Abstract
Nuclear receptor 4A1 (NR4A1, Nur77) is overexpressed in Rhabdomyosarcoma (RMS), and inactivation of NR4A1 (siNR4A1) or treatment with the NR4A1 antagonist 1,1-bis(3’-indoly)-1-(p-hydroxy-phenyl)methane (DIM-C-pPhOH) has antiproliferative and pro-apoptotic effects on RMS cells. However, the mechanism by which NR4A1 inhibition exerts these effects is poorly defined. Here, we report that NR4A1 silencing or inhibition resulted in accumulation of reactive oxygen species (ROS) and ROS - dependent induction of the tumor suppressor - like cytokine interleukin-24 (IL-24) in RMS cells. Mechanistically, NR4A1 was found to regulate the expression of the pro-reductant genes thioredoxin domain-containing 5 (TXNDC5) and isocitrate dehydrogenase 1 (IDH1), which are down-regulated in RMS cells following NR4A1 knockdown or inhibition. Silencing TXNDC5 and IDH1 also induced ROS accumulation and IL-24 expression in RMS cells, suggesting that NR4A1 antagonists mediate their antiproliferative and apoptotic effects through modulation of pro-reductant gene expression. Finally, cotreatment with the antioxidant glutathione or IL-24 blocking antibody reversed the effects of NR4A1 inhibition, demonstrating the importance of both ROS and IL-24 in mediating the cellular responses. Implications: Overall, these data elucidate the mechanism by which NR4A1 inhibition functions to inhibit the proliferation, survival, and migration of RMS cells.
Keywords: NR4A1, antagonists, IL-24, TXNDC5, IDH1
INTRODUCTION
Rhabdomyosarcoma (RMS) is a cancer primarily seen in children and adolescents and represents > 50% of soft tissue sarcomas in this younger age group. The prognosis of patients with RMS is dependent on multiple factors including age of the patient, the site of the tumor, and the tumor size, weight, and degree of metastasis from the tumor site (1–3). Prognosis also differs among patients classified into the major tumor types, namely Embryonal RMS (ERMS) and Alveolar RMS (ARMS) which is characterized by expression of genes resulting from the fusion of PAX3 and PAX7 with FOXO1 (PAX3-FOXO1 and PAX7-FOXO1) (4, 5). The PAX3-FOXO1 fusion gene is the critical prognostic marker for ARMS patients with metastatic disease, with an estimated overall 4 year survival of 8% compared to 75% survival rate of patients with PAX7-FOXO1-expressing tumors (6–8). Five year survival rates can be as high as 83% in younger patients (age 1-4 years) with localized disease (3), however, among childhood and adolescent cancers the decline in mortality has been minimal with RMS patients. Results of PAX3-FOXO1 knockdown or overexpression studies demonstrate the functional importance of this fusion gene in maintaining the aggressive ARMS cancer cell phenotype and this is due, primarily, to the pro-oncogenic PAX3-FOXO1-regulated genes (9–11). Moreover, cancer patients treated with cytotoxic drug therapy for childhood cancers exhibit a high rate of chronic disease incidence (95.5%) in their 40’s (12, 13) indicating that there is a critical need for development of innovative less toxic therapies for treating RMS patients.
Development of new mechanism-based drugs for treatment of RMS is ongoing and this includes ROS – inducing anticancer agents, which have been identified in genomic studies and are effective tumor growth inhibitors in RMS patient-derived xenografts (14–18). The nuclear orphan receptor NR4A1 is overexpressed in tumors from RMS patients and in other solid tumors (19–27) and studies in the laboratory have been investigating the functions of NR4A1 in RMS and solid tumor-derived cancer cell lines by RNA interference (RNAi) (23–25, 27–32). The results indicate that NR4A1 is pro-oncogenic and in RMS NR4A1 regulates cell proliferation, survival and migration/invasion and associated genes. The bis-indole derived compound 1,1-bis(3’-indolyl)-1-(p-hydroxyphenyl) methane (DIM-C-pPhOH/CDIM8) acts as an NR4A1 antagonist and inhibits NR4A1 regulated pro-oncogenic responses and genes (19–27). Analysis of changes in gene expression after knockdown of NR4A1 or PAX3-FOXO1 or treatment with the NR4A1 antagonist DIM-C-pPhOH identified interleukin-24 (IL-24) as a gene induced by all three treatments (33). In this study we demonstrate that silencing of NR4A1 by RNAi or treatment with DIM-C-pPhOH resulted in ROS-dependent induction of IL-24 which is due to downregulation of two pro-reductant NR4A1-regulated genes, namely thioredoxin domain-containing 5 (TXNDC5) and isocitrate dehydrogenase 1 (IDH-1). Results of this study demonstrate a novel pathway for inducing expression of the tumor suppressor-like IL-24 directly in RMS cells. Like most cancer cell lines IL-24 expression in RMS cells is low but is inducible. IL-24 exhibits a broad spectrum of anticancer activities in multiple cancer cell lines and is being developed for clinical applications as a biotherapeutic (34–36). Results of this study illustrate a novel small molecule approach for delivering IL-24 in tumors.
MATERIALS AND METHODS
Cell lines, antibodies and cell proliferation
RD and Rh30 rhabdomyosarcoma cell lines were initially purchased from American Type Culture Collection (Manassas, VA) and authenticated in 2014 (Promega Powerplex 18D) at Duke University DNA analysis Laboritories Mycoplasma contamination was routinely monitored and cell lines were mycoplasma-free. Cells were maintained 37˚C in the presence of 5% CO2 in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium with 10% fetal bovine serum with antibiotic or RPMI-1640 Medium with 10% fetal bovine serum and antibiotic respectively that was purchased from GenDepot (Barker, TX). β-actin antibody Dulbecco’s Modified Eagle’s Medium, and RPMI-1640 Medium, and 36% formaldehyde were purchased from Sigma-Aldrich (St. Louis, MO). Hematoxylin was purchased from Vector Laboratories (Burlingame, CA). Sp1 antibody was purchased from Abcam (Cambridge, MA) and Glutathione (GSH) reduced free acid was purchased from Millipore (Temecula, CA). TXNDC5 and IDH1 antibodies were purchased from Genetex (Irvine, CA). PAX3-FOXO1A were purchased from Cell Signaling technologies (Danvers, MA). NR4A1 antibody was purchased from Novus Biologicals (Littleton, CO). Apoptotic, Necrotic, and Healthy Cells Quantification Kit was purchased from Biotium (Hayward, CA). Cells were visualized under an EVOS fl, Fluorescence microscope, from Advanced Microscopy Group using a multiband filter set for FITC, rhodamine, and DAPI.
RD and Rh30 rhabdomyosarcoma cells (1.0 x 105 per well) were plated in 12 well plates and allowed to attach for 24 hours and cells were treated with DIM-C-pPhOH (dimethyl sulfoxide, DMSO, as empty vehicle or pretreated with GSH 3 hours prior to treatment) for 24 hours or by transfection of siIL24, siTXNDC5, siIDH1, or siNR4A1 (iGL2 as control siRNA with lipofectamine vehicle) for 72 hr. Cells were then trypsinized and counted after respective treatment time intervals using a Coulter Z1 cell counter and growth inhibition was determined. Each experiment was carried out in triplicate, and results were expressed as the mean ± SE for each set of experiments.
Annexin V staining
RD and Rh30 rhabdomyosarcoma cell (1.0 x 105 per well) were seeded in 2-well Nunc Lab-Tek chambered B#1.0 Borosilicate coverglass slides from Thermo Scientific and were allowed to attach for 24 hours. The medium was then changed to DMEM/Ham F-12 medium contained 2.5% charcoal-stripped fetal bovine serum, and after various treatments Annexin V staining was analyzed by apoptotic and necrotic assay kit (Biotium CA), which contained fluorescein isothiocyanate-annexin-V, ethidium homodimer III and Hoechst 3342 as described (30–33).
