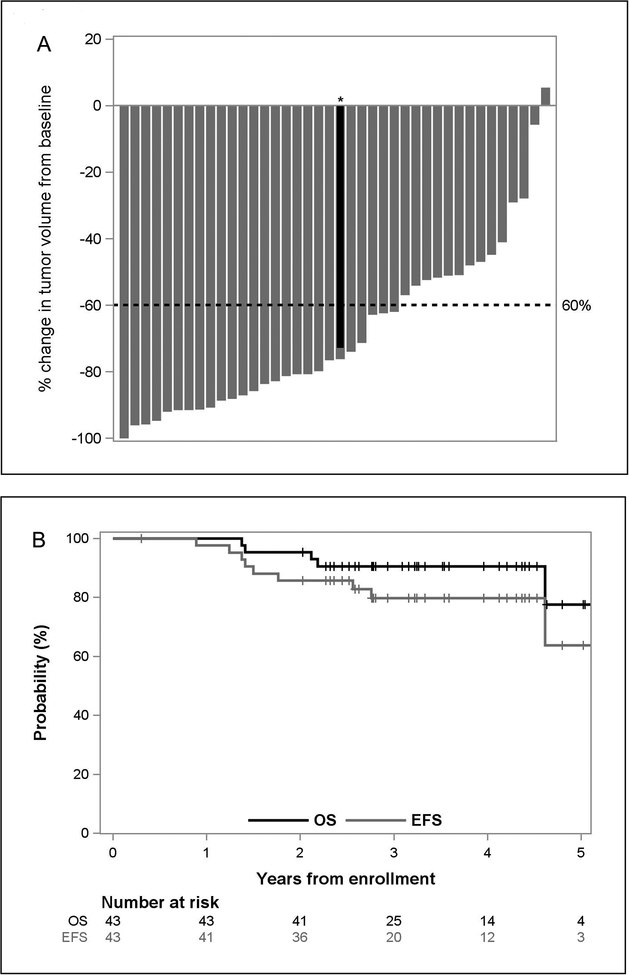
Of the 42 children evaluable for early responses, 32 (76.2%; 95% CI, 60.6–88.0) experienced at least PRs after these first two cycles (Table 3). A waterfall plot illustrating the change in primary tumor volume after the initial two cycles (Fig. 2A) revealed that only one patient did not experience measurable decreases in primary tumor volume (median, –76%; range, –100% to 5%), and 26 of 40 patients experienced reductions of at least 60%. None of these patients experienced disease progression during induction chemotherapy.
Table 3.
Response assessments after the first two cycles of induction therapy and at the end-of-induction therapy
| Assessment time | CR | VGPR | PR | SD | PD | MR/NR | NE | Response rate (95% CI)d |
|---|---|---|---|---|---|---|---|---|
| After two cycles | ||||||||
| NB2012 (n = 43) | 1 | 6 | 25 | 10 | 0 | 0 | 1 | 76% (60.5–87.9) |
| ANBL02P1 (n = 31)a | 2 | 1 | 9 | 0 | 1 | 17 | 1b | 40% (22.7–59.4) |
| End of induction | ||||||||
| NB2012 (n = 43) | 15 | 15 | 10 | 2 | 0 | 0 | 1c | 95% (83.8–99.4) |
| ANBL02P1 (n = 31)a | 7 | 8 | 11 | 0 | 1 | 3 | 1 | 87% (69.3–96.2) |
Abbreviations: CI, confidence interval; CR, complete response; MR/NR, mixed response/no response; NE, not evaluable or not evaluated; PD, progressive disease; PR, partial response; SD, stable disease; VGPR, very good partial response.
Park et al., J Clin Oncol (2011).
One patient had complete resection before start of therapy and was not evaluated after the first two courses.
Patient withdrew after course 4 of therapy.
Exact confidence intervals are reported.
Figure 2.
Patient outcomes with hu14.18K322A during induction therapy. A, Percentage change in primary tumor volume after two courses of chemoimmunotherapy. *One patient had large retroperitoneal mass arising from the celiac axis (red) and a smaller left thoracic paraspinal mass with intraspinal tumor extension at T4 and T5 (gray) and was considered to have two primary tumors. B, Kaplan–Meier estimates of overall survival (OS) and event-free survival (EFS). The median follow-up time for the 32 patients who did not experience an event was 3.3 years (range, 2.0–5.3 years) from study enrollment.
