Abstract
Background:
Aspirin-exacerbated respiratory disease (AERD) is characterized by severe, sometimes life-threatening reactions to non-steroidal anti-inflammatory drugs (NSAID). Mechanisms driving the disease include overproduction of leukotrienes and loss of anti-inflammatory prostaglandin E2 (PGE2) production. Many cell types contribute to the disease, however eosinophils are markedly elevated and are important drivers of pathology.
Objective:
Investigate the capacity of aspirin and NSAIDs to drive eosinophil activation and the ability of PGE2 to inhibit this activation.
Methods:
Eosinophils were purified from blood of healthy non-AERD individuals and stimulated with lysine aspirin (LysASA), ketorolac or sodium salicylate. The role of PGE2 in altering activation was determined by incubating eosinophils with increasing doses of PGE2 prior to LysASA stimulation. Specific PGE2 receptor utilization was determined by incubating eosinophils with receptor agonists and antagonists before aspirin stimulation. Cysteinyl leukotrienes (CysLT), leukotriene B4 (LTB4) and eosinophil derived neurotoxin (EDN) were quantified by ELISA.
Results:
Stimulation of eosinophils with LysASA, ketorolac or sodium salicylate resulted in secretion of CysLTs and LTB4 in the absence of EDN release. Low doses of PGE2 inhibited LTB4 and CysLT release; an effect lost at higher PGE2 concentrations. Use of butaprost, an EP2 receptor agonist, suppressed LysASA stimulation. This mechanism was supported by blocking activity of the EP1 and EP3 receptors.
Conclusion:
Eosinophils can be directly activated by NSAIDs via cyclooxygenase independent pathways to produce CysLTs and LTB4. This effect can be inhibited by PGE2 acting through the EP2 receptor. The recognized loss of EP2 receptor expression combined with low PGE2 levels, explain in part the sensitivity to NSAIDs.
Introduction
Aspirin-exacerbated respiratory disease (AERD) is characterized by severe, sometimes life-threatening reactions to aspirin and other non-selective non-steroidal anti-inflammatory drugs (NSAIDs).1,2 Previous studies have demonstrated that these reactions are triggered by the reduced synthesis of prostaglandin E2 (PGE2) thereby releasing the anti-inflammatory constraints normally mediated by these anti-inflammatory lipids.3,4 However, this model by itself does not explain the mechanisms driving the activation of these cells that need to be constrained by PGE2. Our previous studies demonstrated the capacity of aspirin to activate eosinophils and mast cells in a fashion independent of its ability to inhibit cyclooxygenase (COX).5,6 However, a complicating question raised by these studies was why reactions are not observed in all subjects ingesting these agents but only in those with AERD. An established feature of AERD is a constitutive critically low level of PGE24 related to downregulation of its metabolic synthesis enzymes including COX-2 and microsomal PGE2 synthase7 occurring concomitantly with reduced capacity of PGE2 to signal through its anti-inflammatory receptor EP2.8,9 We therefore reasoned that sufficient persistent tissue production of PGE2 combined with normal EP2 signaling may be enough to prevent reactions from occurring in the non-AERD population.
AERD is characterized by constitutive overproduction of cysteinyl leukotrienes (CysLTs) and a further surge occurs after ingestion of aspirin.10 These CysLTs are derived from numerous cell sources including mast cells, basophils, and, via transcellular lipid transfer from platelet-adherent neutrophils.11 However, a central source remains eosinophils12 and for the purpose of these studies we focused on their role. In addition to CysLT receptor blockade, the superior efficacy of zileuton in the treatment of this disorder13 led us to investigate the role of an alternative 5-lipoxygenase product, leukotriene (LT) B4 in AERD. Our recent studies suggest that eosinophil-derived LTB4 likely contributes to this disorder.6 Previous studies have demonstrated that aspirin as well as other NSAIDs including those that inhibit COX (e.g., ketorolac) and without COX-inhibiting activity (specifically salicylates) are directly able to activate immune cells including eosinophils.5,6,14–16 We therefore investigated the capacity of aspirin and both ketorolac and sodium salicylate to drive eosinophil activation, focusing on production of CysLTs and LTB4 as well as the eosinophil cationic granule product eosinophil-derived neurotoxin (EDN). We further investigated inhibition of this pathway by PGE2. PGE2 can act alternatively through pro-inflammatory EP1/EP3 or anti-inflammatory EP2/EP4 receptors. Receptor involvement was parsed out using the selective EP2 agonist butaprost or a combination of EP1and EP3 antagonists.8
Methods
Eosinophil purification.
