Abstract
Neutrophil infiltration and neutrophil extracellular traps (NETs) in solid cancers are associated with poorer prognosis but the mechanisms are incompletely understood. We hypothesized that NETs enhance mitochondrial function in tumor cells providing extra energy for accelerated growth. Metastatic colorectal cancer tissue showed increased intratumoral NETs and supranormal preoperative serum MPO-DNA, a NET marker. Higher MPO-DNA correlated with shorter survival. In mice, subcutaneous tumor implants and hepatic metastases grew slowly in PAD4-KO mice, genetically incapable of NETosis. In parallel experiments, human cancer cell lines grew slower in nu/nu mice treated with DNAse, which disassembles NETs. PAD4-KO tumors manifested decreased proliferation, increased apoptosis and increased evidence of oxidative stress. PAD4-KO tumors had decreased mitochondrial density, mitochondrial DNA, a lesser degree of ATP production, along with significantly decreased mitochondrial biogenesis proteins PGC-1α, TFAM and NRF-1. In vitro, cancer cells treated with NETs upregulated mitochondrial biogenesis associated genes, increased mitochondrial density, increased ATP production, enhanced the percentage of cancer cells with reduced mitochondrial membrane potential and increased the oxygen consumption rate. Furthermore, NETs increased cancer cell's expression of fission and fusion associated proteins, DRP-1 and MFN-2, and mitophagy-linked proteins, PINK1 and Parkin. All of which were decreased in PAD4-KO tumors. Mechanistically, neutrophil elastase (NE) released from NETs activated TLR-4 on cancer cells leading to PGC-1α upregulation, increased mitochondrial biogenesis and accelerated growth. Taken together, NETs can directly alter the metabolic programming of cancer cells to increase tumor growth. NETs represent a promising therapeutic target to halt cancer progression.
INTRODUCTION
Solid malignant tumors accumulate a diverse collection of inflammatory cells representing both innate and adaptive immune responses as they grow (1,2). Neutrophils account for a significant portion of the inflammatory cells in the tumor microenvironment (TME) of various malignancies (3-6). In addition to serving as a first line of antimicrobial defense, an important role for tumor-associated neutrophils (TAN) has been found to promote tumor growth and metastasis at multiple stages of cancer progression (7). Much current evidence is starting to support the notion that, neutrophils exert these tumor promoting functions, not by phagocytic mechanisms, but rather via the formation of neutrophil extracellular traps (NETs) within tumors a process termed NETosis (8,9).
NETs are produced by extrusion of decondensed DNA chromatin into the extracellular space complexed with citrullinated histones (cit-H3) together with neutrophilic cytoplasmic contents containing granular enzymes, such as myeloperoxidase (MPO) and neutrophil elastase (NE) (10). NETs can augment various inflammatory responses including autoimmune, thrombotic and cardiovascular diseases (11-13). NETosis requires the activation of the enzyme Peptidylarginine deiminase (PAD)-4 which after translocation to the nucleus, citrullinates nuclear histones, inducing chromatin decondensation and release. In a model of surgical stress, sterile inflammation and liver metastases, we have shown that NETs are capable of not only capturing circulating tumor cells, but more importantly increasing their metastatic potential and also promoting the growth of micrometastatic disease (14). Either the prevention of NET formation using mice lacking PAD4 and thus incapable of NET formation, or the administration of deoxyribonuclease (DNAse) to mice to dissolve extruded chromatin as it forms during NETosis, each succeeded in reducing sterile inflammation and significantly decreased metastatic tumor growth in the liver. NETs have also been found in human tumors and their presence confers a worse prognosis. Recently, NETs have also been shown to awaken dormant metastatic foci (15). The mechanisms by which NETs in the TME enhance tumor growth require further clarification.
Solid tumors typically develop in hostile microenvironments but despite that cancer cells continue to exhibit upregulated growth. Recent evidence shows that despite enhanced glycolysis, cancer cells also operate mitochondrial respiration to derive a significant fraction of their adenosine triphosphate (ATP) (16). The variations in metabolic wiring, including change in the bioenergetic profile which favor mitochondrial biogenesis and oxidative phosphorylation, could allow some cancer cells within the TME to be better positioned to survive specific stresses (13). Mitochondrial biogenesis can be defined as the growth and division of preexisting mitochondria. It requires the coordinated synthesis of proteins encoded by the nuclear genome, mitochondrial DNA (mtDNA) replication, as well as mitochondrial fusion and fission must also be coordinated. This process, mainly driven by Peroxisomes proliferator-activated receptor gamma coactivator 1-alpha (PGC1-α), results in an energy boost favorable for anabolic tumor growth. As tumors grow, more NETs are present in the TME which parallels both the increased stress in the environment and the increased cancer cell proliferation (14). We, therefore, hypothesized that a similar metabolic switch is induced by NETs in order to provide the tumor with an adaptive strategy to survive. In this manuscript, we provide evidence that stressed cancer cells release damage associated molecular pattern (DAMP) proteins to recruit neutrophils to the TME and induce NET formation. NETs in turn directly increase energy production and accelerate cancer cell proliferation by promoting mitochondrial homoeostasis primarily through increasing mitochondrial biogenesis. By releasing neutrophil elastase (NE), NETs activate toll-like receptor (TLR)-4 on tumor cells to induce mitochondrial biogenesis and tumor growth. Inhibition of NET formation by genetic alteration, or by DNAse and NE inhibitor (NEi) in vivo, or blocking the NE-TLR4-PGC-1α axis in vitro can inhibit mitochondrial biogenesis and slow tumor growth. Thus, understanding this crosstalk between cancer cells and neutrophils has potential clinical implications in the treatment of metastatic disease.
MATERIALS AND METHODS
Patient samples and data
All the human material utilized during the experiment were obtained under an approved institutional review board (IRB) and Protocol after written informed consent was received from all participants prior to inclusion. Serum samples were obtained pre-operatively within a month of liver resection for metastatic colorectal carcinoma at the University of Pittsburgh Medical Center. We included sequential eligible patients, between the years 2010 and 2016, who were judged to be disease-free at the end of the operation and had a minimum one-year postoperative follow-up. We quantified MPO-DNA complex levels for each serum sample and determined the fold-change compared to healthy control sera from an IRB approved institutional serum bank. Resected tumor tissue and adjacent normal liver were examined by blinded pathologists. Data from the electronic medical record was used to determine disease-free survival and overall survival. A standard protocol of follow up with bi-annual serum carcinoembryonic antigen (CEA) levels and cross-sectional imaging was used to seek recurrent disease. Patients who did not experience an event or were lost to follow-up were censored at the date of last contact or end of the study period.
