Abstract
Pneumonitis may complicate anti-programmed death-1 (PD-1) therapy, although symptoms usually resolve with steroids. The long-term effects on respiratory function, however, are not well-defined. We screened melanoma patients treated with anti–PD-1, with and without ipilimumab (anti–CTLA-4), and identified 31 patients with pneumonitis. Median time to radiographic findings was 4.8 months. Twenty-three patients (74%) presented with respiratory symptoms, whereas 8 (26%) were asymptomatic, and 11 (35%) were hospitalized. With 22.1 months median follow-up, 27 patients (87%) had resolution of symptoms, whereas 4 had persistent cough, dyspnea, and/or wheezing. By contrast, the rate of radiographic resolution was lower: only 11 (35%) had complete radiographic resolution, whereas 14 (45%) had improvement of pneumonitis with persistent scarring or opacities, and 6 (19%) had persistent or worsened groundglass opacities and/or nodular densities. Persistence (vs. resolution) of radiographic findings was associated with older age and initial need for steroids but not with need for hospitalization, timing of onset, or treatment regimen (combination vs. monotherapy). Among patients with serial pulmonary function tests, lung function improved with time. Although symptoms of anti–PD-1–induced pneumonitis resolved quickly, scarring or inflammation frequently persisted on computerized tomography. Therefore, further study of subclinical pulmonary effects of anti–PD-1 is needed.
Keywords: Pneumonitis, PD-1, PD-L1, nivolumab, pembrolizumab, lung, pulmonary
Introduction
Agents blocking programmed death-1 receptor (PD-1) and its ligand, PD-L1, are now approved in 15 different types of metastatic cancer and produce responses in a substantial fraction of patients (1,2), and many responders experience durable benefit lasting for years. These agents are now approved as adjuvant therapy for high-risk melanoma following surgical resection and as maintenance therapy for stage III non-small cell lung cancer (NSCLC) following definitive chemoradiation (3,4). As such, the numbers of patients with long-term survival following anti–PD-1/PD-L1 treatment are increasing substantially and understanding associated long-term therapeutic complications is a critical objective.
Toxicities from anti–PD-1/PD-L1 are triggered by autoreactive T and B cells and can affect any organ in an unpredictable fashion (5–7). Pneumonitis, or inflammation of the lungs, is a particularly important toxicity to evaluate. This event occurs in approximately 3–5% of treated patients and tends to present with a non-productive cough and dyspnea, and/or with variable radiographic findings in asymptomatic patients (8,9). Although occasional cases may be fatal (10), the overt clinical symptoms of pneumonitis usually resolve with corticosteroid administration. The long-term effects on pulmonary function of this inflammatory process, however, are unknown.
In our routine clinical practice, we noted that persistent imaging abnormalities were common in patients who experienced anti–PD-1–induced pneumonitis, even following resolution of symptoms. To investigate this phenomenon further, we reviewed serial imaging and available pulmonary function testing from patients who developed pneumonitis while on anti–PD-1/PD-L1 therapy.
Methods
Patients
Patients were treated in accordance with the Declaration of Helsinki. Each institution obtained IRB consent to perform this retrospective review with waiver of consent. All patients with advanced melanoma from three large academic centers (Vanderbilt University Medical Center, Massachusetts General Hospital, Moffitt Cancer Center) treated with anti–PD-1 therapy with or without ipilimumab were screened. From these, all patients who developed pneumonitis, as determined by the treating physician and confirmed by study radiologists, were identified (31 patients). Retrospective electronic medical record review was performed to identify patient demographics, comorbidities, prior therapies, cancer stage, and treatment response. Symptoms were graded according to the Common Terminology Criteria for Adverse Events [CTCAE] version 5.0. Treatment of pneumonitis, including dose, type, and duration of steroids, and other immunosuppressant treatments, was abstracted from chart review.