Eosinophils were purified from whole blood of healthy non-allergic donors (n=12) with informed consent under a protocol approved by the University of Virginia Institutional Review Board (#14457) via ficoll-hypaque density gradient centrifugation followed by dextran sedimentation. Eosinophils were separated from neutrophils (PMNs) by negative selection using anti-CD16 magnetic affinity beads (Miltenyi Biotec; San Diego, CA) with counts ranging from 20-100×106 total eosinophils. The preparation was shown to be >99% pure by flow cytometry.6
Eosinophil activation.
Blood eosinophils were activated for 30 min at 37°C with lysine aspirin (LysASA) (10 mM: Sanofi-Aventis; Athens, Greece), ketorolac (10 mM: Hospira; Lake Forest, IL), sodium salicylate (NaSal) (10 mM: Acros Organics; Geel, Belgium) or the combination of phorbol 12-myristate 13-acetate (PMA) (100 ng/ml) with ionomycin (2 μg/ml (Sigma)). For studies with PGE2, butaprost, EP3 antagonist (L-798106) or the EP1 antagonist (SC-51089) (Cayman Chemical; Ann Arbor MI), these compounds were preincubated with eosinophils for 30 min prior to stimulation with LysASA for an additional 30 min at 37°C. Supernatants were collected and mediators quantified by enzyme immunoassay (EIA) as described below.
Enzyme immunoassays.
CysLT (Assay Designs, Ann Arbor, MI), LTB4 (ENZO Life Sciences, Farmingdale, NY), and EDN (MBL, Nagoya, Japan) concentrations in culture supernatants were quantified by EIA according to manufacturer’s instructions. The lower limit of detection of the assays were 26.6, 11.7, and 620 pg/ml, respectively.
Statistical analysis.
Data were contrasted between unstimulated and stimulated cells by Wilcoxon Rank Sum for nonparametric data analyses or paired t-test for parametric data with a P value of >0.05 being considered significant. Testing for normality was performed using the Shapiro-Wilks test. Statistical analyses were performed using GraphPad Prism 6 (La Jolla, CA).
Results
Our previous studies showed equivalent ex vivo eosinophil activation with aspirin from healthy and AERD subjects. We studied healthy subjects to focus on the basis by which these drugs do not cause eosinophil activation in this cohort but also to eliminate any influence of drugs taken by the AERD group.5 Stimulation of eosinophils with LysASA, ketorolac or NaSal all resulted in significant and equivalent synthesis and release of CysLTs (Fig 1A) and LTB4 (Fig 1B). EDN release was not increased with any of the NSAIDs, but only upon stimulation with PMA/ionomycin (Fig 1C).
Figure 1. Mediator release from activated eosinophils.