Boyden Chamber Assay
RD and Rh30 rhabdomyosarcoma cells (3.0 x 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 h. Cells were seeded and subsequently treated with DIM-C-pPhOH for 24 hours (+/− GSH 3 h prior to treatment) or with 100 nm of siTXNDC5, siIDH1, siIL-24, or siNR4A1 for 48 hours. Cells were trypsinized, counted then placed in 12-well 8.0μm pore ThinCerts from Greiner bio-one (Monroe, NC) allowed to migrate for 24 hr, fixed with formaldehyde, and then stained with hematoxylin. Cells that migrated through the pores were then counted.
Western blot analysis
RD and Rh30 rhabdomyosarcoma cells (3.0 x 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 h. Cells were seeded and subsequently treated with varying concentration of DIM-C-pPhOH for 24 hours or with 100 nm of respective siRNA for 72 hours. Western blots were determined with Immobilon western chemiluminescence substrates (Millipore, Billerica, MA) to develop images captured on a Kodak 4000 MM Pro image station (Molecular Bioimaging, Bend, OR) as described (30–33).
Small interfering RNA interference assay
RD and Rh30 rhabdomyosarcoma cells were seeded (1.2 x 105 per well) in six well plates in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and left to attach for 24 hours. Knockdown was carried out using Lipofectamine 2000 reagent according to the manufactures protocol. Small inhibitory RNAs and GL2 (non-specific oligonucleotide) were prepared and purchased Sigma-Aldrich (St. Louis MO). The siRNA complexes used in the study are as follows: siGL2-5’, CGU ACG CGG AAU ACU UCG A; siNR4A1, SASI_Hs02_00333289; siTXNDC5, SASI_Hs01_00211116; siIDH1, SASI_Hs02_00340497; siIL-24, SASI_Hs01_00097938
Generation and measurement of ROS
Cellular ROS levels were ascertained using the cell permeable probe CM-H2DCFDA (5-(and-6)-chloromethyl-2’7’ dichlorodihydrofluorescein diacetate acetyl ester) from Invitrogen (Grand Island, NY). CM-H2DCFDA is non-fluorescent until cleavage of the acetyl groups by intracellular esterases and oxidation that transpires within the cell. Following treatment of the cells for 12 hours with DIM-C-pPhOH (+/− 5mM GSH 3 h prior to treatment) for 72 hours, cells plated on a 6-well culture plate were trypsinized, neutralized, then loaded with 10μM of probe for 20 min, washed once with serum free medium, and then ROS was measured by flow cytometry using Accuri’s C6 Flow Cytometer (Accuri, Ann Arbor, MI).
Statistical analysis
Statistical significance of differences between the treatment groups was determined by student’s t test. The results are expressed as means with error bars representing 95% confidence intervals for 3 experiments for each group unless otherwise indicated, and a P value less than 0.05 was considered statistically significant. All Statistical tests were 2-sided.
RESULTS
It was previously reported that inactivation of NR4A1 by RNA interference (RNAi) or treatment with a C-DIM/NR4A1 antagonist resulted in downregulation of PAX3-FOXO1 and induction of IL-24 (33); downregulation of PAX3-FOXO1 by RNAi also resulted in the induction of IL-24. These results suggest that inactivation of NR4A1 using the antagonist DIM-C-pPhOH (CDIM8) would proceed via NR4A1 ⇥ PAX3-FOXO1 ⇥ IL-24. Results in Figure 1A show that DIM-C-pPhOH (CDIM8) induced IL-24 and downregulated PAX3-FOXO1 and PAX3-FOXO1-regulated genes (NMyc, RASSF4, MyoD1, Gremlin, DAPK) in wild-type Rh30 cells however this was not observed in cells where IL-24 is silenced by RNAi and quantitation of the bands is illustrated in Supplemental Figure 1A. These results suggest that in Rh30 cells, CDIM8-mediated downregulation of PAX3-FOXO1 is IL-24-dependent and in cells treated with CDIM8 IL-24 is upstream from PAX3-FOXO1. This observation is supported by the time course study illustrated in Figure 1B showing that DIM-C-pPhOH rapidly induced IL-24 (within 3 hr) and this was followed by downregulation of PAX3-FOXO1 (after 3-6 hr). Moreover, treatment of Rh30 cells also modulates expression of several IL-24 regulated genes (Fig. 1C) (34–36) and similar effects were observed for other NR4A1 ligands (37) (Supplemental Figure 1B). Further evidence that IL-24 is upstream from PAX3-FOXO1 is illustrated in Figure 1D which shows that overexpression of IL-24 in three ARMS cell lines (Rh30, Rh18 and Rh41) decreased expression of PAX3-FOXO1 and PAX3-FOXO1-regulated genes. Moreover, further analysis of tumor lysates from a previous mouse xenograft study (33) showed that administration of adenoviral IL-24 directly into the tumor decreased expression of PAX3-FOXO1 and PAX3-FOXO1-regulated genes (Fig 1E). These results confirm that IL-24 decreases expression of PAX3-FOXO1, suggesting that induction of IL-24 by silencing NR4A1 or by treatment with CDIM8 is the major pathway for downregulation of PAX3-FOXO1.
Figure 1.
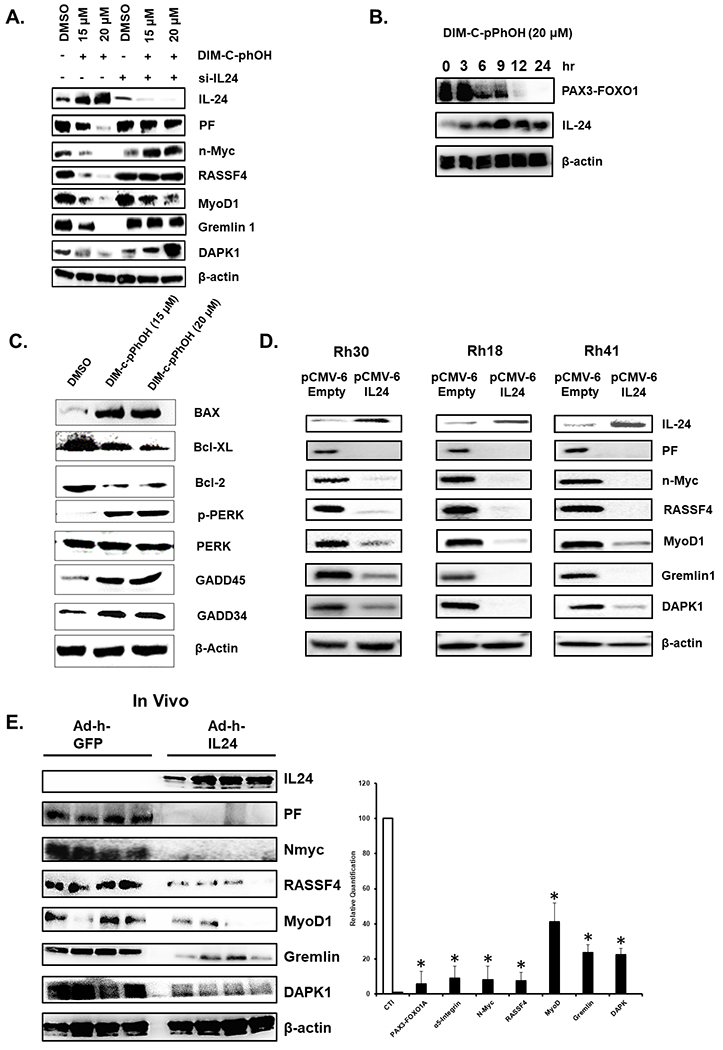
Induction of IL-24 by NR4A1 antagonist. (A) Rh30 cells were treated with CDIM8 in the absence (-) or presence of IL-24 knockdown (siIL-24) and whole cell lysates were analyzed by western blots. (B) Rh30 cells were treated with 20 uM CDIM8 for different times or for 24 hours (C) and whole cell lysates were analyzed by western blots. (D) ARMS cells were transfected with pCMV-IL24 expression plasmid and after 24 hour whole cell lysates were analyzed by western blots. (E) Tumor lysates from athymic nude mice bearing Rh30 cells as xenografts (39) and treated with corn oil (control) or adenoviral – IL-24 were analyzed by western blots. Results are expressed as means ± SD for at three replicate determinations for each treatment group (E) and significant (p<0.05) effects (*) are indicated.
