Abstract
STING (stimulator of IFN genes) signaling is an innate immune pathway for induction of a spontaneous antitumor T cell response against certain immunogenic tumors. Although antigen presenting cells are known to be involved in this process, insight into the participation of tumor cell-intrinsic STING signaling remains weak. In this study, we find diversity in regulation of STING signaling across a panel of human melanoma cell lines. We show that intact activation of STING signaling in a subset of human melanoma cell lines enhances both their antigenicity and susceptibility to lysis by human melanoma tumor infiltrating lymphocytes (TILs) through the augmentation of MHC class I expression. Conversely, defects in the STING signaling pathway protect melanoma cells from increased immune recognition by TILs and limit their sensitivity to TIL lysis. Based on these findings, we propose that defects in tumor cell-intrinsic STING signaling can mediate not only tumor immune evasion but also resistance to TIL-based immunotherapies.
Keywords: STING, melanoma, tumor infiltrating lymphocytes, antigenicity, MHC class I, antitumor immunity
Introduction
Successful cancer immunotherapies are based on knowledge about tumor immune-escape mechanisms and routes to promote antitumor immunity. A network of biological pathways coordinates interactions between tumor cells and the immune system and dictates elimination, establishment, or progression of tumors [1, 2]. Tumors cells can escape the host’s immune recognition through elaborate mechanisms involving tumor-cell-intrinsic and extrinsic elements [3]. A factor limiting the successful use of checkpoint therapy or adoptive cell therapy (ACT) in humans is the loss of immunogenicity and the lack of tumor antigen recognition by cytotoxic T cells [4, 5], which could be due to lack of antigenic mutations, loss of tumor antigen expression, loss of MHC class I expression, or other alterations in antigen processing machinery in tumor cells [6].
Both spontaneous and iatrogenic immunogenicity of tumors can be influenced by type I and type II interferons (IFNs) [7-9]. Indeed, type I IFNs bridge innate and adaptive immunity and provide necessary inflammatory signals. Both in primary carcinogen–induced and transplantable tumor models, spontaneous T cell response depends on endogenous induction of type I IFNs [10]. Studies using gene-targeted mouse models deficient in innate immune pathways have indicated that this response is mediated by STING (stimulator of IFN genes) signaling [11].
STING is a transmembrane protein that is activated by cyclic dinucleotides (CDNs) generated by the cellular synthase, cyclic GMP-AMP synthase (cGAS), following the detection of cytosolic double-stranded DNA (dsDNA) [12, 13]. Activation of STING triggers a signaling cascade involving activation of TANK-binding kinase-1 (TBK1), phosphorylation of IRF-3, and production of type I IFNs [14].
Much work has focused on identifying the innate immune components that contribute to STING-dependent antitumor immunity. Antigen presenting cells (APCs) help induce antitumor immunity in response to both endogenous and enforced activation of STING signaling in the tumor microenvironment [11, 15]. Beside APCs, activation of STING signaling in some tumor cells including lymphoma and prostate cancer cells has been shown to be involved in promoting antitumor immunity [16, 17].
However, disruption of STING signaling through multiple mechanisms has been reported in a number of human cancers, including melanoma [18, 19]. This raises the question of whether tumor cells acquire defective STING signaling as a mechanism to blunt immune recognition. Therefore, in this study we have investigated the function of tumor cell-intrinsic STING signaling in the regulation of tumor immunogenicity using models of STING-intact and STING-defective human melanoma cell lines. We show that intact activation of STING signaling enhances MHC class I surface expression in melanoma cells and sensitizes their lysis by human melanoma tumor infiltrating lymphocytes (TIL) as reflected by increased specific killing and IFN-γ production by TIL. The finding that STING signaling is dysfunctional in a subset of the tested melanoma cell lines suggests that defects in STING signaling can negatively impact immune recognition in melanoma and can promote resistance to T cell-based immunotherapies.
Materials and Methods
Preparation of TIL
Melanoma TILs were established as described previously [20]. Briefly, melanomas were minced into 1–2 mm3 fragments and plated in 24-well plates with 2 mL TIL culture medium (TIL-CM) containing 6000 IU/mL IL-2 (proleukin) per well. The TIL-CM consisted of RPMI 1640, 2.05 mM L–glutamine (HyClone, Thermo Fisher Scientific), 10% heat-inactivated human AB serum (Omega Scientific), 55 μM 2-mercaptoethanol (Invitrogen), 50 μg/mL gentamicin (Invitrogen), 100 IU/mL penicillin, 100 μg/mL streptomycin, and 10 mM HEPES Buffer (Mediatech). Half of the medium was replaced every 2 to 3 days or wells were split when 90% confluent. TILs were expanded for 3–5 weeks. HLA typing of TILs was performed by the HLA Laboratory (American Red Cross, Dedham, MA). TIL 195, TIL 19 and TIL 123 were HLA-A typed as A02, A02/26 and A02/11, respectively.
Melanoma cell lines
Human melanoma cell lines 1205Lu, A375, SBCL2, WM9, WM35, WM39, WM164, WM858, WM1361A, WM1366, WM2032, WM3130, WM3629 (provided in 2011 by Dr. Keiran Smalley, Moffitt Cancer Center, Tampa, FL), 526-MEL and 888-MEL (obtained in 2004 from the Surgery Branch, NCI/NIH, Bethesda, MD) were maintained as monolayers in complete medium consisting of RPMI 1640 supplemented with 10% heat-inactivated FBS and antibiotics. All cell lines were passaged less than 10 times after initial revival from frozen stocks and repeatedly tested for mycoplasma. Cell lines were not authenticated in the past year. HLA typing of melanoma cell lines was performed by the HLA Laboratory (American Red Cross, Dedham, MA). WM39, WM3629, and 526-MEL were HLA-A typed as A01/02, A02/30 and A02/03, respectively.
Knockdown of STING in WM39 cells was achieved using lentiviral particles carrying a target gene sequence for human STING (TMEM173) (catalog no. TL307876V) or scrambled control (catalog no. TR30021V) (Origene Technologies). The targeting sequence for STING was: 5’-GCAACAGCATCTATGAGCTTCTGGAGAAC. Transduced cells were selected by addition of puromycin (0.5 μg/ml) to the medium 24 h after infection.