Cell lines
Murine colorectal (MC38) cells were obtained from Dr. Daolin Tang (University of Pittsburgh, Pittsburgh, PA). HCT116, Hepa1-6 and Huh7 cell lines were purchased from ATCC (Manassas, VA). Cell lines were confirmed by appearance, growth curve analysis and verified to be mycoplasma free using Mycoplasma PCR ELISA (Roche). Cell lines were authenticated using genomic profiling (Genetica, NC).
Animals and Tumor Models
Male wild-type (C57BL/6) mice and athymic nude mice (Nu/J) (8-weeks-old) were purchased from Jackson Immuno-Research Laboratories (West Grove, PA). Peptidyl arginine deiminase type IV knockout (PAD4−/−) mice were provided by Scripps University. Animal protocols were approved by the Animal Care and Use Committee of the University of Pittsburgh and the experiments were performed in adherence to the National Institutes of Health Guidelines. In the subcutaneous flank models, mice were injected with cancer cells (1×106 cells/injection) subcutaneously. The tumor size was measured weekly until sacrifice. Liver metastases were induced in mice as previously described (17). WT and Nude mice in the treatment groups were injected with either DNAse-1 (50µg/mouse, Roche) or Neutrophil Elastase Inhibitor (2 mg/kg) (GW311616 MCE, NJ USA) intraperitoneally every day until the time of sacrifice.
NET isolation and cell treatment
Isolated neutrophils were treated with Phorbol-12-Myristate-13-Acetale (PMA) and collected after 4 hours. Cell were then centrifuged at 480g for 10mins. The supernatant (NET rich media) was then centrifuged at 18000g for 15 mins to form a pellet. The obtained pallet contained the mixture of chromatins and proteins which was then resuspended in cell culture media to treat cancer cells.
Statistical analysis
For animal studies, results are expressed as either standard error of the mean (SEM) or mean standard deviation (SD). Group comparisons were performed using One-way ANOVA with post-hoc Tukey honestly significant difference (HSD) analysis and Student’s t-test. For the human subject’s data analysis, we dichotomized MPO-DNA complex levels at the median and compared the baseline characteristics for each group using chi-square or Fisher’s exact tests for categorical variables and Student’s t or Wilcoxon rank sum tests for continuous variables. We used the Kaplan-Meier estimator to plot survival. For all analyses, two-tailed p-values below 0.05 were considered statistically significant.
RESULTS
NET formation correlates with cancer specific outcomes in patients with metastatic colorectal cancer.
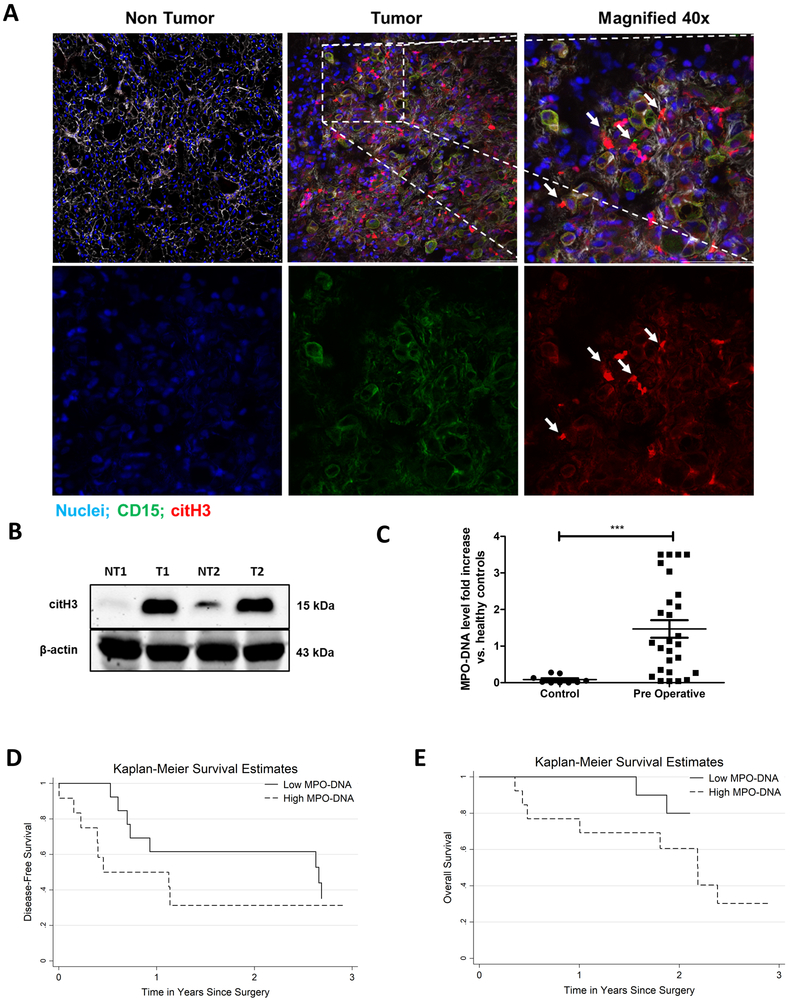
A number of studies have suggested important roles of NETs in tumor progression in animal models (15,18). We found that histopathologic study of colorectal liver metastases (CRLM) in 27 patients undergoing elective curative resection showed a significant increase in tumor associated neutrophils and NETs compared to background liver (Figure 1A). Tumor tissue expressed high levels of citrullinated histones, indicative of NETosis (Figure 1B). In addition, the preoperative serum levels of MPO-DNA, a reliable marker for systemic NETosis (19), in patients with CRLM (n=27) were found to be significantly elevated compared to healthy controls (n=10, p<0.0002) (Figure 1C). We then compared the disease-free and overall survival of patients with CRLM with preoperative MPO-DNA levels above or below the median (Figure 1D and 1E). Of note, all patients had tumor free margins on the resected specimen. Patients with above median (high) MPO-DNA levels had significantly shorter disease-free and overall survival than patients with below median (low) MPO-DNA levels. The median disease-free survival was 5.8 times shorter in patients with high MPO-DNA compared with low MPO-DNA preoperatively (5.5 versus 31.0 months, p<0.001). Similarly, patients with high MPO-DNA had a worst median overall survival after surgery compared to patients with low MPO-DNA (26.2 versus 43.2 months, p<0.001). High MPO-DNA levels were significantly associated with worse survival on multivariable Cox models after adjustment to other known prognostic variables including age, number of tumors, size of largest lesion, and CEA level (HR 2.13 95% CI 1.43-4.17 p<0.01) Supplementary Table 1.