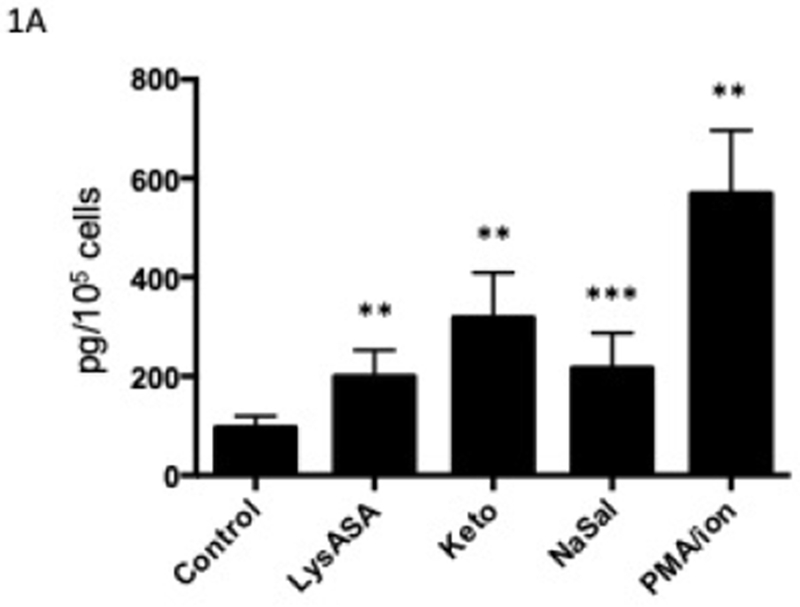
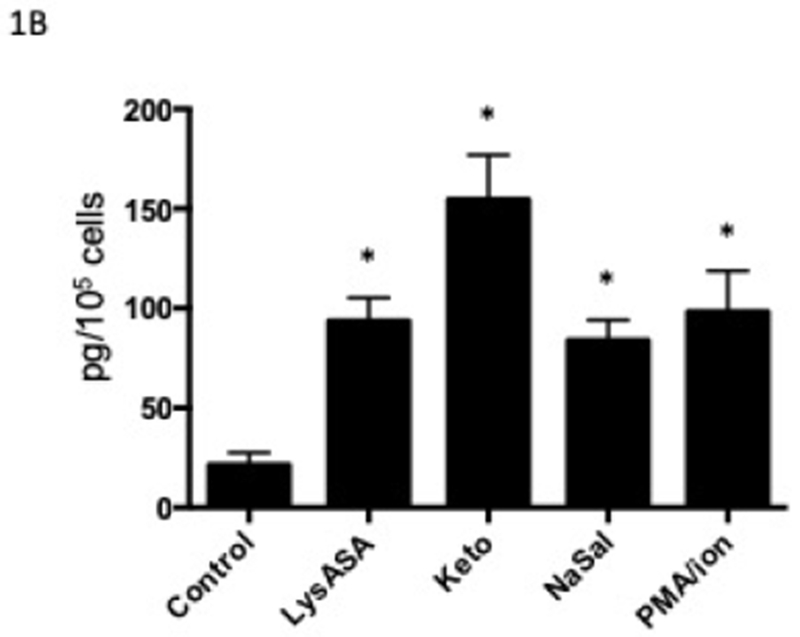
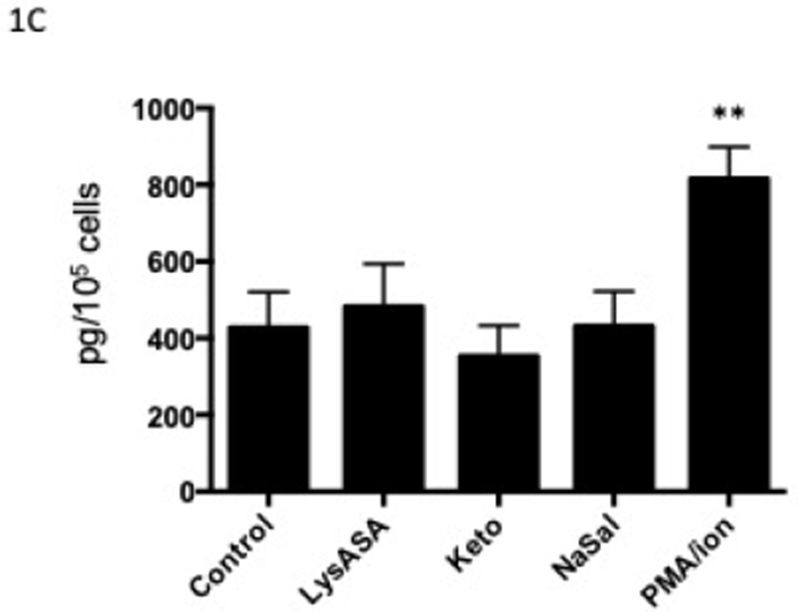
Eosinophils isolated from peripheral blood (n=12) were activated with 10 mM LysASA, ketorolac, NaSal or the combination of ionomycin (2 μg/ml) with PMA (100 ng/ml) for 30 minutes. Supernatants were collected and CysLT (A), LTB4 (B) and EDN (C) levels quantified by EIA and expressed as pg/105 cells. Data are presented as mean±SEM with *p<0.001, **p<0.01 or ***p<0.05 in comparison to unstimulated cells.