STING agonist stimulation
Human melanoma cell lines (4×105 cells/well in 24-well plates) were stimulated with 2’3’-cGAMP (Invitrogen) (10 μg/ml) in the presence of lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. After 4 or 24 hours of incubation at 37°C in a humidified CO2 incubator, the supernatants were collected for detection of CXCL10 and IFN-β release using enzyme-linked immunosorbent assays (Quantikine ELISA Kit, R&D Systems), and cells were scraped, washed and lysed for assessment of IRF3 phosphorylation by immunoblot. For the IFNAR blocking studies, melanoma cells were incubated with anti-IFNAR2 (clone MMHAR-2, PBL Assay Science) at a final concentration of 5 μg/mL for 1 hour at 37°C prior to stimulation with 2’3’-cGAMP.
Immunoblot analysis
Proteins were extracted with RIPA buffer (ThermoFisher Scientific) containing protease inhibitors (Thermo Scientific). Protein extracts from NK92, a natural killer cell line, was used as a positive control for the expression of STING and cGAS [21]. Equal amounts of proteins were resolved on SDS-PAGE gels (Bio-Rad) and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). After blocking with 5% non-fat dry milk, membranes were incubated with 1:1000 dilution of antibodies specific for STING (catalog no. 13647), cGAS (catalog no. 15102), p-IRF3 (catalog no. 4947), IRF3 (catalog no. 11904) (all from Cell Signaling) and 1:5000 dilution of anti-β-Actin (Sigma Aldrich, catalog no. A5316). Following incubation with 1:2000 dilution of HRP-linked anti-rabbit-IgG (catalog no. 7074) or HRP-linked anti-mouse-IgG (catalog no. 7076) (both from Cell Signaling), bands were visualized using an enhanced chemiluminescence detection system.
Coculture assay
1 × 105 of melanoma cells were cultured with TILs at a 1:1 ratio with or without 2’3’-cGAMP (10 μg/ml) in 96-well round-bottom plates. After 24 hours of incubation at 37°C in a humidified CO2 incubator, the supernatant was harvested for detection of IFN-γ release using enzyme-linked immunosorbent assay (Human IFN-γ Quantikine ELISA Kit, R&D Systems). For the MHC class I blocking assay, melanoma cells were incubated with W6/32 (anti-HLA-A,B,C monoclonal antibody, Biolegend) at a final concentration of 10 μg/mL for 1 hour at 37°C prior to the addition of TIL.
51Cr-release assay
Lysis of melanoma cell targets by their HLA-matched TIL cultures was measured in a standard 5lCr release assay, as described previously [22]. Briefly, 1 × 106 melanoma cells were labeled with 100 μCi of 51Cr (Amersham Corp) for 2 h at 37°C. Following three washes with HBSS, labeled target cells were resuspended in TIL CM with or without 2’3’-cGAMP (10 μg/ml) at a concentration of 5 × 104 tumor cells/ml and added to the effector cells at different effector-to-target cell ratios in a 96-well plate and incubated at 37 °C. In addition, two control conditions were included in this assay: a minimum release control containing just the target cells and a maximum release control in which target cells were lysed by TritonX-100. After 4 hours, supernatant was harvested and measured in Trilux (PerkinElmer). Each point represented the average of quadruplicate wells and percentage of specific lysis was calculated by: (experimental release − minimum release)/ (maximum release− minimum release) × 100. Lytic units were calculated as the number of effector cells required to produce 20% lysis of 5 × 103 target cells expressed as the inverse and normalized to 1 × 106 cells [23].
Flow cytometry
Flow cytometry was performed using HLA-A.B.C–PE antibody (1:100, Biolegend, clone W6/32). DAPI (Sigma-Aldrich) was used as a viability dye. Sample acquisition was performed on an LSR II flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (Tree Star).
Statistical methods
Statistical analyses were performed using GraphPad Prism7 software. All data are presented as mean ± SD. Means for all data were compared by one-way ANOVA or unpaired t-test as described in the figure legends. P values of statistical significance are represented as *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Results
Identification of melanoma cell lines with intact STING signaling
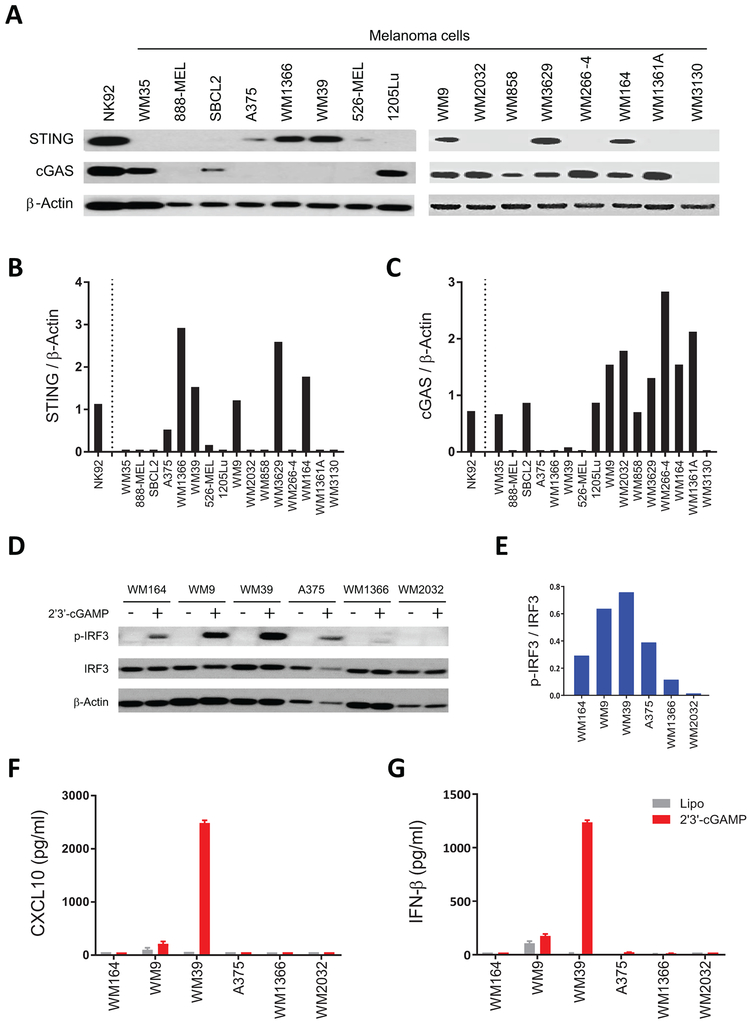
To identify melanoma cell lines with intact STING signaling, we first evaluated expression of STING and cGAS in a panel of human melanoma cell lines by immunoblot (Fig. 1A). We found varying expression of STING and cGAS among these cell lines consistent with a previous report [19]. STING was not detectable in 9 of 16 melanoma cell lines (WM35, 888-MEL, SBCL2, 1205Lu, WM2032, WM858, WM266–4, WM1361A and WM3130). We identified A375, WM1366, WM39, WM9, WM3629 and WM164 as STING+ cell lines (Fig. 1B). cGAS was detected in 10 of 16 cell lines (Fig. 1C), and only three cell lines (WM9, WM3629 and WM164) expressed both cGAS and STING.