MIBG scoring was performed at every evaluation time point (Supplementary Table 2). Of the 43 patients we evaluated, 36 had INSS stage 4 disease. One of these 36 with a complete response after two cycles of induction therapy withdrew (parental preference) before completing induction therapy. CSs for the 36 patients with INSS stage 4 disease ranged from 1 to 28 (median, 16.5) at diagnosis and from 0 to 23 (median, 0) at the end of induction chemotherapy. For the 35 patients with INSS stage 4 disease who completed induction, 31 had end-of-induction CSs of 2 or less, as compared with a median CS of 16.5 at diagnosis.
Only three of 43 evaluable patients showed detectible human anti-human antibody (HAHA) responses to hu14.18K322A after chemoimmunotherapy (Table 4), which is less frequent than the 15 of 37 patients with detectible HAHA responses who were treated with hu14.18K322A without chemotherapy for relapsed/refractory neuroblastoma in a prior phase I trial (11). Moreover, HAHA responses had no influence on hu14.18K322A Cmax levels in subsequent courses, as shown in Table 4 and by Federico et al. (10).
Table 4.
Serum antibody levels in representative patients with and without HAHA development during therapy
| Pt-ID | HAHAa | C1 | C2 | C3 | C4 | C5 | C6 | Int | M1 | M2 | M3 | M4 | M5 | M6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 9 | − | K322Ab | 15.6 | 16.7 | 17.0 | 15.7 | 14.5 | 14.2 | NA | 12.9 | 10.5 | 11.8 | 13.7 | 13.9 | NA |
| HAHA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 12 | − | K322A | 27.7 | 16.1 | 17.9 | 26.7 | 15.3 | 14.4 | 3.5 | 9.4 | 12.0 | 11.8 | 8.8 | 9.8 | NA |
| HAHA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 13 | − | K322A | 12.2 | 23.2 | 21.0 | 23.2 | 20.6 | 17.9 | NA | 11.4 | 10.7 | 13.3 | 9.0 | 13.9 | NA |
| HAHA | 0 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 66 | + | K322A | 12.4 | NA | 7.4 | 15.1 | 13.1 | 13.3 | 12.5 | 12.4 | 11.0 | 14.1 | 11.2 | 18.6 | NA |
| HAHA | 0 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.89 | 0 | 0 | ||
| 69 | + | K322A | 5.9 | 3.8 | 17.0 | 16.2 | 18.3 | 15.4 | 10.3 | 12.7 | 11.5 | 12.1 | 15.8 | 16.8 | NA |
| HAHA | 0 | 0.78 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 112 | + | K322A | 12.4 | 13.9 | 12.8 | 10.1 | 10.8 | 7.6 | 10.5 | 9.3 | 13.3 | 12.1 | 8.4 | 6.2 | NA |
| HAHA | 0.72 | 3.2 | 1.7 | 0.84 | 0.46 | 0.93 | 0.82 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: C, induction course; HAHA, human anti-human antibody; Int, intensification phase; K322A, hu14.18K322A; M maintenance course; Pt-ID, patient identification number.
HAHA levels detected in a bridging ELISA assay with serum obtained at the initiation of the indicated course, before therapy. HAHA+ indicates patients with > 0.7 OD unit increase at any time, as compared with the baseline C1 sample. The three patients who experienced a HAHA response at any time are shown. Three representative patients (of 40) without a HAHA response are also shown.
K322A concentration (μg/mL) per indicated course, obtained 1 hour after completion of K322A infusion on day 1 of that course.