In order to determine the effect of PGE2 on activation, eosinophils were incubated with increasing concentrations of PGE2 for 30 min prior to stimulation with LysASA and suppression of LysASA activation was calculated. No suppression of CysLT production was noted (Fig 2A), but suppression of LTB4 production (Fig 2B) was observed with 10−7 M PGE2. This suppression was lost at higher concentrations, possibly reflecting engagement of competing activities of the proinflammatory PGE2 receptors (EP1 and EP3). To directly assess the role of PGE2 acting through its anti-inflammatory receptor, we investigated the effects of butaprost, a selective EP2 agonist, on LysASA stimulation. Butaprost suppressed both CysLT (Fig 2A) and LTB4 (Fig 2B) release in a dose-dependent manner. This was supported by blocking the activity of EP1 and EP3 with specific antagonists followed by stimulation with LysASA and PGE2. Both CysLT and LTB4 production was inhibited under these conditions (Figure 3A and 3B, respectively), though as observed in Figure 1 the effect was more pronounced for LTB4 than for CysLTs. The inhibition in this case would have to be either through the EP2 or EP4 receptors.
Figure 2. Suppression of mediator release from aspirin-activated eosinophils through EP2 receptor.
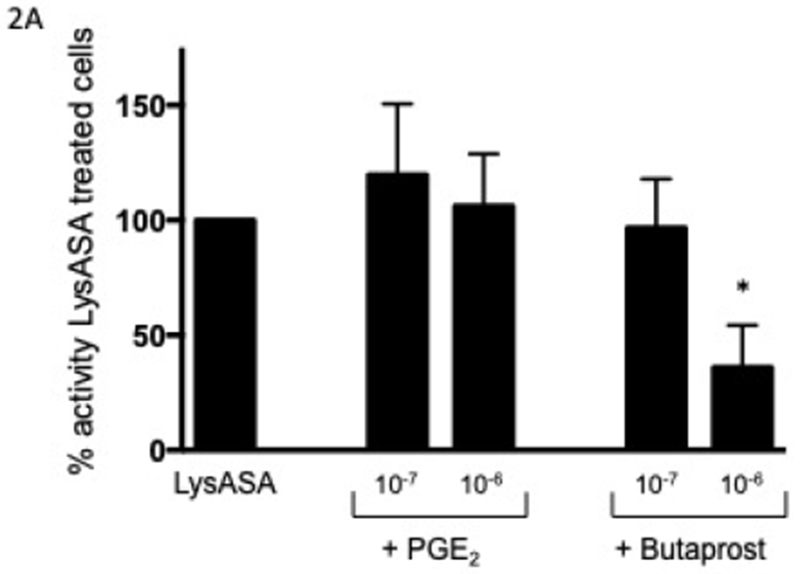
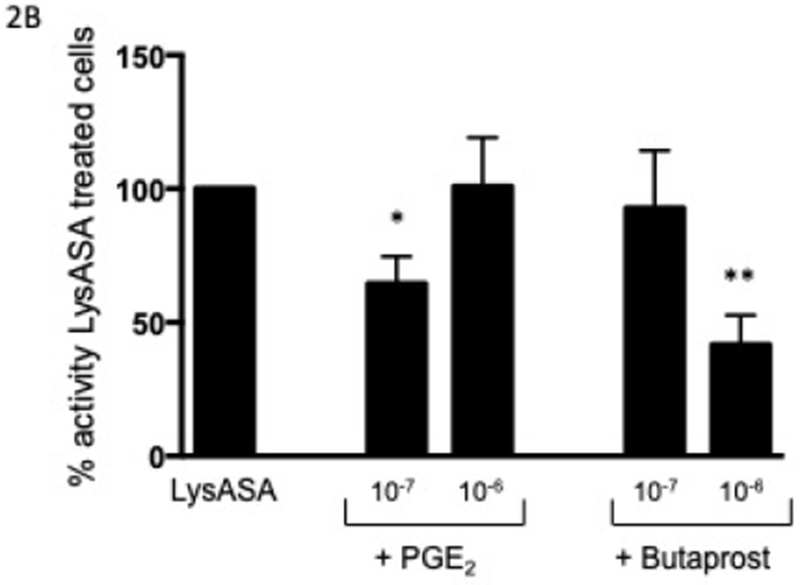
Eosinophils (n=7 subjects) were pre-incubated with PGE2 (10−6 and 10−7 M) or butaprost (10−6 and 10−7 M) for 30 min before activation with 10 mM LysASA for an additional 30 minutes. Supernatants were collected and CysLT (A) and LTB4 (B) levels quantified by EIA. Suppression is presented as percent of activity observed with LysASA stimulated cells. Data are presented as mean±SEM with *p<0.02 or **p<0.002 in comparison to unstimulated cells.
Figure 3. Antagonism of eosinophil activation via EP receptors.