Figure 1. Identification of melanoma cell lines with intact STING signaling.
(A) Immunoblot analysis of STING and cGAS expression in a series of human melanoma cell lines. NK-92 was used as a positive control for the expression of STING and cGAS. Twenty μg of whole-cell lysate was used and β-actin was analyzed as a loading control. (B) Ratio of total STING relative to β-actin, and (C) ratio of total cGAS relative to β-actin for each cell line were quantified using ImageJ software. (D) Immunoblot analysis of p-IRF3 and total IRF3 in five STING+ (WM164, WM9, WM39, A375, and WM1366) and one STING− (WM2032) human melanoma cell lines after 4 h stimulation with 2’3’-cGAMP or lipofectamine. Twenty μg of whole-cell lysate was used and β-actin was analyzed as loading control. (E) Ratio of p-IRF3 relative to IRF-3 for 2’3’-cGAMP stimulated cell lines were quantified using ImageJ software. (F) Induction of CXCL10 and (G) IFN-β in cell culture supernatants of indicated human melanoma cells after stimulation with 2’3’-cGAMP or lipofectamine measured using ELISA and reported as mean ± SD for three biological replicates.
We next investigated the functional STING signaling activation by stimulating melanoma cell lines with the STING agonist 2’3’-cGAMP. As this agonist activates STING signaling in a cGAS-independent manner, we selected 5 STING+ cell lines (WM164, WM9, WM39, A375 and WM1366) and 1 STING- cell line (WM2032). We performed immunoblot analysis on cell extracts after stimulation with 2’3’-cGAMP to assess phosphorylation of the transcription factor IRF3, which is a downstream regulatory element for STING-dependent type I IFN induction [24]. We observed phosphorylation of IRF3 in four of five STING+ cell lines following their stimulation with 2’3’-cGAMP (Fig. 1D and E). As expected, phosphorylation of IRF3 was not detected for the 2’3’-cGAMP stimulated WM2032 (STING−) cell line. We next determined STING-dependent CXCL10 and IFN-β induction in cell culture supernatants of the indicated melanoma cell lines following stimulation with 2’3’-cGAMP or STING-independent cytokine induction following stimulation with polyI:C (Fig. 1F and G and Supplementary Fig.S1A and B). Although WM9 and WM39 induced detectable CXCL10 and IFN-β expression in response to stimulation with 2’3’-cGAMP, polyI:C stimulation resulted in STING-independent induction of CXCL10 and IFN-β for all the cell lines except WM164.
Activation of STING signaling enhanced antigenicity of human melanoma cell lines
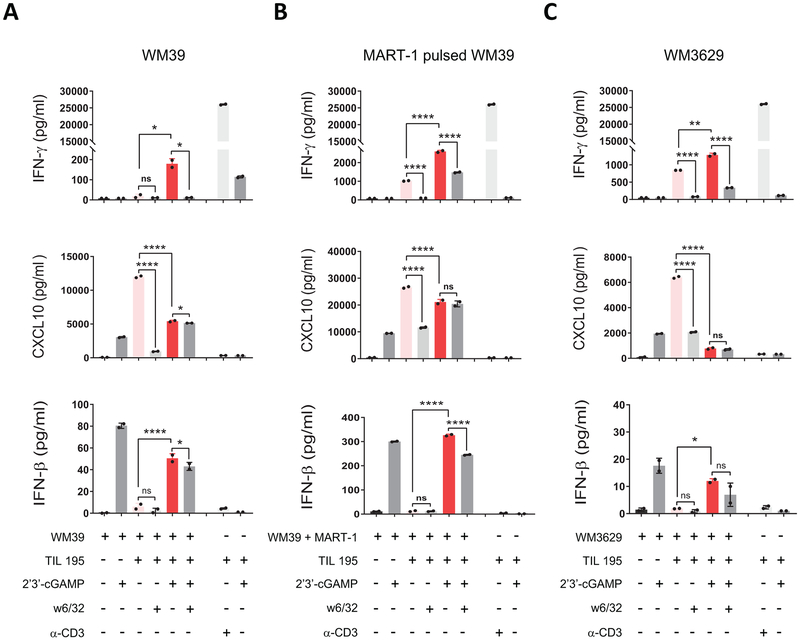
To study the role of STING signaling in antigenicity of melanoma, we initially selected WM39 (HLA-A2), as this cell line responded strongly to STING signaling activation with 2’3’-cGAMP. We used WM39 in cocultures of HLA-A2-restricted human melanoma TILs (TIL 195) in the presence or absence of the STING agonist. We also included experimental conditions in which WM39 cells were pre-incubated with an MHC class I blocking Ab (W6/32), to determine whether IFN-γ release was mediated by CD8+ TIL TCR engagement with peptide/MHC class I.
We assessed the antigenicity by IFN-γ release and found that when cocultures were performed with 2’3’-cGAMP-treatment, there was significantly enhanced IFN-γ secretion by HLA-matched TIL 195 (Fig. 2A). We also observed blockade of IFN-γ release in the presence of the MHC class I blocking Ab (W6/32), which confirmed MHC class I–mediated CD8+ reactivity.
Figure 2. Activation of STING signaling results in enhanced antigenicity of human melanoma cell lines.
(A) WM39, (B) MART-1 pulsed WM39 and (C) WM3629 cells were cocultured with TIL 195 for 24 h with or without 2’3’-cGAMP. IFN-γ (top), CXCL10 (middle) and IFN-β (bottom) concentrations in supernatants were measured using ELISA. Data are presented as mean ± SD of triplicate samples from one representative of three independent experiments. P-values were calculated by one-way ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant).