The role of IL-24 in mediating the downstream effects of DIM-C-pPhOH or knockdown of NR4A1 (siNR4A1) on regulating PAX3-FOXO1 expression and inhibiting cell growth, survival and invasion was investigated in Rh30 cells. Figure 2A shows that CDIM8 or siNR4A1-mediated induction of IL-24 and the subsequent decreased repression of PAX3-FOXO1 are reversed in cells co-transfected with siIL-24. Moreover, CDIM8 and siNR4A1-mediated inhibition of Rh30 cell growth (Fig. 2B), induction of Annexin V staining (Fig. 2C), and inhibition of Rh30 cell migration (Fig. 2D) were also attenuated in cells co-transfected with siIL-24.
Figure 2.
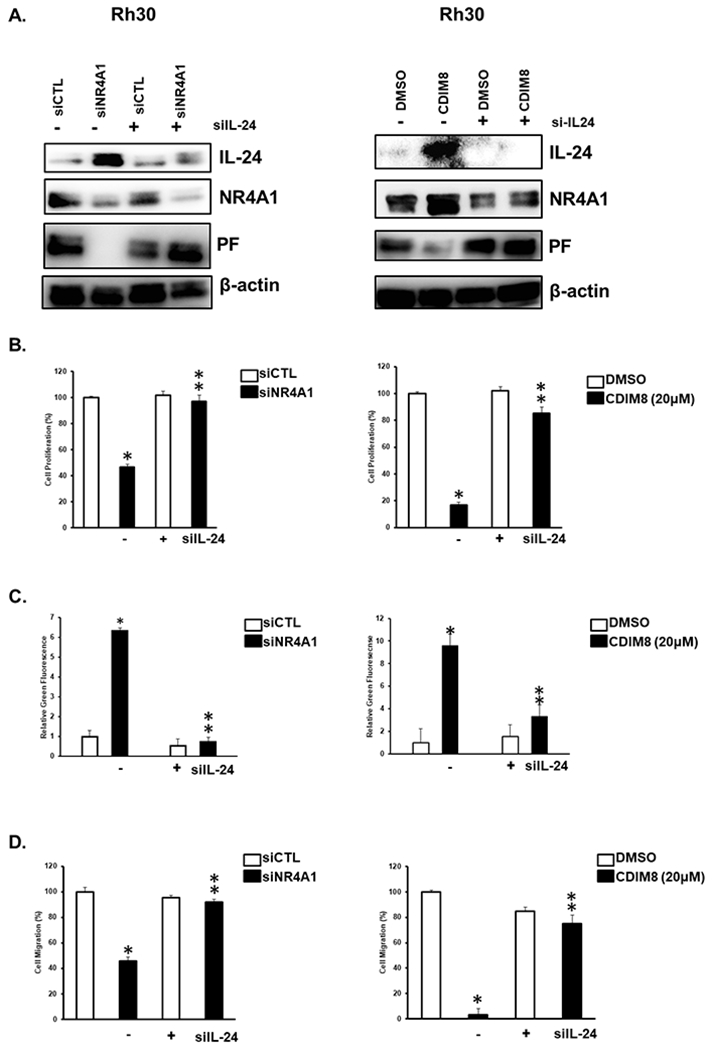
Anticarcinogenic effects of NR4A1 inactivation are due to induction of IL-24. Rh30 cells were transfected with siNR4A1 or treated with 20 mM CDIM8 in the absence or presence of siIL-24; effects on protein expression (A) cell proliferation (B), induction of Annexin V staining (C), and cell migration (D) were determined as outlined in the Materials and Methods. Results are expressed as means ± SD for at three replicate determinations for each treatment group (B, C + D) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
Since previous studies show that IL-24 is induced by reactive oxygen species (ROS) (38) and siNR4A1 and CDIM8 also induce ROS in RMS cells (28), we therefore investigated the role of CDIM8/siNR4A1-induced ROS in mediating induction of IL-24. CDIM8 and NR4A1 silencing induce ROS in Rh30 cells and this response was attenuated in cells cotreated with GSH (Fig. 3A). this same treatment also induces IL-24 and decreases PAX3-FOXO1 expression in Rh30 cells and these responses are also attenuated after co-treatment with GSH (FIG. 3B). The role of siNR4A1- and CDIM8-induced ROS on cell proliferation (Fig. 3C), Annexin V staining (Fig. 3D), and cell migration (Fig. 3E) was confirmed since the siNR4A1/CDIM8-mediated responses were all significantly inhibited after cotreatment with GSH. These results suggest that siNR4A1/CDIM8-mediated induction of ROS (Fig. 3) and subsequent induction of IL-24 (Figs. 1 and 2) play a major role in treatment-related inhibition of Rh30 cell migration and invasion and induction of apoptosis.
Figure 3.