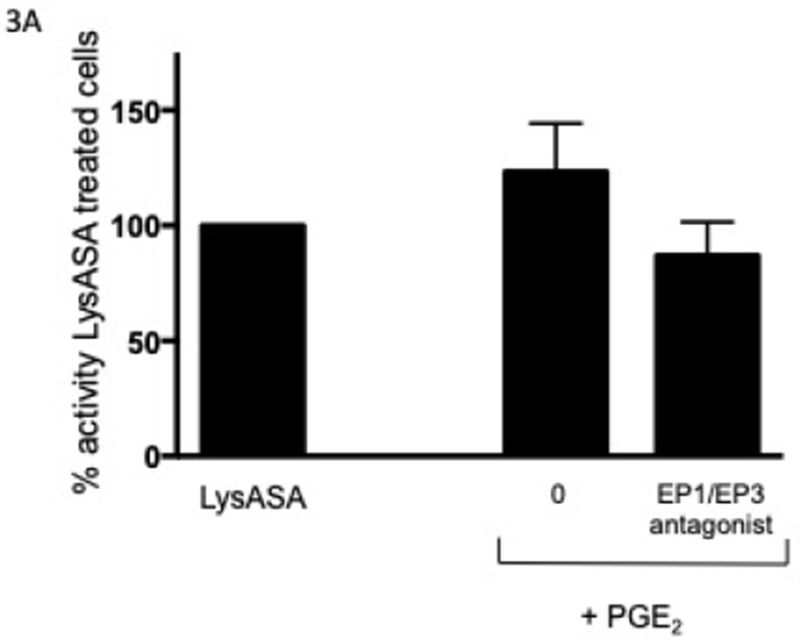
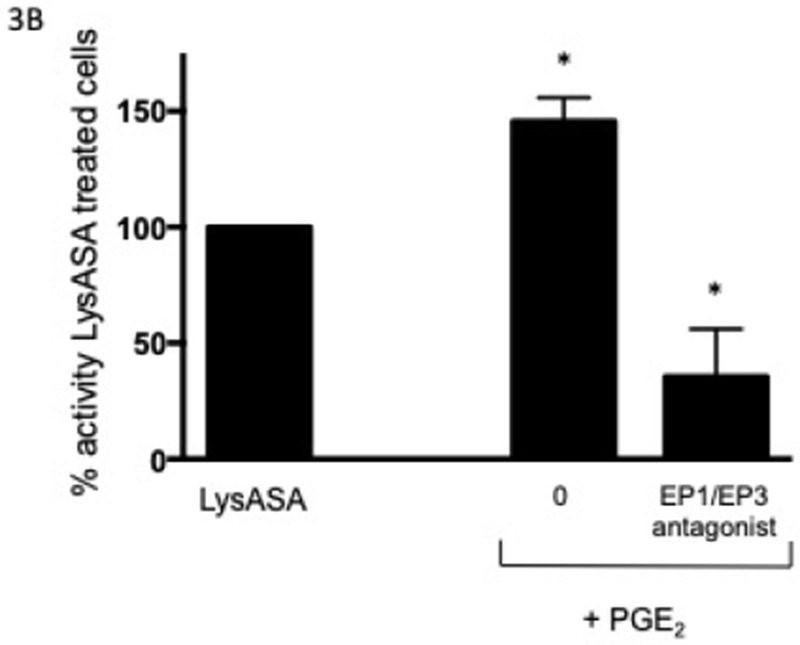
Eosinophils (n=3 subjects) were pre-incubated with an EP1 and EP3 antagonist (10−8 M) for 15 min followed by PGE2 (10−8 M) for 30 min before activation with 10 mM LysASA for an additional 30 minutes. Supernatants were collected and CysLT (A) and LTB4 (B) levels quantified by EIA. Suppression is presented as percent of activity observed with LysASA stimulated cells. Data are presented as mean±SEM with *p<0.05 in comparison to unstimulated cells.
Discussion
We have confirmed that eosinophils can be activated in vitro by aspirin to release leukotrienes (CysLTs and LTB4), without driving substantial release of the cationic peptide EDN (Figure 1). This activity was also observed with two other non-steroidal anti-inflammatory drugs ketorolac and sodium salicylate (Figure 1). It is of interest that sodium salicylate, while able to stimulate leukotriene release in vitro, is generally not thought to elicit symptoms in subjects with AERD. Recent studies suggest efficacy of a stringent low salicylate diet in AERD17,18 suggesting that in some individuals, perhaps in the setting of particularly low constitutive PGE2 expression, may indeed have their pathology exacerbated by dietary salicylates. These observations, along with previous studies that demonstrate eosinophil production of PGD2 following aspirin stimulation,19,20 suggests that activation is in part occurring through a COX-1 independent pathway that is differentially regulated in healthy subjects compared to those with AERD. Support for an independent pathway comes from our previous study that failed to show an increased calcium flux when eosinophils were stimulated with sodium salicylate: a known pathway that leads to COX-1 activation and leukotriene production.5 A human colon cancer cell line that did not express COX