Figure 1. NET formation correlates with cancer specific outcomes in patients with metastatic colorectal cancer.
A, Representative immunofluorescence images by confocal microscopy of human colorectal liver metastases (CRLM) tissue sections showing increased neutrophil infiltration and neutrophil extracellular trap (NET) formation in tumor at 20× magnification compared to non-tumor tissue of the same patient. White arrows showing neutrophils releasing NETs in merge and single staining magnified images at 40×, scale bar 50 µm. B, Protein citH3 levels were increased as evident by western blot image between the tumor (T) and non-tumor (NT) tissue of human CRLM. The blot shown is representative of three independent experiments with similar results. C, Pre-operative MPO-DNA levels detected by Elisa kit are significantly higher in patients with CRLM (n=27) compared to healthy volunteers (n=10, ***p<0.0002). D and E, Kaplan-Meier disease-free and overall survival curves were based on high versus low MPO-DNA levels post-operatively for three years (log-rank test p<0.001 for both D and E).
Tumor growth is reduced in neutrophil extracellular traps (NETs) depleted tumor bearing mice.
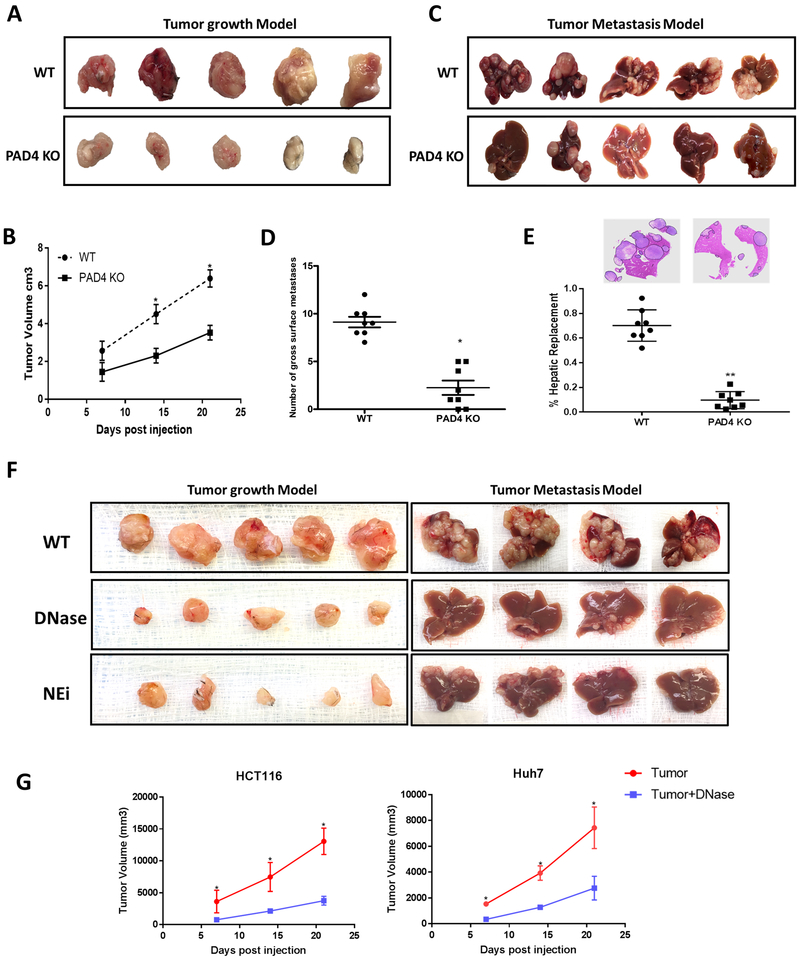
The presence of NETs within CRLM is associated with a worse prognosis in patients as seen above. In addition, we and others have shown that neutrophil infiltration and NET formation is associated with tumor progression and metastases (14,20-22) We therefore compared the rate of tumor growth in WT mice with that in mice genetically incapable of NET formation due to the absence of PAD4 enzyme (23). We compared the growth rates of MC38 cancer cells when inoculated subcutaneously into the flanks of normal C57BL/6 mice and PAD4-KO. Figure 2A and 2B show that subcutaneous tumors grew significantly more slowly in PAD4-KO mice than in WT mice. In a liver metastases model, tumor metastases in PAD4-KO mice were significantly fewer and smaller with less tumor burden than those in wild type mice (Figure 2C and 2D and 2E). Similar results were obtained in NET inhibited WT mice treated daily with intraperitoneal injections of DNAse, which is known to dissolve NETs as they form, and neutrophil elastase inhibitor (NEi) for both subcutaneous and liver metastasis model (Figure 2F). In addition, we also studied the growth of human colorectal and hepatocellular cancer cell lines, HCT116 and Huh7, injected subcutaneously in the flanks of athymic nu/nu mice. DNAse administration to nu/nu mice significantly slowed the growth of tumors to a degree similar to tumors growing in NET inhibited mice (Figure 2G). Thus, inhibition of NETosis is associated with slower tumor growth in a variety of tumor transplants in vivo. Taken together these findings suggest that inhibition of NETs by either method will have similar effects on tumor growth.
Figure 2. Tumor growth is reduced in neutrophil extracellular traps (NETs) depleted mice.
Subcutaneous tumors using MC38 cells (1×106) injected subcutaneously (A) or through the spleen (C) for the metastasis model showing smaller tumors harvested 3 weeks post-inoculation in PAD4 KO compared to WT mice (n=5/group). B and D, Graphs showing significantly decreased tumor volume and surface liver nodules, respectively, in PAD4 KO mice compared to WT control *P <0.05. E, Hematoxylin and Eosin (H&E) staining of liver sections exhibit decreased tumor burden in PAD4 KO mice (n=5) compared to WT **P <0.01. F, Similarly, mice treated daily with DNAse (50ug) or Neutrophil Elastase inhibitor (NEi) (2.0 mg/kg) in both subcutaneous (n=5) and metastatic model (n=4) showed decrease tumor growth compared to controls. G, Graph representing tumor growth curve in DNAse (50ug) treated Nu/Nu athymic mice inoculated with HCT116 and Huh7 cell lines (1×106) (n=5/group). Data presented as mean SEM. *P <0.05.