As of January 2019, 38 of the 43 patients were alive at last follow-up. The median follow-up time for all patients was 3.2 years (range, 1.4–5.3 years) from the time of study enrollment. Two patients died of complications from therapy without evidence of disease. One of these patients died of pulmonary toxicity, presumably from busulfan, which was complicated by underlying Rubinstein Taybi syndrome. The other patient expired at home while completing oral isotretinoin during MRD course 6, with symptoms consistent with overwhelming sepsis. Four patients had persistent disease after completing all therapy: three with persistent MIBG-detected avid bone disease and end-of-treatment CSs of 5, 21, and 22 and one with a residual soft tissue MIBG avid mass near the primary tumor sight. Two of these four patients currently have no evidence of disease after alternative therapy; one has stable disease maintained with an ALK inhibitor, and one died of disease progression. Five additional patients experienced disease recurrence; one after MRD course 3 and four at 4, 4, 16, and 19 months after completing all therapy. Two of these patients died of disease progression, and two are still receiving additional therapy. The end-of-induction response rate (≥ PR) was 95% (95% CI, 83.8–99.4; Table 3), and the overall 2-year EFS and OS rates were 85.7% (95% CI, 70.9–93.3) and 95.3% (95% CI, 82.7–98.8), respectively (Fig. 2B). Univariable survival analysis was unable to identify significant predictors of EFS. The Cox proportional hazards model, considering the effect of early response on EFS, adjusting for age (months) at enrollment, gender, race, INSS stage, and MYCN found none of these variables predictive of EFS. A similar analysis for OS was also unable to identify predictive factors.
Discussion
Our results demonstrate that the concomitant use of an anti-GD2 mAb with intensive induction chemotherapy in children with newly diagnosed high-risk neuroblastoma is feasible and results in an early response rate of nearly 80%. We also evaluated the effect of this therapy on primary tumor volumes and CSs at the end-of-induction chemotherapy as surrogates of OS because both measures predict outcomes in children with newly diagnosed high-risk neuroblastoma (17,19). We initially used a two-stage group sequential design to allow for early stopping if evidence of antitumor activity was lacking. However, the early responses of the first two cohorts (n = 42) provided evidence for improved therapeutic success. Thirty-two of these patients experienced at least PRs (76.2%; 95% CI, 60.6–88.0), in contrast with 12 of 30 (40.0%; 95% CI, 22.7–59.4) in the group without hu14.18K322A (12). The difference in response rates was 36.2% (95% CI, 9.7–56.7; P = 0.003), indicating a significantly higher early response rate in our trial than in ANBL02P1. These findings resulted in an amendment to increase accrual and include an EFS objective in our trial. It is unclear, with this small sample size, whether these improved early responses translated into improved end-of-induction response rates. As illustrated in Table 3, the end-of-induction response rates (≥ PR) of 95% were very similar to the 87% reported in ANBL02P1 (12).
Early response as a surrogate for EFS and OS of high-risk neuroblastoma was reported by Yoo and colleagues (19). In a group of 90 patients with high-risk neuroblastoma, those who experienced a greater than 60% reduction in primary tumor volume after the first two or three cycles of induction chemotherapy had better outcomes. In our study, 65% of evaluable patients experienced a greater than 60% reduction in tumor volume after the first two cycles. However, because of a limited sample size and number of events, statistical conclusions about the prognostic value of primary tumor volume reduction is not possible. Yanik and colleagues (17) evaluated the CSs of patients with INSS stage 4 disease who were treated in the A3973 trial with six courses of similar induction chemotherapy. The EFS of these patients with CSs of 2 or less at the end of induction was markedly improved. In our study, approximately 90% of patients with INSS stage 4 disease had CSs of 2 or less after completing induction chemotherapy. These data suggest that concomitant anti-GD2 mAb administration with induction chemotherapy may lead to improved survival for children with newly diagnosed high-risk neuroblastoma. Randomized controlled clinical trials measuring OS are the gold standard for determining the value of a new therapeutic intervention. Our findings provide evidence to support the development of a randomized controlled trial to test this approach.
The major dose-limiting toxicity of anti-GD2 mAbs is pain, primarily from mAb binding to GD2 on peripheral nerves, resulting in complement activation (21). Dinutuximab, a mouse–human chimeric mAb, also induces hypersensitivity reactions in many patients (1). The hu14.18K322A mAb has several important differences from dinutuximab, including humanization of the antibody to reduce hypersensitivity reactions, elimination of the complement binding domain to reduce pain (22), and production in a cell line to reduce fucosylation and thereby enhance ADCC (11). The tolerability of dinutuximab when added to induction chemotherapy in children with newly diagnosed and frequently widespread metastatic disease has not been tested.
Although early responses were significantly improved with chemoimmunotherapy over those of the historical control group, improved EFS and OS are the most important indicators of successful outcomes. Although our sample size was relatively small and the median follow-up time was 3.2 years, a 2-year EFS of 86% appears to be improved over the estimated 2-year EFS of 66% reported by Yu and colleagues. However, that study included only patients who achieved a PR or better to induction therapy (1), while our estimate included all patients enrolled. Moreover, we did not observe disease progression in any patients during induction, whereas as many as 14% of patients enrolled in past COG trials experienced progression before receiving AHCT (23).