was utilized in a microarray study and demonstrated induction of gene expression by aspirin, again indicating cyclooxygenase-independent pathways were being utilized 21 The ability of aspirin and salicylates to modify gene expression had been specifically demonstrated for IL-4 and IL-13 via the STAT6 and Src kinase pathways.22–24 Both IL-4 and IL-13 are important in driving AERD pathology.1
Our current studies demonstrate that eosinophil activation by aspirin can be inhibited by PGE2, specifically PGE2 acting through its EP2 receptor. This leads us to speculate that salicylates will not activate eosinophils from individuals without AERD, as their persistent synthesis of PGE2 maintains constraints on eosinophils (and presumably also on mast cells, basophils, and platelet-adherent neutrophils). In addition, AERD subjects at baseline have vanishingly low concentrations of PGE2, reflecting their profound downregulation of COX and microsomal PGE2 synthase.4,7 While lowering PGE2 in non-AERD individuals, we would argue that sufficient concentrations remain to prevent activation of inflammatory cells. This constraint is further maintained by the preserved expression and function of anti-inflammatory EP2 and EP4 receptors in healthy subjects. Binding of PGE2 to the EP2 receptor has been shown to inhibit mast cell degranulation and prevent lung fibroblast migration through increases in intracellular cyclic AMP.25–27 This is in contrast to AERD subjects, where reduced EP2 expression and function results in the paradoxical tendency of any PGE2 to only have available pro-inflammatory EP1 and EP3 receptors thereby further driving a pro-inflammatory cascade.8,28 An additional constraint coupled to the lower EP2 expression is the higher affinity of PGE2 for the EP3 receptor.29 As a result, any available PGE2 will likely bind the EP3 pro-inflammatory receptor.