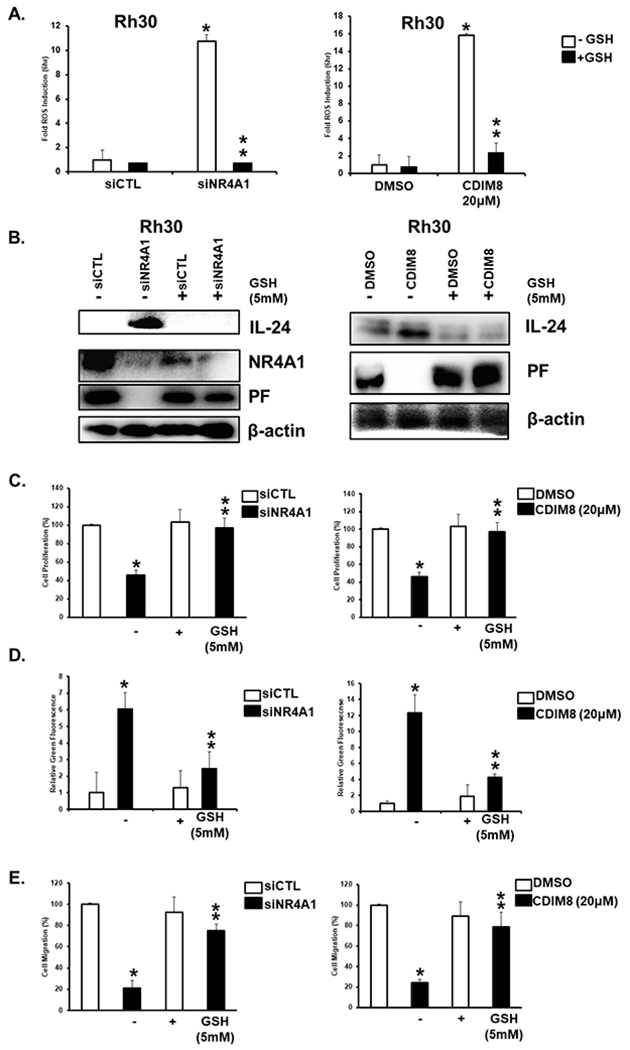
Role of ROS in mediating the anticancer activities of NR4A1 silencing or CDIM8. (A) Rh30 cells were transfected with siNR4A1 or treated with CDIM8 in the presence or absence of 5 mM GSH and induction of ROS was determined by fluorescence using the cell permeable probe as outlined in the Materials and Methods. (B) Cells were treated as described in A and effects on protein expression (from whole cell lysates) were determined by western blots. Rh30 cells were treated as described in A and effects on cell proliferation (C), Annexin V staining (D), and migration (E) were determined as outlined in the Materials and Methods. Results are expressed as means ± SD for at three replicate determinations for each treatment group (A, C, D+E) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
Since induction of ROS and IL-24 by DIM-C-pPhOH or NR4A1 silencing is PAX3-FOXO1-independent, we therefore investigated the importance of this pathway in ERMS (RD) cells that do not express PAX3-FOX01. Treatment of RD cells with siNR4A1 or CDIM8 induced ROS (Fig. 4A), IL-24 (Fig. 4B), inhibited growth (Fig. 4C), induced Annexin V staining (Fig. 4D) and inhibited RD cell migration (Fig. 4E), and all of these responses were significantly attenuated after cotreatment with GSH. Induction of IL-24 (Fig. 4F), inhibition of cell growth (Fig. 4G), induction of Annexin V staining (Fig. 4H) and inhibition of cell migration (Fig. 4I) by CDIM8 or siNR4A1 were also attenuated by simultaneous knockdown of IL-24 (siIL-24). This indicates that the NR4A1 (silencing or inactivation) → ROS → IL-24 is common to both ERMS (RD) and ARMS (Rh30) cell lines and is an important contributor to the anticancer activity observed after inactivation of NR4A1.
Figure 4.
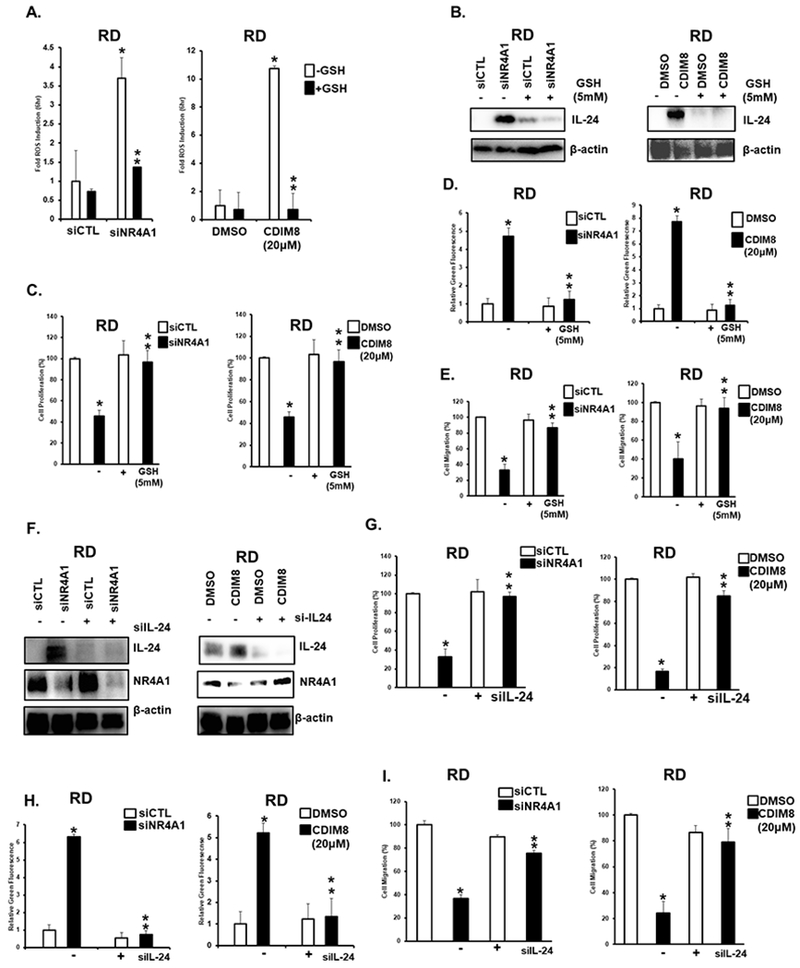
Role of ROS and IL-24 in mediating the anticancer activities of NR4A1 inactivation by siNR4A1 or CDIM8 in RD cells. A. RD cells were transfected with siNR4A1 or treated with CDIM8 in presence or absence of 5 mM GSH and ROS was determined using the cell permeable fluorescent probe as outlined in the Materials and Methods. RD cells were treated as described in A and effects on protein expression (B), cell proliferation (C), Annexin V staining (D) and cell migration (E) were determined as outlined in the materials and methods. (F) RD cells were transfected with siNR4A1 or treated with CDIM8 in the presence or absence of siIL-24 (co-transfected) and whole cell lysates were analyzed by western blots. RD cells were treated as outlined in F and effects on cell proliferation (G), Annexin V staining (H) and cell migration (I) were determined as outlined in the Materials and Methods. Results are expressed as means ± SD for at three replicate determinations for each treatment group (D) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated. Results are expressed as means ± SD for at three replicate determinations for each treatment group (A, C, D, E, G+H) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
TXNDC5 and IDH1 are NR4A1-regulated genes (28–31) that maintain levels of cellular reductants and therefore we further investigated the role downregulation of these genes by CDIM8/siNR4A1 in the induction of ROS and IL-24. Results in Figures 5A and 5B show that NR4A1 silencing or treatment with CDIM8 decreased expression of TXNDC5 and IDH1 in RD and Rh30 cells and this response was not blocked after cotreatment with GSH, suggesting that this response was ROS-independent due to decreased expression of these gene products. In contrast, knockdown of TXNDC5 (Fig. 5C) and IDH1 (Fig. 5D) by RNA interference induced ROS and this response was attenuated in RD and Rh30 cells cotreated with GSH. These results suggest that induction of ROS by CDIM8 or by NR4A1 silencing is due, in part to their effects on decreasing expression of the pro-reductant genes TXNDC5 and IDH1.
Figure 5.