Solid tumors recruit neutrophils and induce NET formation in their microenvironment.
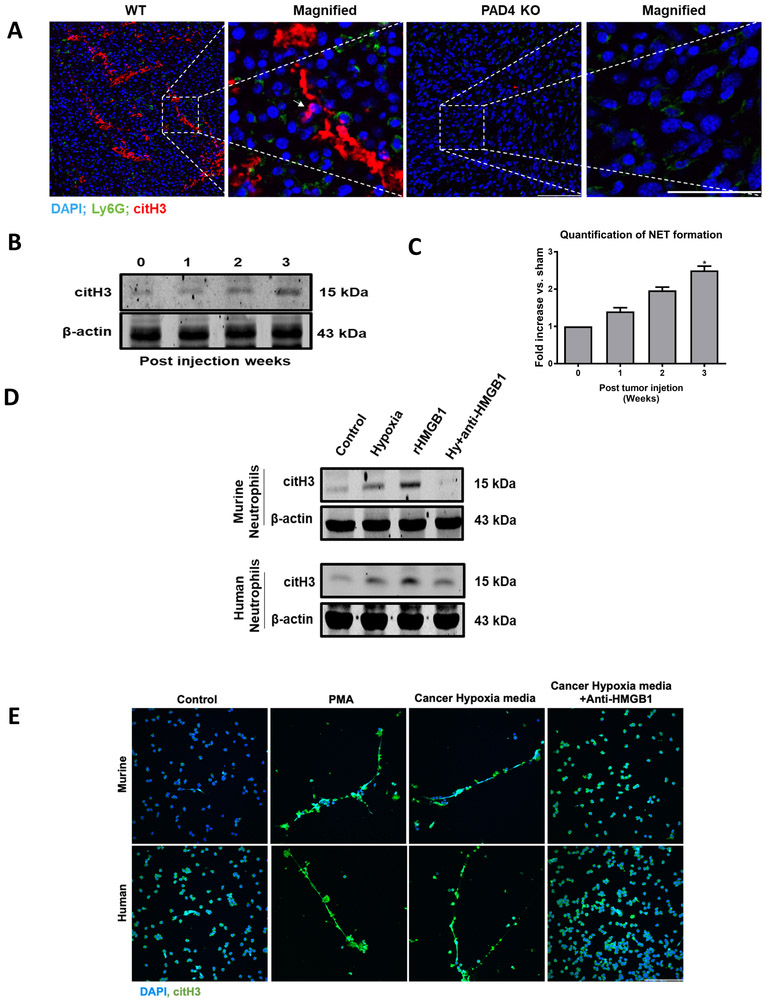
To further investigate the NET-Tumor interaction, tumor tissues from both PAD-KO and WT were harvested after 3 weeks of growth and stained with neutrophil (Ly6G) and NET (citH3) markers (Figure 3A). Immunofluorescent images of tumor tissues revealed that neutrophil infiltration was markedly reduced in the PAD4-KO tumors. NETs were abundant in the tumor tissue in WT mice and absent in PAD-KO mice (Figure 3A). Citrullinated histone characteristic of NET formation was detectible within the growing tumor in WT mice and increased with increasing tumor growth (Figure 3B). Increased levels of serum MPO-DNA, a common marker of in vivo NET formation, were found within the first week and increased in parallel with tumor growth (Figure 3C).
Figure 3. Solid tumors recruit neutrophils and induce NET formation in their microenvironment.
A, Sectional staining of harvested tumor tissue showing neutrophils and neutrophil extracellular traps (NETs) infiltration three weeks after the cancer injection in WT and PAD4 KO mice (magnification 40×, magnified image 60×, scale bar, 50 µm). White arrow showing neutrophils releasing NETs. Nucleus (blue), Ly6G (green) and citH3 (red). B and C, CitH3 protein expression by western blot and Elisa quantification of serum MPO-DNA level (n=3/group) in growing tumors was assessed at indicated time. Invitro, neutrophils isolated from healthy human volunteer (blood) or mouse (bone marrow) when cocultured with 24h hypoxic cancer media (HCT116 and MC38) and rHMGB1 (1µg) for 4h resulted in an increase NET formation as evident by western blot (D) and confocal microscopy (E) at 40× magnification. Neutrophils treated with PMA (250 nM) serves as a positive control. Anti-HMGB1 antibody added to cancer hypoxic media inhibited NET formation when cocultured with neutrophils. Abbreviations Hy, hypoxia. The blots shown are representatives of three independent experiments with similar results. *P <0.05.
The mechanisms by which NETs are generated within tumors deserves study. We therefore sought to evaluate how neutrophils are recruited to the tumor environment and are induced to form NETs. Neutrophils are known to be recruited to sites of acute inflammation and induced to form NETs (24). Hypoxia is commonly present in rapidly growing solid tumors and has been linked to a chronic inflammatory state within the tumor environment. To investigate how tumor cells recruit neutrophils, we performed a neutrophil migration assay. Freshly harvested neutrophils from both human and C57BL/6 mice were studied in trans-well cell migration chambers using media from cultured tumor cells exposed to hypoxic or normoxic conditions. Hypoxic cancer cells have a significant increase in the mRNA expression of neutrophil attractant chemokines (CXCL1/KC, CXCL2/MIP2, and CXCL5/LIX) (Supplementary Figure 1A). Both mouse and human neutrophils exposed to culture media from hypoxic MC38 or HCT116 tumor cells, respectively, migrated more rapidly and in greater number than when exposed to media from of normoxic cultures (Supplementary Figure 1B). In addition to known chemokines, the media of human and murine colorectal and hepatocellular cancer cell lines treated with hypoxia for 24h showed significant increase levels of HMGB1 as measured by Elisa (Supplementary Figure 1C). HMGB1 is known not only to act as a neutrophil chemotactic agent but also can induce NET formation (25). Indeed, neutrophils isolated from humans and mice, when treated with hypoxic media from HCT116 and MC38 tumors, also formed NETs with PMA or recombinant HMGB1 as positive controls (Figure 3D and Figure 3E). The addition of monoclonal HMGB1 neutralizing antibody to hypoxic cancer media markedly inhibited NET formation (Figures 3D and Figure 3E). Taken together one can infer that under conditions of stress, caused partly by the hypoxia in the rapidly growing tumor (26), HMGB1 is released from the tumor cells, and may act along with chemokines to draw neutrophils into the area and act to generate NETs within the tumor microenvironment.