These results, although promising, must be interpreted with caution and within the context of a single-institution pilot study. Although the chemotherapy backbone was identical to that of ANBL02P1, and the major prognostic factors of our patients were very similar to these patients (Table 1), the 2-year EFS (approximately 50%) and OS (approximately 80%) of ANBL02P1 (12) are lower than our results of 85.7% (95% CI, 70.9–93.3) and 95.3% (95% CI, 82.7–98.8), respectively (Fig. 2B). Several differences in therapy may have contributed to our higher response rates. Specifically, GM-CSF was used, rather than filgrastim (12), for its ability to enhance ADCC (24,25) and for primary prophylaxis of febrile neutropenia (26). Additionally, low-dose IL-2 was used for its ability to enhance ADCC (27). The Bu/Mel preparative regimen (28) vs. carboplatin/etoposide/melphalan used in ANBL02P1 (12) and additional course of hu14.18K322A (n = 12) with parental-derived NK cells (n = 27) during consolidation may also have affected EFS. This study was not designed to evaluate the effects of these modifications on the overall outcome.
In conclusion the addition of a unique anti-GD2 mAb to induction chemotherapy nearly doubled early responses, reduced tumor volumes, resulted in no progression during induction, improved CSs, and most importantly yielded a 2-year EFS of 86%. The COG has recently activated a pilot trial to further evaluate this approach, substituting dinutuximab for hu14.18K322A. These results, if validated in a larger trial, may prove practice changing.
Supplementary Material
Translational Relevance.
The current standard treatment for high-risk neuroblastoma includes high-dose induction chemotherapy, surgery, and consolidation with myeloablative chemotherapy, autologous hematopoietic cell transplant, radiotherapy, and treatment of minimal residual disease with a monoclonal antibody (mAb) that targets the disialoganglioside GD2 on neuroblasts. In this paradigm, the anti-GD2 mAb is administered at the end of therapy to avoid chemotherapy-induced immunosuppression, which was thought to adversely affect antibody-dependent cell-mediated cytotoxicity. However, preclinical studies in neuroblastoma models and clinical studies of adult cancers demonstrated that concurrent chemotherapy with various monoclonal antibodies provides additive/synergistic benefits. We postulated that the addition of an anti-GD2 antibody to induction chemotherapy for neuroblastoma would further improve outcomes. Adding the anti-GD2 mAb hu14.18K322A to induction chemotherapy significantly improved early responses, reduced tumor volumes, improved Curie scores at the end of induction, and yielded an encouraging 2-year event-free survival. These results, if validated in a larger trial, may prove practice changing.
Acknowledgements
The authors thank Deanna Welsh for data management and Nisha Badders, PhD, ELS, for scientific editing.
Funding: St. Jude Children’s Research Hospital Comprehensive Cancer Center Support Grant (2 P30 CA021765), American Lebanese Syrian Associated Charities, and Cookies for Kids’ Cancer and Cure Childhood Cancer Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest
All authors declare no conflict of interest in relation to the work described.