In summary, our studies show that eosinophils can be directly activated by NSAIDs via cyclooxygenase independent pathways to produce CysLTs and LTB4. This effect can be inhibited by PGE2 acting through the EP2 receptor. Loss of EP2 receptor expression combined with low PGE2 levels, explain, at least in part, the sensitivity of AERD but not healthy controls on exposure to NSAIDs.
Acknowledgments
Conflict of interest statement: Larry Borish is the primary investigator to whom funding was awarded by the NIH. All other authors report no conflicts of interest with data presented in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinke JW, Borish L. Factors driving the AERD phenotype. Am J Rhinol Allergy 2015;29:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laidlaw TM. Clinical updates in aspirin-exacerbated respiratory disease. Allergy Asthma Proc 2019;40;4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sestini P, Armetti L, Gambaro G, Pieroni MG, Refini RM, Sala A, et al. Inhaled PGE2 prevents aspirin-induced bronchoconstriction and urinary LTE4 excretion in aspirin-sensitive asthma. Am J Respir Crit Care Med 1996;153:572–5. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Novo CA, Watelet JB, Claeys C, van Cauwenberge P, Bachert C. Prostaglandin, leukotriene, and lipoxin balance in chronic rhinosinusitis with and without nasal polyposis. J Allergy Clin Immunol 2005;115:1189–96. [DOI] [PubMed] [Google Scholar]
- 5.Steinke JW, Negri J, Liu L, Payne SC, Borish L. Aspirin activation of eosinophils and mast cells:implications in the pathogenesis of aspirin-exacerbated respiratory disease. J Immunol 2014;193:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal K, Feng X, Steinke JW, Burdick MD, Shim YM, Sung SS, et al. Leukotriene A4 hydrolase activation and leukotriene B4 production by eosinophils in severe asthma. Am J Respir Cell Mol Biol 2018; 60:413–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci USA 2013;110:16987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng C, Beller EM, Bagga S, Boyce JA. Human mast cells express multiple EP receptors for prostaglandin E2 that differentially modulate activation responses. Blood 2006;107:3243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serra-Pages M, Olivera A, Torres R, Picado C, de Mora F, Rivera J. E-prostanoid 2 receptors dampen mast cell degranulation via cAMP/PKA-mediated suppression of IgE-dependent signaling. J Leukoc Biol 2012;92:1155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson AP, Cowburn AS, Sladek K. Profound overexpression of leukotriene C4 synthase in bronchial biopsies from aspirin-intolerant asthmatic patients. Int Arch Allergy Immunol 1997;113:355–7. [DOI] [PubMed] [Google Scholar]
- 11.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 2012;119:3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Payne SP, Early SB, Huyett P, Han JK, Borish L, Steinke JW. Evidence for distinct histologic profile of nasal polyps with and without eosinophilia. Laryngoscope 2011;121:2262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlen B, Nizankowska E, Szczeklik A, Zetterstrom O, Bochenek G, Kumlin M, et al. Benefits from adding the 5-lipoxygenase inhibitor zileuton to conventional therapy in aspirin-intolerant asthmatics. Am J Respir Crit Care Med 1998;157:1187–94. [DOI] [PubMed] [Google Scholar]