NETs in the tumor microenvironment correlate with evidence of increased mitochondrial biogenesis and more rapid tumor growth.
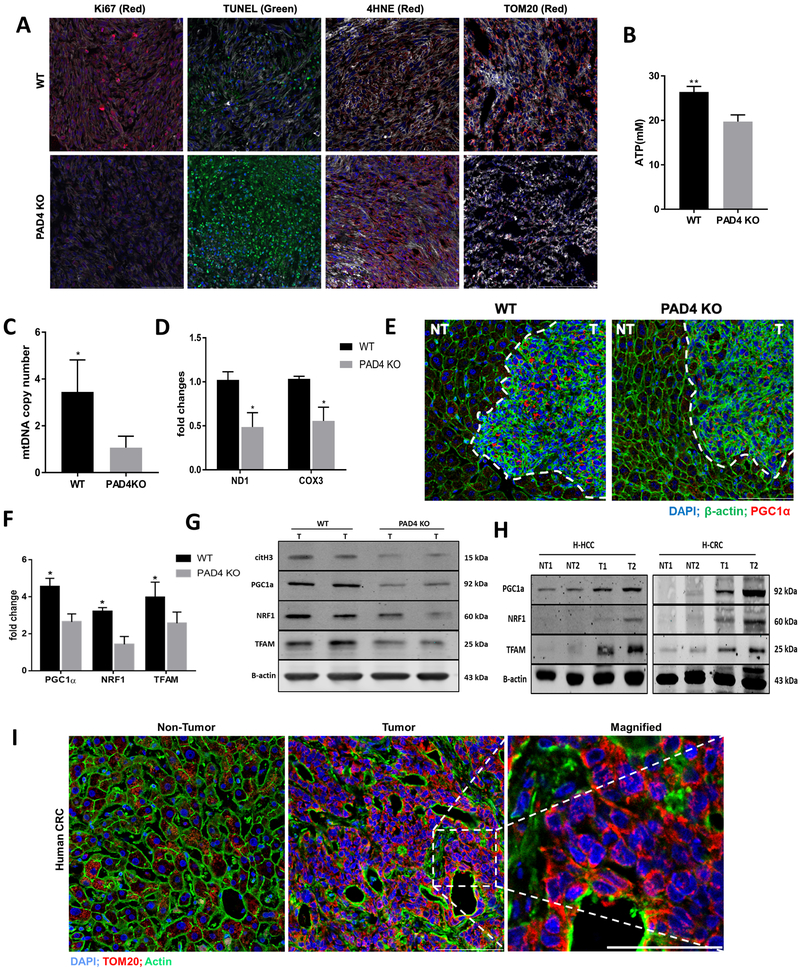
Figure 4A compares the immunohistochemical markers of cell proliferation and active mitosis (Ki67), apoptosis (TUNEL), oxidative stress (4-HNE; 4-hydroxynenal Michael adducts), and expression of the mitochondrial protein translocase on the mitochondrial outer membrane-a common measure of overall mitochondrial function and density (TOM20). Tumors in PAD4-KO, DNAse and NEi treated mice showed lesser degrees of cellular proliferation, and lesser maintenance of mitochondrial function. Concurrently there was marked evidence of tumor cell apoptosis and greater evidence of lipid peroxidation with elevated 4HNE expression in PAD4-KO mice than seen in tumors grown in WT mice (Figure 4A and Supplementary Figure 2A). These changes are consistent with the findings in WT treated DNAse, NEi and PAD4-KO mice of lesser degrees of ATP production, reduced Mitochondrial DNA (mtDNA) copy number, and poor expression of NADH-ubiquinone oxidoreductase chain 1 [ND1] and cyclooxygenase 3 (Figure 4B, 4C, 4D and Supplementary Figure 2B and 2C). In short, the presence of abundant NETosis in WT tumors is associated with a pro-proliferative active metabolic response and preservation of mitochondrial function, mitochondrial homeostasis, coincident with more energy production and more rapid tumor growth. As seen in the experiments above, tumors of various types grow less vigorously, fail to protect their mitochondrial integrity, and generate far less ATP than WT mice when NET production is genetically prevented. Conversely, invasion by neutrophils and NET formation enhances energy production within tumors and augments tumor growth in vivo.
Figure 4. NETs in the tumor microenvironment correlate with evidence of increased mitochondrial biogenesis and more rapid tumor growth.
A, A marked decrease in cell proliferation rate (Ki67) and increased apoptosis (TUNEL) was observed in PAD4 KO tumors. Ki67+ (median 25 [3-44] Ki67+ cells/106 µm2 in PAD4KO tumor tissue versus 120 [62-159] Ki67+ cells/106 µm2 in WT and TUNEL (median 31 [5-49.3] TUNEL+ cells/103 µm2 in PAD4KO tumor tissue versus 17 [9-35] TUNEL+ cells/103 µm2 in WT, P <0.05). Increase in the oxidative stress observed by positive 4-HNE staining in PAD4 KO tissue (median 0.193 [0.067-0.438] in PAD4 KO versus 0.053 [0.005-0.079] in WT control, P<0.05. PAD4 KO tumors showed impaired mitochondrial density compared with WT tumors when stained for TOM20. The median normalized TOM20+ area in PADKO tumor tissue was 0.087 (range 0.0065-0.197) versus 0.438 (range 0.292-0.504) in control tumors. Nuclei (blue), actin (white), Ki67/4HNE/TOM20 (red), TUNEL (green). Scale bars, 100 µm, n=5 mice/group. Values were based on analysis of tumors from five mice for each group. There was a significant decrease in mtDNA copy numbers measured by RT-PCR (B) and lesser degree of ATP levels (C) in tumors lacking NETs. **P < 0.01, *P < 0.05. D, COX3 and ND1 expression was analyzed by western blot showing significantly low in protein density, *P < 0.05. E, Three weeks after splenic injection the liver tumor area of WT mouse showing upregulated PGC-1α expression as evident by immunofluorescence comparing PAD4 KO. No differences in the non-tumor section of both mice were observed. Magnification 40×, scale bars 50 µm. F and G, Genes associated with mitochondrial biogenesis were assessed by both Real-time PCR and western blot in the tumor tissue of PAD4 KO and WT mice, *P < 0.05. H, Protein expression of citH3, PGC-1α, NRF1, and TFAM in human hepatocellular (H-HCC) and colorectal (H-CRC) tumor tissue (T) comparing its non-tumor (NT) counterpart. The blots shown are representatives of three independent experiments with similar results I, The human colorectal liver metastasis (CRLM) tissue showing an increased mitochondrial density in the tumor section compared to non-tumor area as observed by immunofluorescence staining of TOM20 staining (magnification 40×, magnified 60×, scale bars 50 µm). Abbreviations; TUNEL, terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling.