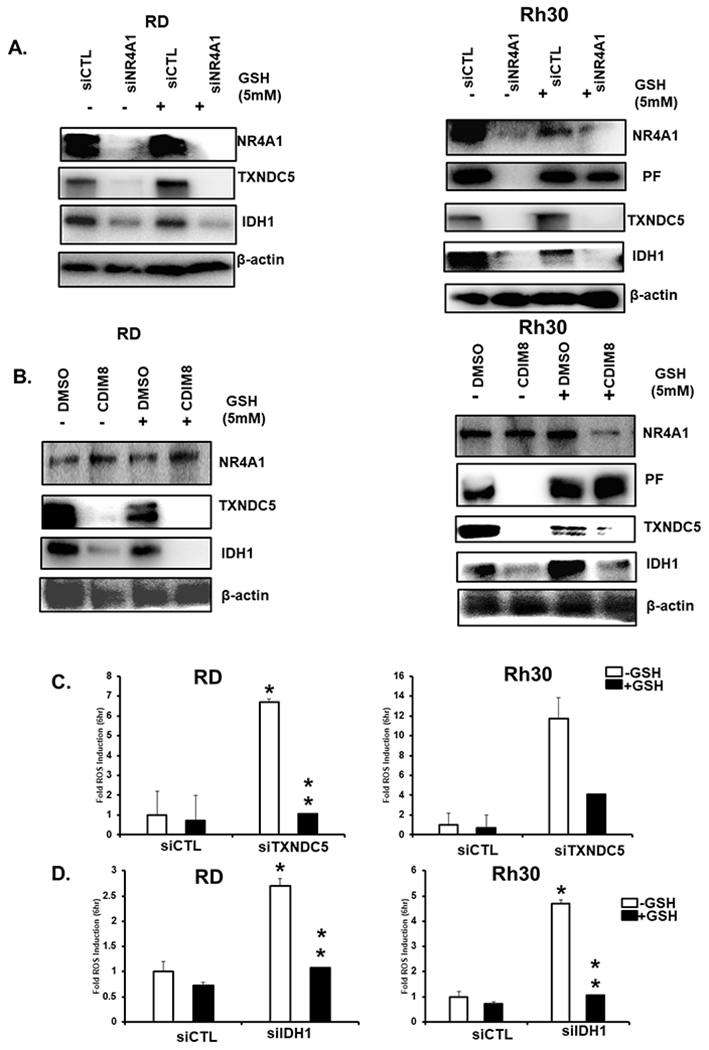
Regulation of TXNDC5 and IDH1 by NR4A1 and the effects of knockdown of TXNDC5 and IDH1 on ROS. RD and Rh30 cells were transfected with siNR4A1 (A) or treated with 20mM CDIM8 (B) in the presence or absence of GSH and after 24 hour whole cell lysates were analyzed by western blots. RD and Rh30 cells were transfected with siTXNDC5 (C) or siIDH-1 (D) to knockdown the TXNDC5 and IDH-1 genes respectively and induction of ROS was determined using the fluorescent cell-permeable probe. Results are expressed as means ± SD for at three replicate determinations for each treatment group (C+D) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
Since induction of ROS is important for enhancing expression of IL-24, and its subsequent inhibition of cell growth, survival and migration, we examined the effects of knockdown of TXNDC5 and IDH1 on these same parameters in RD and Rh30 cells. Transfection of RD and Rh30 cells with siTXNDC5 (Fig. 6A) or siIDH1 (Fig. 6B) increased expression of IL-24 and this was abrogated after cotreatment with GSH. Using this same protocol, we also observed that siTXNDC5 and siIDH1 decreased RD and Rh30 cell proliferation (Fig. 6C), induced Annexin V staining (Fig. 6D), and decreased invasion (Fig. 6E) and these responses were inhibited by cotreatment with GSH.
Figure 6.
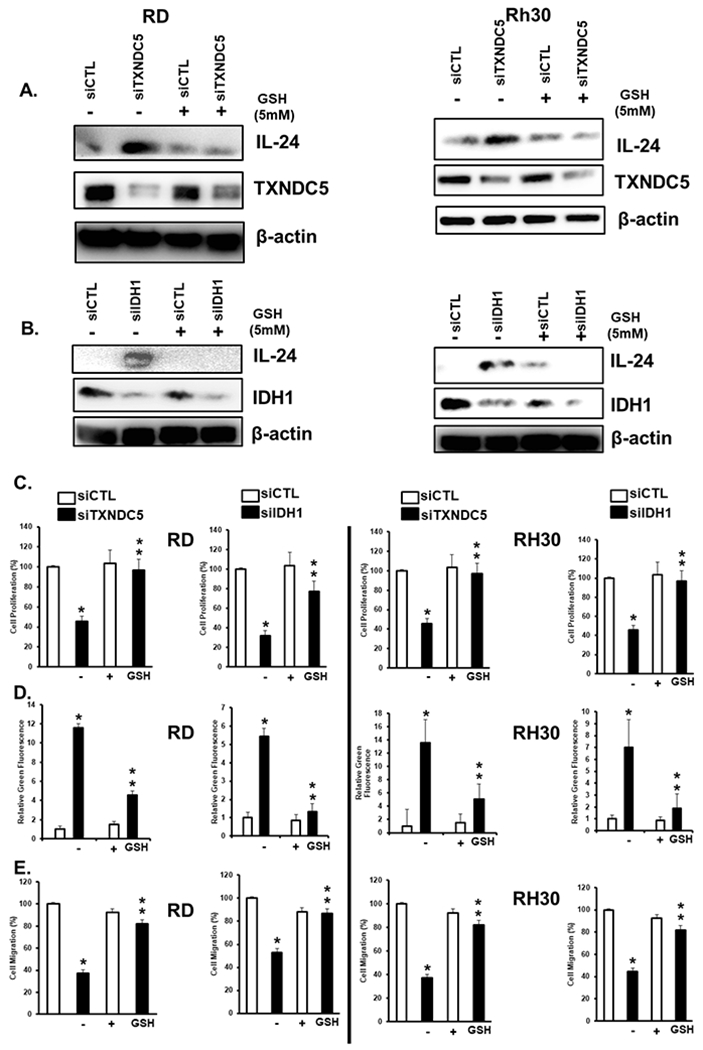
Knockdown of TXNDC5 and IDH-1 induced ROS-dependent anticancer activities. RD and Rh30 cells were transfected with siTXNDC5 (A) or siIDH-1 (B) in the presence or absence of 5 mM GSH and after 24 hours, whole cell lysates were analyzed by western blots. RD and Rh30 cells were transfected with siTXNDC5 or siIDH-1 in the presence or absence of GSH and the effects on cell proliferation (C), Annexin V staining (D), and cell migration (E) were determined as outlined in the Materials and Methods. Results are expressed as means ± SD for at three replicate determinations for each treatment group (C, D+E) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
The role of NR4A1-mediated downregulation of TXNDC5 and IDH1 as intermediates in generating ROS-dependent induction of IL-24 was investigated in RD and Rh30 cells transfected with oligonucleotides targeting TXNDC5 (siTXNDC5) (Fig. 7A) and IDH1 (siIDH1) (Fig. 7B). Both oligonucleotides induced IL-24 expression and in Rh30 cells, PAX3-FOXO1 expression was decreased in Rh30 cells and these responses were blocked in cells co-transfected with siIL-24. Results in Figures 6C–6E demonstrate that siTXNDC5/siIDH1-mediated inhibition of cell growth, survival (induced Annexin V staining), and migration was ROS-dependent (inhibited by GSH). Using this same experimental approach, we show that the effects of siTXNDC5/siIDH1 on cell proliferation, Annexin V staining and migration of RD and RH30 cells (Figs. 7C–7E) were also blocked by silencing of IL-24. Thus, a major pathway associated with the anticancer activity of CDIM8/NR4A1 antagonists involves initial decreased transcription of TXNDC5 and IDH1, induction of ROS, and induction of the tumor suppressor-like cytokine IL-24 which triggers downstream inhibition of RMS cell growth and migration and induction of apoptosis (Fig. 7F).
Figure 7.