- 14.Perez GM, Melo M, Keegan AD, Zamorano J. Aspirin and salicylates inhibit the IL-4 and IL-13-induced activation of STAT6. J Immunol 2002;168:1428–34. [DOI] [PubMed] [Google Scholar]
- 15.Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994;265:956–9. [DOI] [PubMed] [Google Scholar]
- 16.Aceves M, Duenas A, Gomez C, San Vicente E, Crespo MS, Garcia-Rodriguez C. A new pharmacological effect of salicylates: inhibition of NFAT-dependent transcription J Immunol 2004;173:5721–9. [DOI] [PubMed] [Google Scholar]
- 17.Sommer DD, Hoffbauer S, Au M, Sowerby LJ, Gupta MK, Nayan S. Treatment of aspirin exacerbated respiratory disease with a low salicylate diet: a pilot crossover study. Otolaryngol Head Neck Surg 2015;152:42–7. [DOI] [PubMed] [Google Scholar]
- 18.Sommer DD, Rotenberg BW, Sowerby LJ, Lee JM, Janjua A, Witterick IJ, et al. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a mutlicenter randomized control crossover trial. Int Forum Allergy Rhinol 2016;6:385–91. [DOI] [PubMed] [Google Scholar]
- 19.Luna-Gomes T, Magalhaes KG, Mesquita-Santos FP, Bakker-Abreu I, Samico RF, Molinaro R et al. Eosinophils as a novel cell source of prostaglandin D2: autocrine role in allergic inflammation. J Immunol 2011;187:6518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng X, Ramsden MK, Negri J, Baker MG, Payne SC, Borish L, et al. Eosinophil production of prostaglandin D2 in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;138:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin H, Xu H, Zhao Y, Yang W, Cheng J, Zhou Y. Cyclooxygenase-independent effects of aspirin on HT-29 human colon cancer cells, revealed by oligonucleotide microarrays. Biotechnol Lett 2006;28:1263–70. [DOI] [PubMed] [Google Scholar]
- 22.Cianferoni A, Schroeder JT, Kim J, Schmidt JW, Lichtenstein LM, Georas SN, et al. Selective inhibition of interleukin-4 gene expression in human T cells by aspirin. Blood 2001;97:1742–49. [DOI] [PubMed] [Google Scholar]
- 23.Perez-G M, Melo M, Keegan AD, Zamorano J. Aspirin and salicylates inhibit the IL-4- and IL-13-induced activation of STAT6. J Immunol 2002;168;1428–34. [DOI] [PubMed] [Google Scholar]
- 24.Steinke JW, Culp JA, Kropf E, Borish L. Modulation by aspirin of nuclear phospho-signal transducer and activator of transcription 6 expression: possible role in therapeutic benefit associated with aspirin desensitization. J Allergy Clin Immunol 2009;124:724–30. [DOI] [PubMed] [Google Scholar]
- 25.Kay LJ, Yeo WW, Peachell PT. Prostaglandin E2 activates EP2 receptors to inhibit human lung mast cell degranulation. Br J Pharmacol 2006;147:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White ES, Atrasz RG, Dickie EG, Aronoff DM, Stambolic V, Mak TW, et al. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol 2005;32:135– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol 2004;173:559–65. [DOI] [PubMed] [Google Scholar]
- 28.Corrigan CJ, Napoli RL, Meng Q, Fang C, Wu H, Tochiki K, et al. Reduced expression of the prostaglandin E2 receptor E-prostanoid 2 on bronchial mucosal leukocytes in patients with aspirin-sensitive asthma. J Allergy Clin Immunol 2012;129:1636–46. [DOI] [PubMed] [Google Scholar]
- 29.Markovič T, Jakopin Ž, Dolenc MS, Mlinarič-Raščan I. Structural features of subtype-selective EP receptor modulators. Drug Discov Today 2017;22:57–71. [DOI] [PubMed] [Google Scholar]