We hypothesized that NETs would provide a signal for mitochondrial biogenesis and sought evidence for increased PGC-1α expression and upregulation of the downstream proteins NRF1 and TFAM which lead to mitochondrial biogenesis. PGC-1α is a transcriptional coactivator that regulates the genes involved in energy metabolism. It is the master regulator of mitochondrial biogenesis (27). Immunohistological comparison of MC38 subcutaneous tumors reveals that PGC-1α expression was enhanced in WT mice but poorly expressed in PAD4-KO mice (Figure 4E). We then compared the expression of the main regulators, PGC-1α, NRF1 and TFAM, involved in the regulation of mitochondrial biogenesis in the growing tumors of WT treated with NET inhibitors, PAD4-KO vs control mice. Figure 4F, 4G and Supplementary Figure 2D show that the expression of these proteins parallels the level of citrullinated histone in these tumors and are reduced in tumors growing in WT treated with NET inhibitor and PAD4-KO mice compared to tumors growing in untreated WT mice. These findings are consistent with the idea that NETs in the tumor microenvironment contribute to the upregulation of tumor mitochondrial functions and accelerated tumor growth.
To determine if fresh human surgically removed tissues showed evidence of mitochondrial biogenesis, human colorectal tumor metastatic to the liver showed marked expression of mitochondrial protein translocase TOM20 on the mitochondrial outer membrane - as a marker of healthy mitochondrial function and increased expression of PGC-1α, NRF1 and TRAM in comparisons with adjacent liver tissue (Figure 4H and 4I). These preliminary observations suggest that human tumors may have undergone metabolic reprogramming with upregulation of mitochondrial biogenesis without affecting adjacent liver tissue.
NETs directly alter the metabolism of cancer cells by enhancing mitochondrial function and biogenesis in vitro.
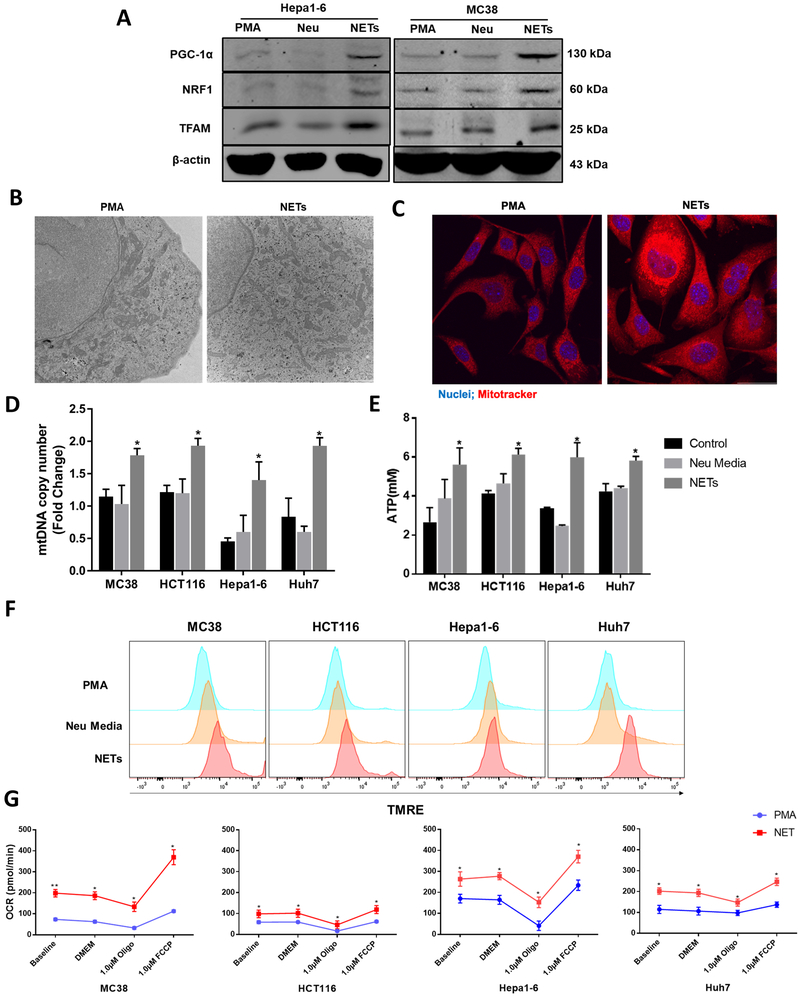
We isolated the neutrophils from the bone marrow of untreated WT mice. The isolated neutrophils were treated with or without PMA and incubated for 4h in order to generate NETs. The media from the stimulated neutrophils was then spun down and the NET pallet was resuspended to incubated with the MC38 cancer cells. Cancer cells treated with NETs showed a significant upregulation in the expression of the genes associated with mitochondrial biogenesis PGC-1α, NRF1 and TFAM compared to PMA or media from unstimulated neutrophils (Figure 5A). To determine whether increased expression of genes associated with mitochondrial biogenesis correlated with increased mitochondria number and mitochondrial respiration, we measured mitochondrial density, mitochondria DNA, ATP production and the oxygen consumption rate in cancer cells exposed to NETs. Electron Microscopy of the MC38 cancer cells showed an increase in the number of mitochondria within those cancer cell co-cultured with NETs (Figure 5B and Supplementary Figure 2E) but not with PMA. Similarly, the PMA treated neutrophils media which had generated NETs induced an increase in mitochondrial mass when the cancer cells were stained with Mitotracker Red (Figure 5C). Mitochondrial DNA copy number from all four tumor cell lines, MC38, HCT116, Hep 1-6 and Huh7, were also increased (Figure 5D). The increases in the overall mitochondrial DNA copy number and biogenesis translated to increases in the levels of ATP molecules in all four cancer cells treated with isolated NETs (Figure 5E). To further probe mitochondrial function, the membrane potential-sensitive probe tetramethylrhodamine ethyl ester (TMRE) was used to detect mitochondrial membrane potential by flow cytometry. Media in which NETs had been resuspended significantly enhanced the percentage of cells with reduced mitochondrial membrane potential (Figure 5F). Figure 5G demonstrates that each of the four cancer cell lines had a significantly increased oxygen consumption rate (OCR), a common measure of mitochondrial respiration. It is also notable that cancer cells exposed to NETs containing media showed a significant increase in basal respiration and after inhibition of non-mitochondrial respiration compared with tumor cells exposed to PMA alone (Figure 5G). These results suggest that NETs directly enhance oxidative phosphorylation in cancer cells and control its bioenergetics by increasing the number of mitochondria per cell through mitochondrial biogenesis. This finding suggests that the effect of NETs on the bioenergetics of tumor growth in vivo is not the downstream effect of NETs on other components of the in vivo inflammatory cellular mix, but is rather a direct effect of NET components on the tumor cells themselves.