Pneumonitis
Pneumonitis resulting from anti–PD-1 treatment was diagnosed by the treating physician with characteristic radiographic findings on computed tomography (CT) scans, and absence of infectious or neoplastic etiology explaining these findings. A board-certified radiologist reviewed serial CT images from all patients with pneumonitis and were reviewed at each site to confirm the presence of pneumonitis and standardize classifications and measurements. Longitudinal imaging findings were evaluated, including the time of maximal opacities and resolution. Imaging findings of pneumonitis, including groundglass opacities, septal thickening, consolidation, and nodular opacities, were noted if present. Patients were categorized as having complete resolution of imaging findings (e.g. complete resolution of all groundglass opacities, septal thickening, etc. as noted above), partial resolution/improvement of imaging abnormalities, or lack of improvement or worsening. Clinical symptoms, as noted in the electronic medical record, were extracted and correlated with imaging findings. Pulmonary function tests (PFTs), when obtained at the discretion of the treating provider, were reviewed, and evaluated longitudinally. Histopathologic evaluation of lung biopsies, when performed (in 6 patients), was reviewed. Steroid treatment was defined as receiving at least 0.5mg/kg prednisone daily or equivalent.
Statistics
Patient demographics were assessed descriptively (Table 1). Time to symptom onset, imaging abnormalities, symptom resolution, and imaging improvement were evaluated using the Kaplan-Meier method. Age, treatment regimen (combination vs. single-agent), symptoms at presentation, need for hospitalization, and need for steroids were compared among patients with persistent imaging abnormalities vs. with patients with resolution using chi-square and Mann-Whitney U tests. Similarly, baseline imaging characteristics were correlated with resolution using chi-square testing. All statistical tests were performed using GraphPad version 6 (GraphPad Software, La Jolla, CA). P values of <0.05 were considered statistically significant.
Table 1:
Demographics and clinical features of pneumonitis
| Feature | Complete radiographic response (n=11) Number (%) |
Incomplete radiographic response (n=20) Number (%) |
p-value |
|---|---|---|---|
| Age (median) | 53 | 72.5 | 0.002 |
| Treatment | |||
| Ipilimumab + Nivolumab | 5 (45) | 8 (40) | 0.768 |
| Anti-PD-1 Monotherapy | 6 (55) | 12 (60) | 0.768 |
| Initially symptomatic | 6 (55) | 17 (85) | 0.064 |
| Symptoms resolved | 10 (91) | 16 (80) | 0.429 |
| Prior thoracic radiation | 0 (0) | 2 (10) | N/A |
| Prior lung surgery | 3 (27) | 1 (5) | 0.078 |
| Current smoker | 1 (9) | 2 (10) | 0.933 |
| Former smoker | 6 (55) | 11 (55) | 0.975 |
| Required steroids | 5 (45) | 18 (90) | 0.007 |
| Required infliximab | 0 | 1 (5) | 0.451 |
| Time to radiographic findings (median, months) | 5.7 | 4.5 | 0.869 |
| Radiographic features | |||
| Septal thickening | 1 (9) | 8 (40) | 0.070 |
| Groundglass opacities | 11 (100) | 16 (80) | 0.112 |
| Consolidation | 3 (27) | 9 (45) | 0.332 |
| Nodular opacities | 3 (27) | 7 (35) | 0.660 |
Results
Patients and clinical presentation
Among the three centers, we screened 821 patients treated with anti–PD-1 therapy (alone or in combination with ipilimumab) and identified 31 patients with anti–PD-1–induced pneumonitis (3.8%). Of these, 13 (42%) received combination ipilimumab and nivolumab, whereas 18 (58%) received anti–PD-1 monotherapy (10 with pembrolizumab, 8 with nivolumab) (Table 1). Median age was 60 years (range 35 – 82), and all patients had advanced melanoma.
Of these patients, 23 (74%) presented with respiratory symptoms (CTCAE version 5.0 grade 2–4 pneumonitis), whereas 8 (26%) were asymptomatic and diagnosed based on imaging abnormalities alone (CTCAE grade 1 pneumonitis). Of symptomatic patients, the most common symptoms were shortness of breath (n=16; 70%) and cough (n=14, 61%). Less common symptoms included pleuritic pain (n=2), wheezing (n=1), fever (n=1), and hemoptysis (n=1). No asymptomatic patients were hospitalized whereas 11 of 23 symptomatic patients were admitted to the hospital (48%). Similarly, no asymptomatic patients received corticosteroids, and only one discontinued anti–PD-1 (for pneumonitis and concurrent refractory anti–PD-1–induced arthritis). In contrast, 22 of 23 (96%) symptomatic patients received corticosteroids, and all discontinued anti–PD-1 for pneumonitis. One patient received infliximab for lack of improvement on an initial course of steroids, resulting in slow improvement of symptoms, although inhaled bronchodilators and corticosteroids provided more symptom relief. The median time from treatment start to symptom onset was 4.8 months (range 0.2–21.8 months).