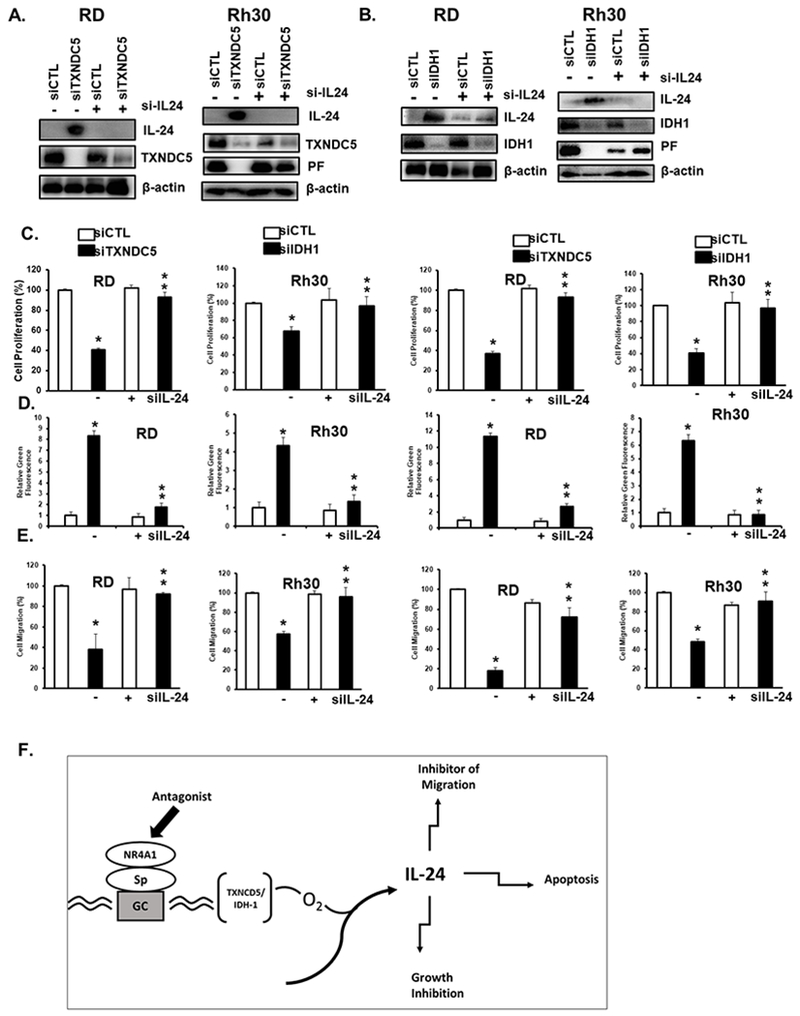
IL-24 – induced anticancer activities are due to silencing of TXNDC5 and IDH-1. RD and Rh30 cells were transfected with siTXNDC5 (A) or siIDH-1(B) in the presence or absence of siIL-24 and whole cell lysates were analyzed by western blots. RD and Rh30 cells were treated as described in (A)/(B) in the presence or absence of co-transfected siIL-24 and the effects on cell proliferation (C), Annexin V staining (D), and cell migration (E) were determined as outlined in the Materials and Methods, (F) Model describing the anticancer activities of NR4A1 antagonists. Results are expressed as means ± SD for at three replicate determinations for each treatment group (C, D+E) and significant (p<0.05) effects (*) and reversal of these responses (**) are indicated.
DISCUSSION
Interleukin-24 [or melanoma differentiation associated gene-7 (MDA-7)] is a member of the IL-10 family of cytokines secreted by immune cells. IL-24 was initially identified and cloned from terminally differentiated human metastatic melanoma cells (34–36). Expression of MDA-7/IL-24 protein is low or absent in the majority of cancer cells compared to their normal counterpart however it can be induced by appropriate treatments. Adenoviral-IL-24 has been used in preclinical studies to investigate the anticancer activities of this biotherapeutic alone and in combination therapies using several different models (34, 35, 39, 40). Fisher and coworkers demonstrated that ROS-inducing agents, including HDAC inhibitors, overcame resistance to IL-24-mediated therapy in cancer cell lines (34–36, 39–41), and the HDAC inhibitors trichostatin and butyrate induced IL-24 in melanoma cells (42). This observation is highly relevant for RMS since genomic analysis of ERMS patients “identified oxidative stress as a pathway of therapeutic relevance for RMS” and this was confirmed using ROS-inducing HDAC inhibitors (17).
Analysis of RNAseq data after treatment of Rh30 cells with DIM-C-pPhOH or knockdown of NR4A1 and PAX3-FOXO1 showed that all three treatments induced IL-24 and we characterized induction of IL-24 after PAX3-FOXO1 knockdown (33). These observations raised some interesting but unresolved questions; namely, in cells transfected with siNR4A1 or treated with the receptor antagonist DIM-C-pPhOH is IL-24 upstream or downstream from PAX3-FOXO1 and if it is upstream then what is the mechanism of IL-24 induction by NR4A1. Results illustrated in Figures 1 and 2 demonstrate CDIM8-mediated downregulation of PAX3-FOXO1 was IL-24-dependent and the IL-24 was upstream from PAX3-FOXO1. Moreover, the inhibition of cell growth and migration and induction of apoptosis by CDIM8 or siNR4A1 in Rh30 and RD (ERMS) cells was also inhibited by si-IL24 suggesting that induction of IL-24 is a major pathway associated with the anticancer activities of the NR4A1 antagonist CDIM8 and related compounds (34–36, 39–41) (Suppl. Fig. 1B) (24, 25, 27–33, 37). Previous studies with IL-24 demonstrated that this tumor suppressor-like cytokine regulated and large number of pathways and genes associated with the overall anticancer activity observed after induction or treatment with IL-24. Results in Figure 1C and Supplemental Figure S1B show that NR4A1 antagonists not only induce IL-24 but also several IL-24 – mediated responses including activation of Bax, decrease bcl-2 and bcl-x, activation of PERK phosphorylation and induction of GADD 45 and GADD 34 (43–47). Induction of these IL-24 – mediated responses undoubtedly contributes to the overall anticancer activities of NR4A1 antagonists which includes induction of apoptosis and activation of stress pathways. Moreover, there is also evidence that the effects of IL-24 are highly selective in targeting cancer cells and not normal cells (48) and similar results have been observed for CDIM/NR4A1 ligands (37) including the effects on C2C12 normal muscle cells (Suppl. Fig. S1C).
ROS-inducing anticancer drugs induce IL-24 (34–36, 39–41) and we observed that siNR4A1 or CDIM8 induced ROS in Rh30 and RD cells and effects of these treatments on inhibition of RMS cell proliferation, survival and migration were inhibited after co-treatment with the antioxidant GSH (Fig 3 and 4). Previous studies in RMS and other cancer cell lines (34–37) show that NR4A1 regulates expression of TXNDC5 and IDH1 which help to maintain cellular reductant levels (49, 50). In RMS cells, transfection with siNR4A1 or treatment with DIM-C-pPhOH decreased TXNDC5 and IDH1 expression and the responses observed were duplicated in separate experiments where TXNDC5 and IDH1 were silenced by RNAi (Fig 5 and 6). Decreased expression of both genes induced ROS, decreased RMS cell growth, survival and migration, these responses were attenuated after co-treatment with GSH, and comparable effects were observed in RMS cells in which NR4A1 was inactivated (Figs 6 and 7). These results suggested that induction of IL-24 by CDIM8/siNRA1 was due to decreased expression of TXNDC5and IDH1, which resulted in the induction ROS, and ROS-mediated induction of IL-24 (Fig 7F). Evidence that IL-24 was downstream from TXNDC5/IDH1 is summarized in Figure 7 which shows that inhibition of cell growth and migration and induction of apoptosis observed after silencing of TXNDC5/IDH1 (Fig 6C and 6E) were attenuated by silencing IL-24 (Fig 7A–7E).