We also measured CXCL10 and IFN-β (Fig. 2A) expression in coculture supernatants to confirm 2’3’-cGAMP-triggered activation of STING signaling. Although we observed CXCL10 induction in the coculture group without 2’3’-cGAMP, this effect was related to STING-independent and IFN-γ-mediated induction of CXCL10 in WM39 cells [25]. Induction of IFN-β in coculture groups with 2’3’-cGAMP confirmed activation of STING signaling. We did not observe IFN-β induction for the control group containing TILs with 2’3’-cGAMP which suggests tumor cells are the main source of IFN-β expression in the coculture group in response to stimulation with the agonist. Taken together, these data indicate that activation of STING signaling enhances antigenicity of WM39 cells.
To further investigate the impact of STING activation on antigen-presentation and immune T cell activity, we pulsed WM39 cells with MART-1 (a melanoma specific peptide recognized by HLA-A2-restricted TILs [26]), and cocultured them with TIL 195 in the presence or absence of the STING agonist 2’3’-cGAMP (Fig. 2B). Similarly, we found a significantly increased (p < 0.0001) secretion of IFN-γ by TIL 195 in the 2’3’-cGAMP-treated coculture group. Consistent with the WM39 cocultures, we observed similar patterns of CXCL10 and IFN-β (Fig. 2B) induction for MART-1-pulsed WM39 cocultures, which confirmed activation of STING signaling in 2’3’-cGAMP-treated groups.
We also cocultured MART-1 pulsed WM39 cells with two additional HLA-A2 TILs (Supplementary Fig.S2A and B). Similarly, we found higher IFN-γ production (p < 0.01) by both TIL samples in 2’3’-cGAMP-treated cocultures compared to the untreated cocultures. Furthermore, we tested the WM3629 (HLA-A2) melanoma cell line that in our earlier experiments responded to 2’3’-cGAMP stimulation but to a lesser extent than WM39 (Supplementary Fig. S3), and used it in cocultures with TIL 195 in the presence or absence of the STING agonist (Fig. 2C). Stimulation with 2’3’-cGAMP similarly resulted in increased (p < 0.01) IFN-γ release in WM3629/TIL 195 cocultures, indicating that the enhancing effect of 2’3’-cGAMP was not restricted to the WM39 melanoma cell line.
We observed a decrease (p < 0.0001) in the expression of CXCL10 in the agonist-treated cocultures compared to the untreated cocultures (Fig. 2A-C). As tumor cells are the primary source of CXCL10 expression, this is likely an indication that more tumor cells were lysed by TILs in the agonist-treated groups compared to the untreated controls.
STING signaling is required in melanoma cells for agonist-induced improved antigenicity
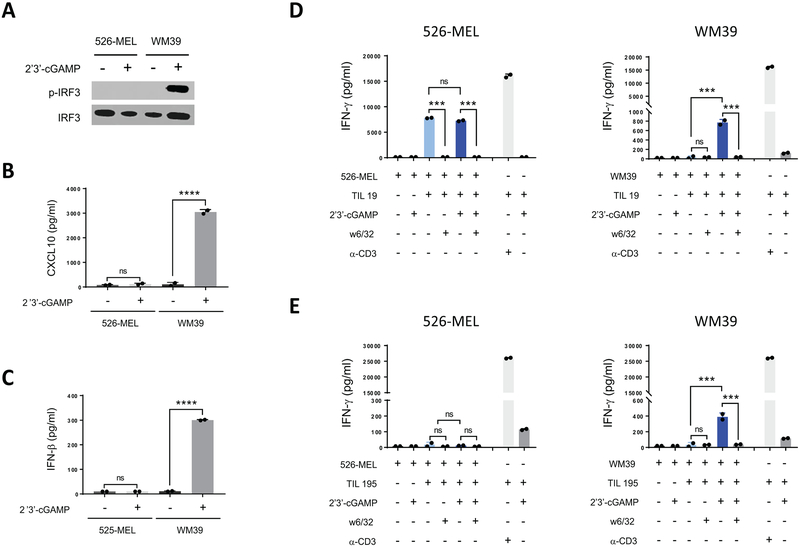
Given the impact of the STING agonist on IFN-γ release in melanoma/TIL cocultures, we next sought to determine whether this was due to the direct activation of STING signaling in melanoma cells. To address this possibility, we cocultured the 526-MEL (HLA-A2) melanoma cell line that did not respond to 2’3’-cGAMP stimulation (Fig. 3A-C) with two HLA-A2-restricted TILs (TIL 19 and TIL 195) in the presence or absence of the STING agonist. In parallel cocultures we used WM39 cells with the same TIL samples (Fig. 3D and E). 526-MEL stimulated higher IFN-γ release from TIL 19 compared to WM39 cells. However, in contrast to WM39/TIL 19 cocultures for which stimulation with 2’3’-cGAMP resulted in 24-fold higher (p < 0.001) IFN-γ release than the untreated group, agonist treatment did not induce any increased IFN-γ secretion for 526-MEL/TIL 19 coculture (Fig. 3D). Similarly, we did not find any increase in IFN-γ release for the 526-MEL/TIL 195 cocultures in the presence of the agonist (Fig. 3E), suggesting that STING agonist-mediated enhanced antigenicity is driven by activation of STING signaling in melanoma cells.
Figure 3. STING signaling is required in melanoma cells for agonist-induced improved antigenicity.
(A) Immunoblot analysis of p-IRF3 and total IRF3 in 526-MEL and WM39 melanoma cells after stimulation with 2’3’-cGAMP. (B) Induction of CXCL10 and (C) IFN-β in 526-MEL and WM39 cells after 24 h stimulation with 2’3’-cGAMP measured using ELISA. (D) 526-MEL and WM39 cells were cocultured with TIL 19 and (E) TIL 195 for 24 h with or without 2’3’-cGAMP. IFN-γ amounts in supernatants were measured using ELISA. Data are presented as mean ± SD of duplicate samples from one representative of three independent experiments. P-values were calculated by one-way ANOVA (***P < 0.001, ****P < 0.0001, ns = not significant).