Six patients had bronchoscopy with transbronchial biopsies to establish the diagnosis. Of these, two biopsies demonstrated normal lung parenchyma, two showed organizing pneumonia, one showed non-caseating granulomas (in a patient with bibasilar patchy and nodular infiltrates and lack of hilar adenopathy), and one showed lymphocytes with histoplasma (in a hilar lymph node biopsy in a patient with patchy groundglass opacities and negative histoplasma antigens from blood and urine; all symptoms resolved with steroids). All biopsies were performed in the initial diagnostic setting rather than for delayed and persistent imaging findings.
Radiographic findings
The median time to first radiographic finding was 4.8 months (range 0.6–20 months), similar to time of first symptom onset (median 4.8 months). Asymptomatic patients generally had a later onset of radiographic findings than symptomatic patients (median radiographic onset 8.2 months vs. 3.7 months, logrank p=0.35), although this was not statistically significant (Supplementary Fig. S1). Imaging findings were diverse and included groundglass opacities (n=27, 87%), consolidation (n=12, 39%), nodular opacities (n=10, 32%), and septal thickening (n=9, 29%). In most patients (58%), the initial scan represented the peak imaging findings, with stability or improvement in future scans. In the remaining 42%, the median time from initial imaging to peak imaging abnormalities (i.e. the “worst” scan) was 44 days (range 7–366 days).
Resolution
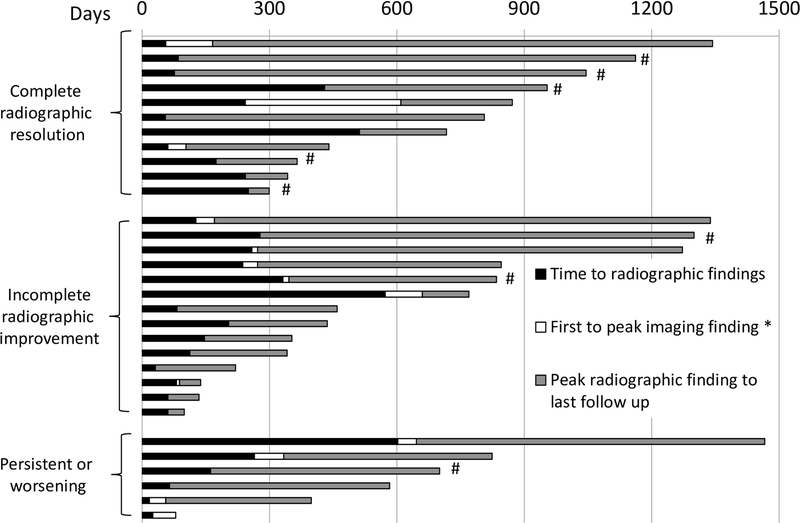
With a median follow up of 22.1 months, 19 of 23 (83%) symptomatic patients had resolution of their respiratory symptoms. Of the 4 with persistent symptoms, one had wheezing, one had cough, and two had dyspnea on exertion, although all were substantially improved from their initial presentation. By contrast, only 11 of 31 patients (35%) had complete resolution of all imaging findings at the time of last follow-up. Of these, 6 (19%) had <6 months follow-up from their initial imaging findings. Two patients (33%) had complete resolution, whereas 4 (67%) retained imaging abnormalities. Twenty-five (81%) had imaging follow-up >6 months (median 17.2 months, range 6.2–42.4 months), and of these, only 9 (36%) had complete resolution, suggesting that persistent findings did not simply represent short follow-up time (Fig. 1). Most patients with persistent imaging findings (14 of 20; 70%) had some improvement but residual scarring (Fig. 2), nodularity, or groundglass opacities. Six patients (30%) had no improvement or worsening at their most recent scans.