Thus, results of this study demonstrated that NR4A1 antagonists represent a novel class of drugs that induce IL-24 in RMS and represent an alternative to adenoviral delivery for inducing IL-24 expression in tumors from RMS patients (35, 36, 39). In addition, we also demonstrate that the mechanism of ROS-induced IL-24 involves inactivation of NR4A1, which results in the induction of oxidative stress due to the decreased expression of the reductant genes TXNDC5 and IDH1 (Fig 7F). Since RMS tumors and particular ERMS are sensitive to inducers of oxidative stress, results of this study and previous reports (27, 28) support clinical applications of NR4A1 antagonists for RMS chemotherapy and ongoing studies are focused on developing more potent bis-indole derived compounds for treatment of this disease.
Supplementary Material
ACKNOWLEDGMENTS
The financial assistance of the Kleberg Foundation, the National Institutes of Health (P30-ES029067), and the Syd Kyle Chair endowment are gratefully acknowledged.
Footnotes
Conflict of Interest: There are no conflicts of interest to declare.
References
- 1.Parham DM, Ellison DA. Rhabdomyosarcomas in adults and children: an update. Arch Pathol Lab Med. 2006;130(10):1454–65. Epub 2006/11/09. doi: 10.1043/1543-2165(2006)130[1454:RIAACA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Ren S, Wang Z, Huang X, Sun L, Shao J, Ye Z. Prognostic factors for postoperative survival among patients with rhabdomyosarcoma of the limbs. Cancer Manag Res. 2018;10:4181–9. Epub 2018/10/17. doi: 10.2147/CMAR.S175734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Punyko JA, Mertens AC, Baker KS, Ness KK, Robison LL, Gurney JG. Long-term survival probabilities for childhood rhabdomyosarcoma. A population-based evaluation. Cancer. 2005;103(7):1475–83. Epub 2005/02/16. doi: 10.1002/cncr.20929. [DOI] [PubMed] [Google Scholar]
- 4.Barr FG, Galili N, Holick J, Biegel JA, Rovera G, Emanuel BS. Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;3(2):113–7. Epub 1993/02/01. doi: 10.1038/ng0293-113. [DOI] [PubMed] [Google Scholar]
- 5.Davis RJ, D’Cruz CM, Lovell MA, Biegel JA, Barr FG. Fusion of PAX7 to FKHR by the variant t(1;13)(p36;q14) translocation in alveolar rhabdomyosarcoma. Cancer Res. 1994;54(11):2869–72. [PubMed] [Google Scholar]
- 6.Missiaglia E, Williamson D, Chisholm J, Wirapati P, Pierron G, Petel F, Concordet JP, Thway K, Oberlin O, Pritchard-Jones K, Delattre O, Delorenzi M, Shipley J. PAX3/FOXO1 fusion gene status is the key prognostic molecular marker in rhabdomyosarcoma and significantly improves current risk stratification. J Clin Oncol. 2012;30(14):1670–7. doi: 10.1200/JCO.2011.38.5591. [DOI] [PubMed] [Google Scholar]
- 7.Davicioni E, Anderson MJ, Finckenstein FG, Lynch JC, Qualman SJ, Shimada H, Schofield DE, Buckley JD, Meyer WH, Sorensen PH, Triche TJ. Molecular classification of rhabdomyosarcoma--genotypic and phenotypic determinants of diagnosis: a report from the Children’s Oncology Group. Am J Pathol. 2009;174(2):550–64. doi: 10.2353/ajpath.2009.080631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davicioni E, Anderson JR, Buckley JD, Meyer WH, Triche TJ. Gene expression profiling for survival prediction in pediatric rhabdomyosarcomas: a report from the children’s oncology group. J Clin Oncol. 2010;28(7):1240–6. doi: 10.1200/JCO.2008.21.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lae M, Ahn EH, Mercado GE, Chuai S, Edgar M, Pawel BR, Olshen A, Barr FG, Ladanyi M. Global gene expression profiling of PAX-FKHR fusion-positive alveolar and PAX-FKHR fusion-negative embryonal rhabdomyosarcomas. J Pathol. 2007;212(2):143–51. doi: 10.1002/path.2170. [DOI] [PubMed] [Google Scholar]
- 10.Ahn EH, Mercado GE, Lae M, Ladanyi M. Identification of target genes of PAX3-FOXO1 in alveolar rhabdomyosarcoma. Oncol Rep. 2013;30(2):968–78. doi: 10.3892/or.2013.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan J, Simon R, Bittner M, Chen Y, Leighton SB, Pohida T, Smith PD, Jiang Y, Gooden GC, Trent JM, Meltzer PS. Gene expression profiling of alveolar rhabdomyosarcoma with cDNA microarrays. Cancer Res. 1998;58(22):5009–13. [PubMed] [Google Scholar]
- 12.Smith MA, Altekruse SF, Adamson PC, Reaman GH, Seibel NL. Declining childhood and adolescent cancer mortality. Cancer. 2014;120(16):2497–506. Epub 2014/05/24. doi: 10.1002/cncr.28748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, Green DM, Armstrong GT, Nottage KA, Jones KE, Sklar CA, Srivastava DK, Robison LL. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. Epub 2013/06/13. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham J, Nunez-Alvarez Y, Hettmer S, Carrio E, Chen HI, Nishijo K, Huang ET, Prajapati SI, Walker RL, Davis S, Rebeles J, Wiebush H, McCleish AT, Hampton ST, Bjornson CR, Brack AS, Wagers AJ, Rando TA, Capecchi MR, Marini FC, Ehler BR, Zarzabal LA, Goros MW, Michalek JE, Meltzer PS, Langenau DM, LeGallo RD, Mansoor A, Chen Y, Suelves M, Rubin BP, Keller C. Lineage of origin in rhabdomyosarcoma informs pharmacological response. Genes Dev. 2014;28(14):1578–91. doi: 10.1101/gad.238733.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jothi M, Mal M, Keller C, Mal AK. Small molecule inhibition of PAX3-FOXO1 through AKT activation suppresses malignant phenotypes of alveolar rhabdomyosarcoma. Mol Cancer Ther. 2013;12(12):2663–74. doi: 10.1158/1535-7163.MCT-13-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrero Martin D, Boro A, Schafer BW. Cell-based small-molecule compound screen identifies fenretinide as potential therapeutic for translocation-positive rhabdomyosarcoma. PLoS One. 2013;8(1):e55072. doi: 10.1371/journal.pone.0055072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Stewart E, Shelat AA, Qu C, Bahrami A, Hatley M, Wu G, Bradley C, McEvoy J, Pappo A, Spunt S, Valentine MB, Valentine V, Krafcik F, Lang WH, Wierdl M, Tsurkan L, Tolleman V, Federico SM, Morton C, Lu C, Ding L, Easton J, Rusch M, Nagahawatte P, Wang J, Parker M, Wei L, Hedlund E, Finkelstein D, Edmonson M, Shurtleff S, Boggs K, Mulder H, Yergeau D, Skapek S, Hawkins DS, Ramirez N, Potter PM, Sandoval JA, Davidoff AM, Mardis ER, Wilson RK, Zhang J, Downing JR, Dyer MA, St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome P. Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell. 2013;24(6):710–24. doi: 10.1016/j.ccr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Erp AEM, Versleijen-Jonkers YMH, van der Graaf WTA, Fleuren EDG. Targeted Therapy-based Combination Treatment in Rhabdomyosarcoma. Mol Cancer Ther. 2018;17(7):1365–80. Epub 2018/07/04. doi: 10.1158/1535-7163.MCT-17-1131. [DOI] [PubMed] [Google Scholar]
- 19.Safe S, Jin UH, Hedrick E, Reeder A, Lee SO. Minireview: role of orphan nuclear receptors in cancer and potential as drug targets. Mol Endocrinol. 2014;28(2):157–72. doi: 10.1210/me.2013-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang JR, Gan WJ, Li XM, Zhao YY, Li Y, Lu XX, Li JM, Wu H. Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. 2014;35(11):2474–84. doi: 10.1093/carcin/bgu157. [DOI] [PubMed] [Google Scholar]