STING activation in melanoma lines improves cytotoxic T lymphocyte-mediated lysis
We next performed 51Cr release cytotoxicity assays using WM39, MART-1 pulsed WM39, WM3629, and 526-MEL as target cells and TIL 195 as effector cells in the presence or absence of 2’3’-cGAMP to determine cytolytic activity of TILs against melanoma cells stimulated with the agonist. Similar to our finding of increased TIL production of IFN-γ, STING activation in both WM39 and MART-1 pulsed WM39 cells increased their lysis by TIL 195 (>2-fold, p < 0.05) (Fig. 4A and B). Blocking MHC class I in WM39 targets inhibited specific TIL lysis in agonist treated groups by up to 60% (p < 0.05) (Fig. 4C), indicating that the enhanced cytotoxic activity in response to activation of STING signaling was driven by MHC class I restricted TIL. To confirm that this effect was mediated by cytolytic activity of TILs per se and not by the STING agonist, we included two control groups in which WM39 target cells were incubated with or without 2’3’-cGAMP in the absence of TILs (Supplementary Fig. S4). We found no significant difference of cytotoxicity in these two groups, which argued that stimulation with the STING agonist alone did not result in any major cytotoxicity. We also found increased (p < 0.05) cytotoxicity in 2’3’-cGAMP-treated WM3629 cells compared to the controls (Fig. 4D). However, 2’3’-cGAMP stimulation of 526-MEL targets, which were defective in STING signaling, did not alter their specific lysis by TIL 195 (Fig. 4E). To better compare the cytolytic activity of TILs against different agonist-treated and untreated melanoma targets, we calculated lytic units (Fig. 4F). We found more than a 10-fold increase in lytic potential of TIL 195 against 2’3’-cGAMP-treated WM39 and MART-1-pulsed WM39 cells compared to their controls. Similarly, activation of STING in WM3629 cells resulted in in greater lysis by TIL 195. In contrast, 2’3’-cGAMP-treated 526-MEL cells did not induce any increase in lysis by TIL 195.
Figure 4. Activation of STING pathway in human melanoma cell lines improves cytotoxic T lymphocyte-mediated lysis.
51Cr cytotoxicity assay using (A) WM39 (B) MART-1 pulsed WM39, (C) WM39 plus W6/32, (D) WM3629, and (E) 526-MEL cells as target cells and TIL 195 as effector cells at the indicated effector/target (E/T) ratios with or without 2’3’-cGAMP. Data represent the mean ± SD of quadruplicate wells (representative of three independent experiments). (F) Lytic activity of TIL 195 against different agonist-treated and untreated melanoma targets was measured in lytic units (106 divided by the number of effector cells required to cause 20% lysis of 5 × 103 tumor cells). Data shown are representative of three independent experiments.
STING activation in human melanoma cell lines induces up-regulation of MHC class I
Following our observation of enhanced antigenicity of human melanoma cell lines triggered by agonist-induced activation of STING signaling, we next examined the expression of MHC class I on 2’3’-cGAMP-stimulated melanoma cells. We found that surface expression of MHC class I in all four cell lines with functional STING signaling (WM9, WM3629, A375 and WM39) was significantly increased (p< 0.01, fold change > 1.6) following stimulation with the STING agonist (Fig. 5A and B). In contrast, there were no significant changes in the expression of MHC class I in 1205Lu, WM266–4, WM2032 and 526-MEL cell lines with impaired STING signaling following their exposure to the agonist. To determine whether the up-regulation of MHC class I in response to activation of STING occurs through type I IFN signaling, we blocked type I IFN receptor using an IFNAR blocking antibody in WM39 cells and performed stimulation with 2’3’-cGAMP. We found blockade of IFNAR inhibited agonist-induced upregulation of MHC class I (Supplementary Fig.S5A and B). Together, these results indicated that activation of STING signaling could induce up-regulation of MHC class I in a subset of melanoma cell lines, leading to more effective immune recognition and antigen presentation to TIL.
Figure 5. STING activation in human melanoma cell lines induces up-regulation of MHC class I (HLA-A.B.C).
(A) Representative histograms of HLA-A.B.C expression on four STING-defective (1205Lu, WM266–4, WM2032, and 526-MEL) and four STING-intact (WM9, WM3629, A375, and WM39) human melanoma cell lines with or without 2’3’-cGAMP stimulation. Data shown are representative of 3 independent experiments. (B) Mean fluorescence intensity (MFI) of HLA-A.B.C on indicated human melanoma cells. Data are mean ± SD of three biological replicates. Statistical significance was determined by unpaired t-test (**P < 0.01, ***P < 0.001, ****P < 0.0001).
Knockdown of STING blocks agonist-induced upregulation of MHC class I
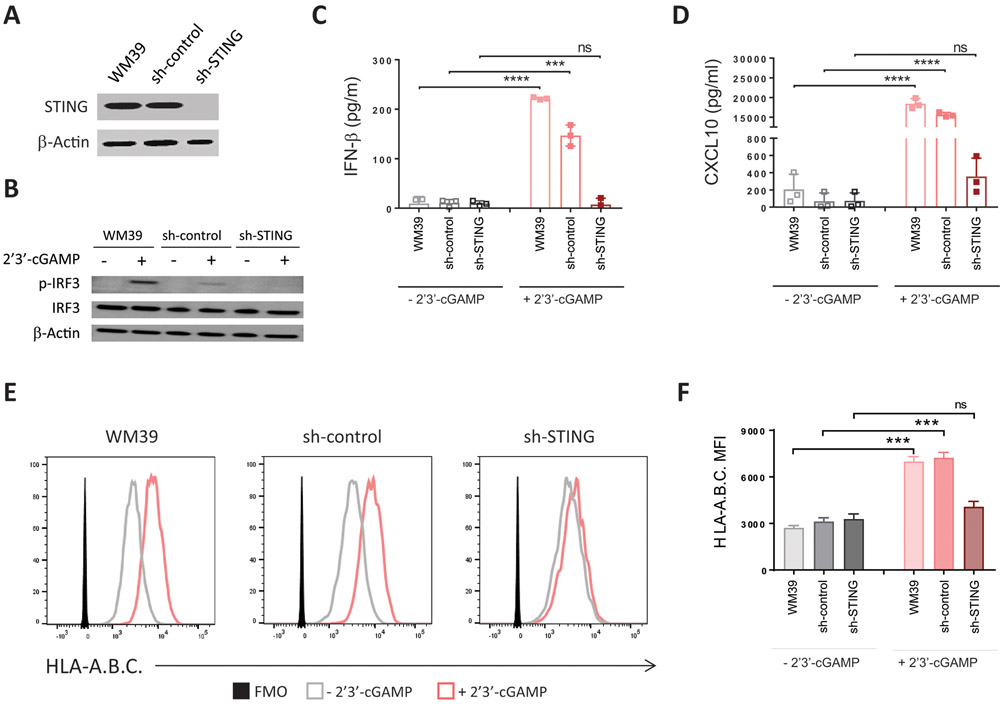
We next knocked down STING expression in WM39 cells with lentivirus-based short hairpin RNA targeting STING (sh-STING). We used WM39 cells expressing a nontargeting hairpin as control cells (sh-control). Immunoblot analysis confirmed knockdown of STING in WM39 cells transduced with sh-STING (Fig. 6A). Knockdown of STING blocked phosphorylation of IRF3 and agonist-induced induction of CXCL10 and IFN-β in WM39 cells in response to stimulation with 2’3’-cGAMP (Fig. 6B-D) but did not affect STING-independent induction of CXCL10 and IFN-β in response to stimulation with polyI:C (Supplementary Fig. S6A and B). Unlike WM39 and sh-control, for which stimulation with the agonist resulted in more than 2.5-fold higher surface expression of MHC class I (p < 0.01), stimulation with the agonist did not cause any increase in MHC class I for sh-STING cells (Fig. 6E and F) demonstrating that upregulation of MHC class I in response to stimulation with the agonist occurs through activation of STING signaling.