Figure 1:
Timing of radiographic findings based on CT scans in 31 patients with pneumonitis, including initial and peak imaging findings; each bar represents one patient. Day 0 represents first treatment with anti–PD-1. *Time from initial radiographic findings to peak findings, if applicable; #Asymptomatic initial presentation
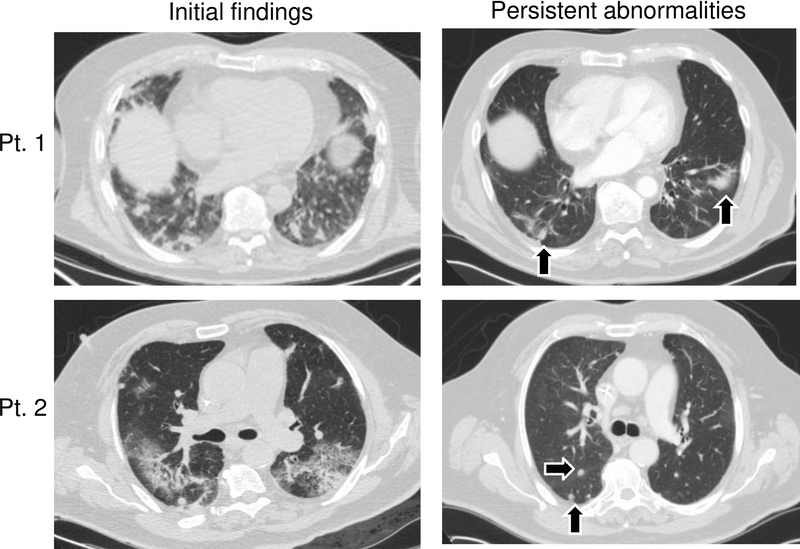
Figure 2:
Representative cross-sectional lung CT imaging of initial pneumonitis radiographic findings and persistent scarring following >6 months after initial findings (patient 1) and nodularity (patient 2) denoted by black arrows. Representative of the 31 patients.
Among 5 patients with serial pulmonary function tests (PFTs), lung function generally improved, although 1 patient had persistent moderate to severe obstruction with persistent wheezing (Supplementary Table S1). This 82-year-old patient had a history of childhood asthma that had been quiescent for many years. He had improvement in his shortness of breath and cough with steroids and infliximab, but the wheezing persisted and was managed successfully with inhaled corticosteroids and bronchodilators.
We then assessed whether initial presentation or treatment regimens correlated with radiographic resolution. Patients without imaging resolution were older than patients with complete resolution (median 72.5 vs. 53 years, p=0.002, Supplementary Fig. S2A), more frequently required steroids at initial presentation (90% vs. 45%, p=0.007, Supplementary Fig. S2B) and were more often symptomatic at presentation (85% vs. 55%, p=0.06, Supplementary Fig. S2C), although the latter was not statistically significant. Regimen type (anti–PD-1/ipilimumab vs. anti–PD-1 alone), median time to radiographic findings (5.7 vs. 4.5 months, p=0.87), and need for hospitalization (73% vs. 60%, p=0.48) did not predict imaging resolution. Radiographically, septal thickening at initial presentation appeared to be associated with lack of complete resolution (9% of those with complete resolution vs. 40% without; p=0.07), whereas groundglass opacities seemed to be more common in those with resolution (100% vs. 80%, p=0.11), although neither analysis was statistically significant. Neither consolidation at initial presentation (27% vs. 45%, p=0.33), nor nodular opacities (27 vs. 35%, p=0.66) were associated with radiographic resolution.
Discussion
In this study, we observed that most patients with anti–PD-1–induced pneumonitis had resolution of their overt clinical symptoms. However, about two-thirds had persistent imaging abnormalities with lung scarring and opacities, even with prolonged follow-up. Although most experienced radiographic improvement after a course of high-dose steroids, about 20% lacked improvement, and several patients had persistent respiratory symptoms and PFT abnormalities. This study demonstrated long-term evaluation of radiographic and clinical features of anti–PD-1–induced pneumonitis, which adds to the current knowledge of long-term effects using these therapies.
The long-term consequences of anti–PD-1 therapy remain incompletely studied, but their importance is increasing with more prevalent use of these agents across cancer types and into the adjuvant setting. Although most immune-related adverse events resolve following immunotherapy discontinuation and/or high-dose steroids, a subset of toxicities persist and become chronic. These include arthralgias/arthritis, endocrinopathies, and neurotoxicities (11–13). The longitudinal history of pneumonitis, by contrast, has not been closely investigated, likely because clinical symptoms tend to improve with steroid administration. However, persistent inflammation could be hypothesized to induce subtler but clinically meaningful deficits in pulmonary function or exercise capacity. Determining this with serial pulmonary function and exercise testing will be a critical objective, but will require a coordinated, multicenter, systematic effort involving pre-treatment and longitudinal screening of a large number of patients.