- 21.Zhou F, Drabsch Y, Dekker TJ, de Vinuesa AG, Li Y, Hawinkels LJ, Sheppard KA, Goumans MJ, Luwor RB, de Vries CJ, Mesker WE, Tollenaar RA, Devilee P, Lu CX, Zhu H, Zhang L, Dijke PT. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nature communications. 2014;5:3388. doi: 10.1038/ncomms4388. [DOI] [PubMed] [Google Scholar]
- 22.Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, Wang H, Safe S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer research. 2010;70(17):6824–36. Epub 2010/07/28. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscat GE, Eriksson NA, Byth K, Loi S, Graham D, Jindal S, Davis MJ, Clyne C, Funder JW, Simpson ER, Ragan MA, Kuczek E, Fuller PJ, Tilley WD, Leedman PJ, Clarke CL. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Mol Endocrinol. 2013;27(2):350–65. Epub 2013/01/08. doi: 10.1210/me.2012-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–76. Epub 2011/11/15. doi: 10.1038/onc.2011.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho SD, Yoon K, Chintharlapalli S, Abdelrahim M, Lei P, Hamilton S, Khan S, Ramaiah SK, Safe S. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer research. 2007;67(2):674–83. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, Xie L, Jiang F, Qin B, Yan T, Chen L, Zhao Y, Cao X, Wu Y, Lin B, Zhou H, Wong AS, Zhang XK, Zeng JZ. Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB J. 2011;25(1):192–205. Epub 2010/09/18. doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lacey A, Rodrigues-Hoffman A, Safe S. PAX3-FOXO1A expression in rhabdomyosarcoma is driven by the targetable nuclear receptor NR4A1. Cancer research. 2017;77(3):732–41. doi: 10.1158/0008-5472.CAN-16-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS). Oncotarget. 2016;7(21):31257–69. doi: 10.18632/oncotarget.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SO, Jin UH, Kang JH, Kim SB, Guthrie AS, Sreevalsan S, Lee JS, Safe S. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Molecular cancer research : MCR. 2014;12(4):527–38. Epub 2014/02/12. doi: 10.1158/1541-7786.MCR-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocr Relat Cancer. 2015;22(5):831–40. doi: 10.1530/ERC-15-0063. [DOI] [PubMed] [Google Scholar]
- 31.Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, Abudayyeh A. Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma. PLoS One. 2015;10(6):e0128308. doi: 10.1371/journal.pone.0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit b1-integrin-dependent breast cancer cell migration. Mol Cell Biol. 2016;36(9):1383–94. doi: 10.1128/MCB.00912-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacey A, Hedrick E, Cheng Y, Mohankumar K, Warren M, Safe S. Interleukin-24 (IL24) Is Suppressed by PAX3-FOXO1 and Is a Novel Therapy for Rhabdomyosarcoma. Mol Cancer Ther. 2018;17(12):2756–66. Epub 2018/09/08. doi: 10.1158/1535-7163.MCT-18-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, Bachelor MA, Grant S, Curiel DT, Dent P, Fisher PB. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111(3):596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panneerselvam J, Munshi A, Ramesh R. Molecular targets and signaling pathways regulated by interleukin (IL)-24 in mediating its antitumor activities. J Mol Signal. 2013;8(1):15. doi: 10.1186/1750-2187-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menezes ME, Bhoopathi P, Pradhan AK, Emdad L, Das SK, Guo C, Wang XY, Sarkar D, Fisher PB. Role of MDA-7/IL-24 a Multifunction Protein in Human Diseases. Adv Cancer Res. 2018;138:143–82. Epub 2018/03/20. doi: 10.1016/bs.acr.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedrick E, Li X, Cheng Y, Lacey A, Mohankumar K, Zarei M, Safe S. Potent inhibition of breast cancer by bis-indole-derived nuclear receptor 4A1 (NR4A1) antagonists. Breast Cancer Res Treat. 2019. Epub 2019/05/24. doi: 10.1007/s10549-019-05279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lebedeva IV, Su ZZ, Vozhilla N, Chatman L, Sarkar D, Dent P, Athar M, Fisher PB. Mechanism of in vitro pancreatic cancer cell growth inhibition by melanoma differentiation-associated gene-7/interleukin-24 and perillyl alcohol. Cancer Res. 2008;68(18):7439–47. Epub 2008/09/05. doi: 10.1158/0008-5472.CAN-08-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11(1):160–72. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Inoue S, Shanker M, Miyahara R, Gopalan B, Patel S, Oida Y, Branch CD, Munshi A, Meyn RE, Andreeff M, Tanaka F, Mhashilkar AM, Chada S, Ramesh R. MDA-7/IL-24-based cancer gene therapy: translation from the laboratory to the clinic. Curr Gene Ther. 2006;6(1):73–91. [DOI] [PubMed] [Google Scholar]
- 41.Hamed HA, Yacoub A, Park MA, Archer K, Das SK, Sarkar D, Grant S, Fisher PB, Dent P. Histone deacetylase inhibitors interact with melanoma differentiation associated-7/interleukin-24 to kill primary human glioblastoma cells. Mol Pharmacol. 2013;84(2):171–81. Epub 2013/05/11. doi: 10.1124/mol.113.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pan L, Pan H, Jiang H, Du J, Wang X, Huang B, Lu J. HDAC4 inhibits the transcriptional activation of mda-7/IL-24 induced by Sp1. Cell Mol Immunol. 2010;7(3):221–6. Epub 2010/04/13. doi: 10.1038/cmi.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarkar D, Su ZZ, Lebedeva IV, Sauane M, Gopalkrishnan RV, Valerie K, Dent P, Fisher PB. mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc Natl Acad Sci U S A. 2002;99(15):10054–9. Epub 2002/07/13. doi: 10.1073/pnas.152327199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su ZZ, Madireddi MT, Lin JJ, Young CS, Kitada S, Reed JC, Goldstein NI, Fisher PB. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A. 1998;95(24):14400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yacoub A, Park MA, Gupta P, Rahmani M, Zhang G, Hamed H, Hanna D, Sarkar D, Lebedeva IV, Emdad L, Sauane M, Vozhilla N, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, Fisher PB, Dent P. Caspase-, cathepsin-, and PERK-dependent regulation of MDA-7/IL-24-induced cell killing in primary human glioma cells. Mol Cancer Ther. 2008;7(2):297–313. doi: 10.1158/1535-7163.MCT-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, Fisher PB, Dent P. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4(4):513–5. Epub 2008/02/27. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pei DS, Yang ZX, Zhang BF, Yin XX, Li LT, Li HZ, Zheng JN. Enhanced apoptosis-inducing function of MDA-7/IL-24 RGD mutant via the increased adhesion to tumor cells. J Interferon Cytokine Res. 2012;32(2):66–73. Epub 2012/01/18. doi: 10.1089/jir.2011.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer research. 2005;65(22):10128–38. Epub 2005/11/17. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 49.Chawsheen HA, Ying Q, Jiang H, Wei Q. A critical role of the thioredoxin domain containing protein 5 (TXNDC5) in redox homeostasis and cancer development. Genes Dis. 2018;5(4):312–22. Epub 2018/12/29. doi: 10.1016/j.gendis.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molenaar RJ, Maciejewski JP, Wilmink JW, van Noorden CJF. Correction: Wild-type and mutated IDH½ enzymes and therapy responses. Oncogene. 2018;37(43):5810 Epub 2018/08/25. doi: 10.1038/s41388-018-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