Figure 6. Knockdown of STING blocks agonist-induced upregulation of MHC class I in melanoma cells.
WM39 cells were stably transduced with a lentiviral shRNA specific for STING (sh-STING) or non-target shRNA (sh-control). (A) Immunoblot analysis of STING expression in WM39, sh-control, and sh-STING cells. Whole-cell lysate (20 μg) was used and β-actin was analyzed as a loading control. (B) Immunoblot analysis of p-IRF, total IRF3, and β-actin in WM39, sh-control and sh-STING cells after stimulation with 2’3’-cGAMP or lipofectamine. (C) Induction of CXCL10 and (D) IFN-β in WM39, sh-control and sh-STING cells after stimulation with 2’3’-cGAMP or lipofectamine. (E) Representative histograms of HLA-A.B.C expression on indicated cells with or without 2’3’-cGAMP stimulation. (F) Mean fluorescence intensity (MFI) of HLA-A.B.C on indicated cells. Data are presented as mean ± SD of three biological replicates. P-values were calculated by one-way ANOVA (**P < 0.01, ***P < 0.001, ****P < 0.0001).
STING is essential for agonist-induced enhanced antigenicity in melanoma cells
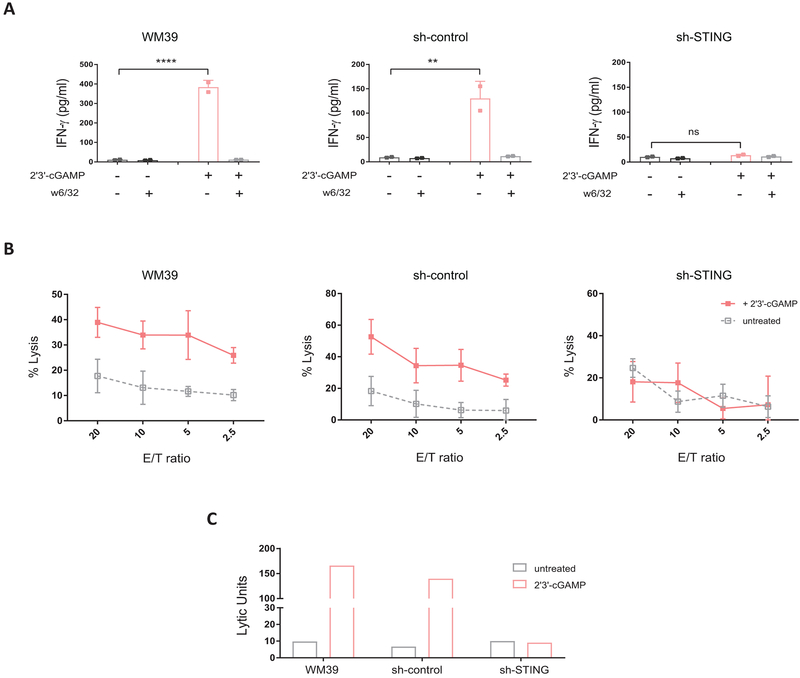
To further confirm the role of STING signaling in enhancing antigenicity of melanoma, we used sh-STING, sh-control and non-transfected WM39 cells in cocultures with TIL 195 in the presence and absence of 2’3’-cGAMP. In contrast to WM39/TIL 195 and sh-control/TIL 195 cocultures, for which stimulation with the agonist resulted in more than 25-fold higher IFN-γ release (P < 0.01), we did not find any increase in IFN-γ induction in sh-STING/TIL 195 cocultures in the presence of the agonist (Fig. 7A). We also performed 51Cr cytotoxicity assays using sh-STING, sh-control and WM39 as target cells and TIL 195 as effector cells at different effector to target ratios with or without 2’3’-cGAMP. Although we found increased cytolytic activity with TIL 195 against both WM39 and sh-control in the presence of the agonist, inhibition of STING signaling in sh-STING blocked this response (Fig. 7B), as reflected by the corresponding lytic units (Fig. 7C).
Figure 7. STING is essential for agonist-induced enhanced antigenicity in melanoma cells.
(A) WM39, sh-control and sh-STING cells were cocultured with TIL 195 for 24 h in the presence or absence of 2’3-cGAMP. IFN-γ levels in supernatants were measured using ELISA. Data are presented as mean ± SD of duplicate samples. P-values were calculated by one-way ANOVA (**P < 0.01, ***P < 0.001, ****P < 0.0001, ns = not significant). (B) 51Cr cytotoxicity assay using WM39, sh-control and sh-STING cells as target cells and TIL 195 as effector cells at the indicated effector/target (E/T) ratios with or without 2’3’-cGAMP. Data represent the mean ± SD of quadruplicate wells. (C) Lytic activity of TIL 195 against indicated targets with or without 2’3’-cGAMP stimulation was measured in three independent experiments, one of which is shown.
Discussion
Immunotherapies, including adoptive cell transfer of tumor infiltrating lymphocytes and immune checkpoint inhibitor antibodies, have shown efficacy in patients with metastatic melanoma [5, 20, 27]. However, there remains a subset of melanoma patients treated with immune-based therapies who do not achieve clinical benefit [28-30]. Understanding the mechanisms underlying both successful and failed immune responses may help improve immunotherapeutic approaches.