Figure 5. NETs directly alter the metabolism of cancer cells by enhancing mitochondrial function and biogenesis in vitro.
Neutrophils isolated from mice were stimulated with PMA for 4 hours to form NETs. Extracted NETs were cocultured with cancer cells (MC38 and Hepa-1-6) overnight. A, Representative images of proteins expressing PGC-1α, NRF1 and TFAM by western blot in indicated groups. The blots shown are representatives of three independent experiments with similar results. B and C, Electron microscopy and confocal microscopy of NETs treated MC38 cells showing significant increase in the number of mitochondria within the cells (magnification 10×, scale bar 50 µm) P<0.05. Mitotracker (red), nuclei (blue). The Real-Time PCR of mtDNA copy number (D) and the production of intracellular ATP levels (E) was observed in both human and mouse cancer cells between groups treated with PMA, neutrophil media and NETs, *P < 0.05. F, As witnessed by the staining of Tetramethylrhodamine ethyl ester (TMRE) flowcytometry analysis showing increased mitochondrial membrane potential following NET treatment in cancer cells. G, The mitochondrial respiratory capacity of cancer cells was elevated in the NET treated group when cell oxygen consumption rate (OCR) was tested, *P < 0.05, **P < 0.01. PMA (250nM) group was added to serve as a control group in each experiment.
NETs preserve mitochondrial homeostasis and dynamics: fission, fusion and mitophagy.
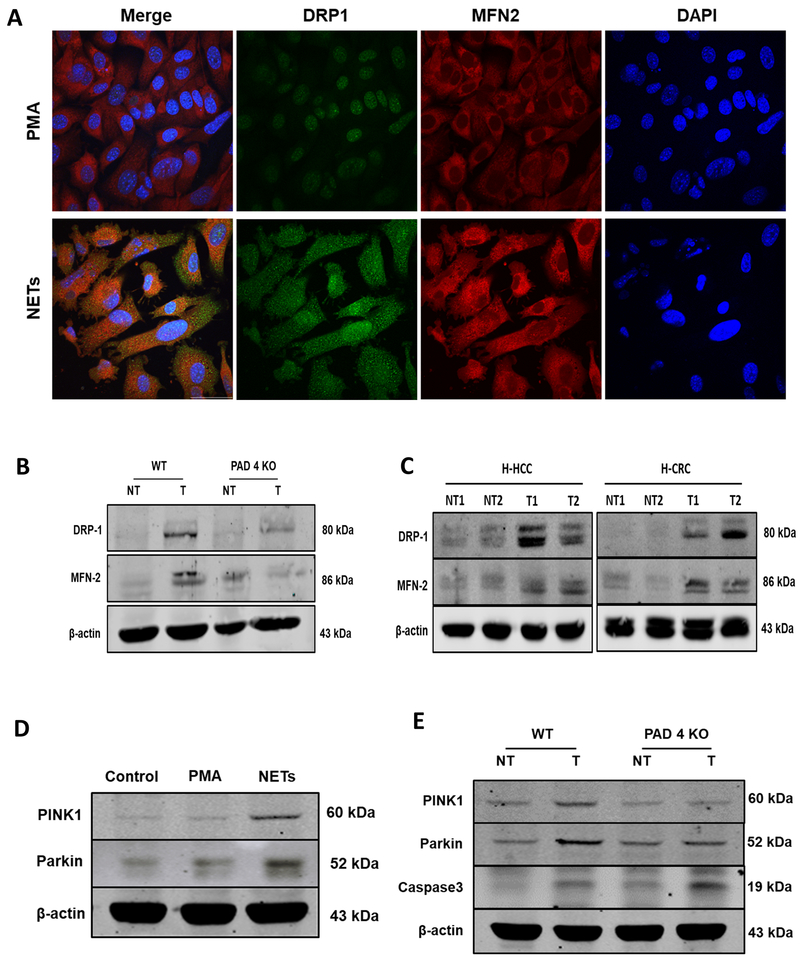
Although depicted as oval-shaped structures, healthy mitochondria form a highly dynamic network in which they constantly undergo fission and fusion. The proteins involved in these changes are generally known as part of the Dynamin family. To see if NETs also regulate mitochondrial dynamics in cancer cells, we compared the expression of DRP1, an essential protein in mitochondrial fission, and MFN2, a critical protein in mitochondrial fusion, after exposure to PMA or media in which NETs had been resuspended. Immunofluorescence images show a significant increase in the expression of DRP1 and MFN2 after exposure to NETs (Figure 6A). To see if this change in mitochondrial dynamics in vitro was associated with tumor growth in vivo, we compared the expression of fission and fusion regulators in tumors growing rapidly in WT mice vs genetically identical tumors growing less robustly in PAD4-KO mice (Figure 6B). The immunoblotting assay revealed lesser expression of both DRP1 and MFN2 in PAD4-KO tumor tissue compared to WT tumor tissue. We did not see any difference of expression of these proteins between the adjacent peri-tumoral (nontumor) tissues. We repeated these immunoblotting studies using freshly excised primary human HCC tumors and human colorectal cancer metastases to the liver. Figure 6C shows that both human tumor tissue (HCC and CRC) expressed elevated fission and fusion markers when compared to adjacent normal liver. It is also known that mitochondrial dynamins are closely associated with increases in mitophagy (28). These mitophagy regulators are responsible for eliminating unhealthy mitochondria to preserve optimal cellular damage control. Interestingly, we also found that NET-treated cancer cells in vitro showed higher levels of mitophagy-linked proteins, as observed by the expression of PINK1 and Parkin (Figure 6D). Figure 6E compares the expression of these mitophagy associated proteins in tumors growing rapidly in WT mice with slow growing tumors in PAD4 KO mice. PINK1 and Parkin proteins were downregulated in the PAD4 KO tumor tissue suggesting that this process is dysregulated in the absence of NETs. The lack of NETs in the PAD4 KO tumor tissue was associated with increases in Caspase 3 expression consistent with increased degrees of the cellular apoptosis (Figure 4A and Figure 6E).