Another unaddressed question is whether active inflammation persists or whether imaging abnormalities simply represent scarring. In our study, most patients experienced resolution of their symptoms and improvement of their imaging findings, thus, suggesting that unrestrained inflammation (and progressive interstitial lung disease) did not occur (or was unlikely). Performing biopsies in patients with persistent imaging abnormalities will help address whether these lesions represent inflammation or simply scarring. We found that septal thickening correlated with more adverse radiographic outcomes, although the reason for this is not clear. However, one could speculate that inflammation in these areas predispose patients to a more vigorous scarring response. Finally, the role of thoracic radiation therapy and risk of developing pneumonitis with persistent radiographic changes and prolonged symptoms has not been well-studied, and although evaluating patients with melanoma treated with immune checkpoint inhibitors is not ideal to understand this phenomenon, it is logical to extend this type of analysis to patients with NSCLC who are often treated with radiation therapy to the thorax prior to immune checkpoint inhibition. However, the type of underlying cancer (specifically, lung cancer with prior radiation and pre-existing pulmonary comorbidities) may affect the long-term clinical and radiographic course of patients with pneumonitis.
Limitations of this study include the retrospective design. As such, patients did not have a standard follow-up time, and, thus, slower developing resolution may have not been captured in some patients. Further, no functional analysis was performed in most patients to determine whether the imaging abnormalities corresponded with lung function deficits.
In conclusion, we observed resolution of clinical symptoms from anti–PD-1–induced pneumonitis in most patients, but frequent persistence of scarring, nodularity, and groundglass opacities. Performing serial pulmonary function testing and obtaining tissue diagnoses are critical next steps to understand the pathogenesis of these findings and determine whether subclinical effects on respiratory function occur. Understanding the long-term effects of these commonly-used therapeutics will be a major issue over the next decade.
Supplementary Material
Acknowledgements:
DBJ is funded by NCI/NIHK23 CA204726, the American Cancer Society Institutional Resource Grant, the Melanoma Research Foundation/BMS Young Investigator Award, and the James C. Bradford Jr. Melanoma Fund. ZE was supported by NCI Skin SPORE 5P50CA168536
Conflicts of interest: DBJ is on advisory boards for Array Biopharma, BMS, Incyte, Merck, and Novartis, and receives research funding from BMS and Incyte. FJF receives research support from BTG PLC. RJS is a consultant/serves on advisory boards for Array Biopharma, Amgen, Merck, Novartis, Compugen, Replimmune, Syndax, and receives research funding from Amgen and Merck. ZE was on advisory boards for Array Biopharma and Regeneron.
References
- 1.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med. 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolchok JD. PD-1 Blockers. Cell. 2015;162:937. [DOI] [PubMed] [Google Scholar]
- 3.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med. 2017;377:1824–35. [DOI] [PubMed] [Google Scholar]
- 4.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 5.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med. 2018;378:158–68. [DOI] [PubMed] [Google Scholar]
- 6.Wang DY, Johnson DB, Davis EJ. Toxicities Associated With PD-1/PD-L1 Blockade. Cancer J. 2018;24:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson DB, Chandra S, Sosman JA. Immune Checkpoint Inhibitor Toxicity in 2018. JAMA. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishino M, Sholl LM, Hodi FS, Hatabu H, Ramaiya NH. Anti-PD-1-Related Pneumonitis during Cancer Immunotherapy. N Engl J Med. 2015;373:288–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DB, Friedman DL, Berry EG, Decker I, Ye F, et al. Survivorship in immune therapy: assessing chronic immune toxicities, health outcomes, and functional status among long-term ipilimumab survivors at a single referral center. Cancer Immunol Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faje AT, Sullivan R, Lawrence D, Tritos NA, Fadden R, et al. Ipilimumab-induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients with Metastatic Melanoma. J Clin Endocrinol Metab. 2014:jc20142306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.