TIL-based immunotherapies have been developed on the basis of expanding tumor-reactive T cells from the tumor microenvironment. Thus a spontaneous adaptive immunity exists within tumors, although in a dysfunctional state [31, 32]. Moreover, a pre-existing CD8+ T cell infiltrate within tumors has been associated with clinical response to checkpoint blockade immunotherapies in melanoma patients [33], indicating the prognostic significance of endogenous T cell responses. These observations have led to a question regarding how the innate immune system could detect cancer and initiate a spontaneous adaptive T cell response against tumor antigens without the presence of infectious pathogens [34].
Studies using gene-targeted mouse models deficient in specific innate immune pathways have identified STING pathway to be the innate immune sensing mechanism for the detection of immunogenic tumors and initiation of a spontaneous T cell response [11]. Based on this finding, multiple studies have been conducted to evaluate whether direct activation of STING signaling using pharmacologic STING agonists could be used in facilitating antitumor immune responses in mouse models [15, 35, 36]. Although these studies find enhanced therapeutic activity by intratumoral administration of STING agonists, mechanistic details regarding how direct activation of STING signaling could potentiate antitumor immunity remain largely unknown. In fact, it is unclear how STING agonists could impact cell types other than APCs in a tumor microenvironment, in particular tumor cells.
Although evidence suggests STING signaling is frequently impaired in human melanoma cells [19], there remains a subset of melanomas with STING expression for which the function of STING activation has not been well explored. In this study, we have shown that many melanoma cell lines have lost expression of STING and are therefore defective in responding to stimulation with the STING agonist 2’3’-cGAMP. We have also observed impaired functional responses to stimulation with the STING agonist in some melanoma cells that expressed STING, suggesting that STING signaling can be inhibited not only by suppression of STING/cGAS expression but also through other molecular mechanisms that remain to be determined [19, 37]. We have found that stimulation with the 2’3’-cGAMP agonist can induce STING activation in a subset of human melanoma cell lines leading to downstream production of IFN-β and CXCL10. Such agonist-induced activation of the STING pathway can increase antigenicity of melanoma cells through augmentation of MHC class I expression and result in better tumor-antigen recognition by immune T cells.
Down-regulation of MHC class I is used by tumor cells to evade host immune recognition [38, 39]. Loss or down-regulation of MHC class I was associated with fewer tumor infiltrating lymphocytes and poor clinical outcomes in patients with metastatic melanoma. Such correlations were found in melanoma cell lines derived from both recurrent metastases in patients who had initially experienced clinical responses to TIL-based therapies [40-42] or from previously untreated melanoma patients who showed resistance to anti–CTLA-4 therapy later [4]. In contrast, tumor regression was correlated with positive tumor MHC class I expression, highlighting the functional significance of antigen presentation by tumor cells in the initiation of successful anti-tumor responses [43].
The molecular mechanism(s) underlying STING agonist-induced up-regulation of MHC class I remains undefined. We however have shown that it depends on activation of STING signaling, as up-regulation of MHC class I was not observed in melanoma cell lines with defective STING signaling following their stimulation with 2’3’-cGAMP. Also, our findings suggest that STING agonist-mediated up-regulation of MHC class I occurs through type I IFN dependent mechanisms, as this effect was found in tumor cells stimulated with the agonist in the absence of TILs and IFN-γ. In addition, inhibition of this effect by blocking IFNAR in tumor cells further supported the hypothesis that up-regulation of MHC class I in response to activation of STING occurs through type I IFN signaling. Taken together, our data support the concept that agonist-induced activation of STING signaling in melanomas could be considered as a therapeutic intervention to restore MHC class I surface expression and subsequently to enhance tumor antigen recognition and tumor cell destruction by immune T cells.
We have shown that activation of STING signaling in melanoma cell lines results in downstream induction of IFN-β. STING-mediated IFN-β induction in dendritic cells has been found to be essential for their activation and the cross-priming of cytotoxic T cells [11]. Similarly, others have shown that initiation of an adaptive immune response to radiation therapy requires STING-mediated IFN-β induction in dendritic cells [44].
Here, we showed that agonist-mediated induction of IFN-β in melanoma cell lines cocultured with their HLA-matched TILs correlates with increased TIL production of IFN-γ and T lymphocyte-mediated cytotoxicity. IFN-β induction in tumor cells initiates a series of events involving autocrine and paracrine signals that affect both tumor cells and antigen presenting cells, regulating antigen processing, peptide transfer, peptide-loading complex, MHC class I expression, or downstream induction of other cytokines and/or chemokines such as CXCL10 [7, 45]. Therefore, it may be of benefit to investigate if and how IFN-β induced by tumor cells in response to the STING agonist initiates antitumor immunity.
We observed that CXCL10, one of the chemokines induced by type I IFN immune response and STING signaling [46, 47], was induced in melanoma cell lines with intact STING signaling following their stimulation with 2’3’-cGAMP. Along with CXCL9, another CXCR3-binding chemokine, CXCL10 mediates recruitment of CXCR3+ tumor-specific T lymphocytes into the tumors and its intratumoral expression correlated with favorable clinical outcomes in patients with melanoma and colorectal cancer [48-50]. CXCL10 is also one of the chemokines in our earlier reported 12-chemokine gene signature classifier predicting the presence of the tumor-localized, tertiary lymphoid structures, which correlate positively with overall survival in some patients with metastatic melanoma [51]. In addition, CXCL10 promotes the generation and function of effector T cells [52]. Given the role of CXCL10 in mediating T cell recruitment and its positive prognostic value, it seems likely that STING-agonist mediated induction of CXCL10 by tumor cells could facilitate recruitment of effector T cells into the tumor microenvironment. Indeed, CXCL10 could be used to recruit higher numbers of T cells into the tumors that lack T cell infiltration and therefore increase the likelihood of patients responding to current immune checkpoint antibody therapies. And, CXCL10 could be used in TIL-based therapies prior to tumor resection and TIL expansion to attract higher numbers of tumor-specific T cells into the tumors with the aim of increasing the probability of successful expansion of tumor-reactive TILs ex vivo. Finally, in adoptive T cell therapy, STING agonist-mediated CXCL10 induction in tumor cells could be used to improve TIL trafficking into the tumor sites.
In summary, we have shown how human melanoma cell lines respond to STING signaling activation after stimulation with a STING agonist. Our data suggest that activation of STING signaling in melanomas can promote antitumor immunity by regulating tumor cell-intrinsic factors that improve tumor-antigen presentation and recognition by immune T cells, as well as their trafficking. Analysis of the mechanism by which tumor cell intrinsic STING signaling contributes to antitumor immunity will need to be addressed by future in vivo studies. Further understanding of the regulation and function of STING in melanomas and other tumor types may lead to the development of strategies that target STING pathway to improve the efficacy of adoptive cell therapy and other immunotherapies in patients who do not currently benefit from these interventions.