References
- 1.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003;63(15):4490–6. [PubMed] [Google Scholar]
- 3.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer 2005;5(5):397–405 doi 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 4.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106(12):3725–32. [DOI] [PubMed] [Google Scholar]
- 5.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346(4):235–42. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N EnglJ Med 2004;350(23):2335–42. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344(11):783–92. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida S, Kawaguchi H, Sato S, Ueda R, Furukawa K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun terminal kinase) activation. JpnJ Cancer Res 2002;93(7):816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kowalczyk A, Gil M, Horwacik I, Odrowaz Z, Kozbor D, Rokita H. The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Lett 2009;281(2):171–82. [DOI] [PubMed] [Google Scholar]
- 10.Federico SM, McCarville MB, Shulkin BL, Sondel PM, Hank JA, Hutson P, et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin Cancer Res 2017;23(21):6441–9 doi 10.1158/1078-0432.CCR-17-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Navid F, Sondel PM, Barfield R, Shulkin BL, Kaufman RA, Allay JA, et al. Phase I Trial of a Novel Anti-GD2 Monoclonal Antibody, Hu14.18K322A, Designed to Decrease Toxicity in Children With Refractory or Recurrent Neuroblastoma. J Clin Oncol 2014;32(14):1445–52 doi 10.1200/JCO.2013.50.4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JR, Scott JR, Stewart CF, London WB, Naranjo A, Santana VM, et al. Pilot Induction Regimen Incorporating Pharmacokinetically Guided Topotecan for Treatment of Newly Diagnosed High-Risk Neuroblastoma: A Children’s Oncology Group Study. J Clin Oncol 2011;29(33):4351–57 doi 10.1200/jco.2010.34.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol 1993;11(8):1466–77. [DOI] [PubMed] [Google Scholar]
- 14.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr 2005;17(1):7–13. [DOI] [PubMed] [Google Scholar]
- 15.Hank JA, Gan J, Ryu H, Ostendorf A, Stauder MC, Sternberg A, et al. Immunogenicity of the hu14.18-IL2 immunocytokine molecule in adults with melanoma and children with neuroblastoma. Clin Cancer Res 2009;15(18):5923–30 doi 10.1158/1078-0432.CCR-08-2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talleur AC, Triplett BM, Federico S, Mamcarz E, Janssen W, Wu J, et al. Consolidation Therapy for Newly Diagnosed Pediatric High-Risk Neuroblastoma Patients Using Busulfan/Melphalan, Autologous Hematopoietic Cell Transplant, Anti-GD2 Antibody, GM-CSF, IL-2 and Haploidentical NK Cells. Biol Blood Marrow Transplant 2017;23(11):1910–7 doi 10.1016/j.bbmt.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yanik GA, Parisi MT, Shulkin BL, Naranjo A, Kreissman SG, London WB, et al. Semiquantitative mIBG scoring as a prognostic indicator in patients with stage 4 neuroblastoma: a report from the Children’s oncology group. J Nucl Med 2013;54(4):541–8 doi 10.2967/jnumed.112.112334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiong X A class of sequential conditional probability ratio tests. Journal of American Statistical Association 1995;90(432):1463–73. [Google Scholar]
- 19.Yoo SY, Kim JS, Sung KW, Jeon TY, Choi JY, Moon SH, et al. The degree of tumor volume reduction during the early phase of induction chemotherapy is an independent prognostic factor in patients with high-risk neuroblastoma. Cancer 2013;119(3):656–64 doi 10.1002/cncr.27775. [DOI] [PubMed] [Google Scholar]
- 20.Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 With GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Frontiers in immunology 2018;9:1355 doi 10.3389/fimmu.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorkin LS, Otto M, Baldwin WM 3rd, Vail E, Gillies SD, Handgretinger R, et al. Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 2010;149(1):135–42 doi 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anghelescu DL, Goldberg JL, Faughnan LG, Wu J, Mao S, Furman WL, et al. Comparison of pain outcomes between two anti-GD2 antibodies in patients with neuroblastoma. Pediatr Blood Cancer 2015;62(2):224–8 doi 10.1002/pbc.25280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non-purged peripheral blood stem-cell transplantation for high-risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncol 2013;14(10):999–1008 doi 10.1016/S1470-2045(13)70309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood 1989;73(7):1936–41. [PubMed] [Google Scholar]
- 25.Mueller BM, Romerdahl CA, Gillies SD, Reisfeld RA. Enhancement of antibody-dependent cytotoxicity with a chimeric anti-GD2 antibody. J Immunol 1990;144(4):1382–6. [PubMed] [Google Scholar]
- 26.Furman WL, Fairclough DL, Huhn RD, Pratt CB, Stute N, Petros WP, et al. Therapeutic effects and pharmacokinetics of recombinant human granulocyte-macrophage colony-stimulating factor in childhood cancer patients receiving myelosuppressive chemotherapy. J Clin Oncol 1991;9(6):1022–8. [DOI] [PubMed] [Google Scholar]
- 27.Meropol NJ, Porter M, Blumenson LE, Lindemann MJ, Perez RP, Vaickus L, et al. Daily subcutaneous injection of low-dose interleukin 2 expands natural killer cells in vivo without significant toxicity. Clin Cancer Res 1996;2(4):669–77. [PubMed] [Google Scholar]
- 28.Ladenstein R, Potschger U, Pearson AD, Brock P, Luksch R, Castel V, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 2017;18(4):500–14 doi 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.