Supplementary Material
Acknowledgements
This work was supported by the Flow Cytometry Shared Resource at the H. Lee Moffitt Cancer Center & Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292).
Financial support:
This work was funded by: NCI-NIH (1R01 CA148995, 1R01 CA184845, P30 CA076292, P50 CA168536), Cindy and Jon Gruden Fund, Chris Sullivan Fund, V Foundation, Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Footnotes
Competing interests
The authors declare no competing interests.
Conflict of interest statement:
No competing financial interests.
References
- 1.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- 3.Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and-extrinsic factors. Immunity. 2016;44:1255–69. [DOI] [PubMed] [Google Scholar]
- 4.Rodig SJ, Gusenleitner D, Jackson DG, Gjini E, Giobbie-Hurder A, Jin C, et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Science translational medicine. 2018;10:eaar3342. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. Journal of immunotherapy (Hagerstown, Md: 1997). 2004;27:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. Journal of Experimental Medicine. 2011;208:1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuertes MB, Kacha AK, Kline J, Woo S-R, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. Journal of Experimental Medicine. 2011;208:2005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107. [DOI] [PubMed] [Google Scholar]
- 10.Dunn GP, Bruce AT, Sheehan KC, Shankaran V, Uppaluri R, Bui JD, et al. A critical function for type I interferons in cancer immunoediting. Nature immunology. 2005;6:722. [DOI] [PubMed] [Google Scholar]
- 11.Woo S-R, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MY, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burdette DL, Monroe KM, Sotelo-Troha K, Iwig JS, Eckert B, Hyodo M, et al. STING is a direct innate immune sensor of cyclic di-GMP. Nature. 2011;478:515–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrales L, Glickman LH, McWhirter SM, Kanne DB, Sivick KE, Katibah GE, et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. Cell reports. 2015;11:1018–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam AR, Le Bert N, Ho SS, Shen YJ, Tang ML, Xiong GM, et al. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer research. 2014;74:2193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho SS, Zhang WY, Tan NYJ, Khatoo M, Suter MA, Tripathi S, et al. The DNA structure-specific endonuclease MUS81 mediates DNA sensor STING-dependent host rejection of prostate cancer cells. Immunity. 2016;44:1177–89. [DOI] [PubMed] [Google Scholar]
- 18.Xia T, Konno H, Ahn J, Barber GN. Deregulation of STING signaling in colorectal carcinoma constrains DNA damage responses and correlates with tumorigenesis. Cell reports. 2016;14:282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia T, Konno H, Barber GN. Recurrent loss of STING signaling in melanoma correlates with susceptibility to viral oncolysis. Cancer research. 2016;76:6747–59. [DOI] [PubMed] [Google Scholar]
- 20.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban S, et al. Brief communication: efficacy of adoptive cell transfer of tumor infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. Journal of immunotherapy 2012;35:615–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souza-Fonseca-Guimaraes F, Parlato M, de Oliveira RB, Golenbock D, Fitzgerald K, Shalova IN, et al. Interferon-gamma and granulocyte/monocyte colony-stimulating factor production by natural killer cells involves different signaling pathways and the adaptor stimulator of interferon genes (STING). Journal of Biological Chemistry. 2013;288:10715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu G, Nemoto S, Mailloux AW, Perez-Villarroel P, Nakagawa R, Falahat R, et al. induction of Tertiary lymphoid structures With antitumor Function by a lymph node-Derived stromal cell line. Frontiers in immunology. 2018;9:1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant J, Day R, Whiteside TL, Herberman RB. Calculation of lytic units for the expression of cell-mediated cytotoxicity. Journal of immunological methods. 1992;146:91–103. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Science signaling. 2012;5:ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer research. 2012;72:5209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. Journal of Experimental Medicine. 1994;180:347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. New England Journal of Medicine. 2015;372:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nature Reviews Cancer. 2016;16:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T cell transfer immunotherapy. Clinical Cancer Research. 2011;17:4550–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Topalian S, Solomon D, Rosenberg S. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. The Journal of Immunology. 1989;142:3714–25. [PubMed] [Google Scholar]
- 32.Robbins PF, Lu Y-C, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nature medicine. 2013;19:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corrales L, McWhirter SM, Dubensky TW, Gajewski TF. The host STING pathway at the interface of cancer and immunity. The Journal of clinical investigation. 2016;126:2404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5, 6-dimethylxanthenone-4-acetic acid. The Journal of Immunology. 2013;190:5216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Science translational medicine. 2015;7:283ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Sun L, Chen ZJ. Regulation and function of the cGAS–STING pathway of cytosolic DNA sensing. Nature immunology. 2016;17:1142–9. [DOI] [PubMed] [Google Scholar]
- 38.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunology today. 2000;21:455–64. [DOI] [PubMed] [Google Scholar]
- 39.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. The Journal of clinical investigation. 1998;101:2720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. JNCI: Journal of the National Cancer Institute. 1996;88:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garrido F, Aptsiauri N, Doorduijn EM, Lora AMG, van Hall T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Current opinion in immunology. 2016;39:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang C-C, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot β2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. The Journal of Immunology. 2005;174:1462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carretero R, Wang E, Rodriguez AI, Reinboth J, Ascierto ML, Engle AM, et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon‐mediated rejection genes. International journal of cancer. 2012;131:387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sistigu A, Yamazaki T, Vacchelli E, Chaba K, Enot DP, Adam J, et al. Cancer cell–autonomous contribution of type I interferon signaling to the efficacy of chemotherapy. Nature medicine. 2014;20:1301–9. [DOI] [PubMed] [Google Scholar]
- 46.Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends in immunology. 2014;35:88–93. [DOI] [PubMed] [Google Scholar]
- 47.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Type I interferons in anticancer immunity. Nature Reviews Immunology. 2015;15:405–14. [DOI] [PubMed] [Google Scholar]
- 48.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–40. [DOI] [PubMed] [Google Scholar]
- 49.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob J-J, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. New England Journal of Medicine. 2017;377:1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mikucki M, Fisher D, Matsuzaki J, Skitzki J, Gaulin N, Muhitch J, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nature communications. 2015;6:7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Scientific reports. 2012;2:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. The Journal of Immunology. 2002;168:3195–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.