Figure 6. NETs preserve mitochondrial homeostasis and dynamics: fission, fusion and mitophagy.
A, After PMA stimulation for 4h, neutrophil extracellular traps (NETs) were cocultured with MC38 cancer cell line and confocal microscopy was utilized to analyze proteins regulating mitochondrial fission and fusion. NET treatment upregulated the expression of DRP1 and MFN2 proteins compared to cells treated with PMA as a control, nuclei (blue), DRP1 (green), and MFN2 (red). magnification 40× scale bar 50 µm. B, 3 weeks after the splenic injection the liver tumor tissue of PAD4 KO mice showing downregulated expression of DRP1 and MFN2 compared to its non-tumor background. Similarly, human hepatocellular (H-HCC) and human colorectal (H-CRC) tumor (T) sections showing upregulation in these proteins comparing non-tumor (NT) counterparts. D, Representative western blot analysis image showing increased induction of mitophagy as evident by the upregulation in the expression of protein PINK1 and Parkin in MC38 cancer cells when treated with NETs. E, Parallel results can be seen in the tumor tissues of the WT mice 3 weeks after injection as analyzed by western blot. The blots shown are representatives of three independent experiments with similar results.
Neutrophil Elastase released by NETs induces mitochondrial biogenesis through TLR4-p38-PGC-1α pathway.
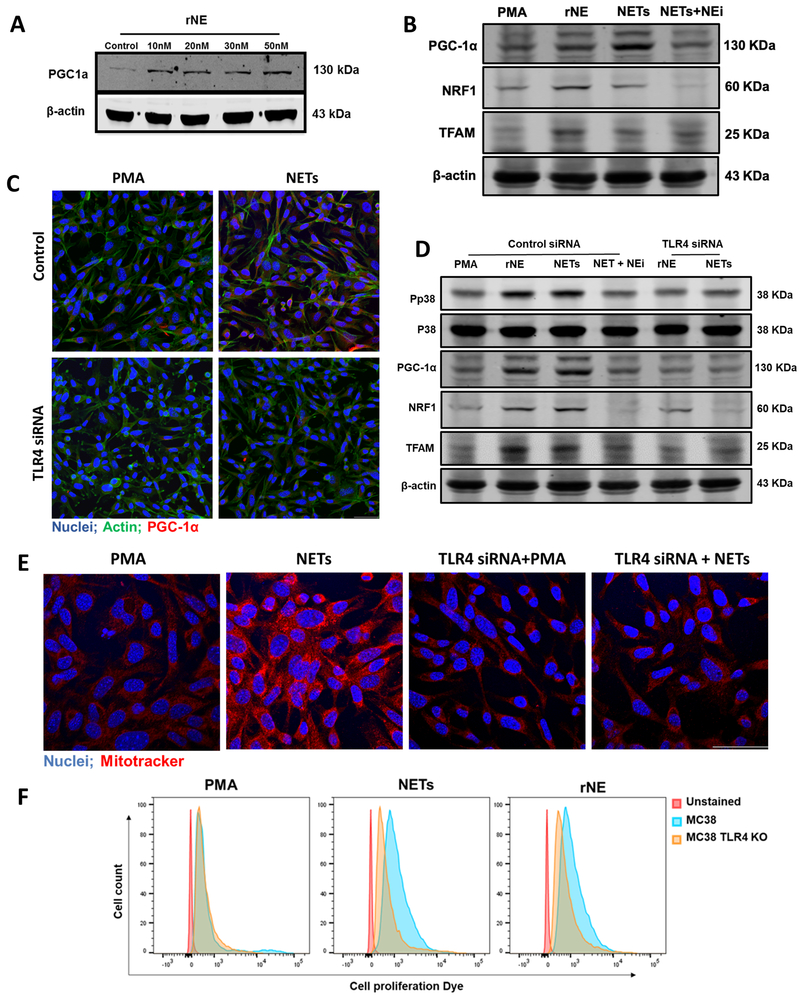
The presence of Neutrophil Elastase (NE) within several human tumor types has been found to be an independent factor for poorer prognosis (29-33). Given the fact that NE is released during NETosis, we hypothesized that NETs may alter cancer cell metabolism through the release of NE or directly via the presentation of NE on NET chromatin. Indeed, immunofluorescence revealed high levels of NE expression in human colorectal metastatic tumor tissue (Supplementary Figure 2F). In vitro, we confirmed that NE is expressed on the extruded chromatin of NETs after neutrophils were stimulated with PMA (Supplementary Figure 2G). Treatment of MC38 cells in vitro with recombinant NE led to a dose dependent increase in PGC-1α (Figure 7A). Recombinant NE was able to induce the expression of PGC-1α, NRF1, and TFAM, the genes associated with mitochondrial biogenesis albeit to a lesser extent than when MC38 cells were treated with media containing NETs (Figure 7B). The effect of NETs on the expression of these genes was significantly reduced when NE inhibitor, monoclonal anti-NE immunoglobulin, was added.
Figure 7. Neutrophil Elastase released by NETs induces mitochondrial biogenesis through TLR4-p38-PGC1-α pathway in MC38 cells.
A, Western blot analysis showing expression of protein PGC-1α in cancer cells treated endogenously with recombinant neutrophil elastase (rNE) at indicated concentration. B, Addition of NETs or rNE (30nM) in the culturing cancer cells increased the expression of PGC-1α, NRF1 and TFAM when determined. However, the addition of neutrophil elastase inhibitor (NEi) together with NETs inhibited this effect. C, Immunofluorescence staining of TLR4 transfected cells cocultured with NETs showing decreased PGC-1a expression with the staining of nuclei (blue) PGC-1α (red) and actin (green). Magnification 20×, scale bar 50 µm. D, Western blot analysis showing downregulated expression of phospho-p38, PGC-1α, NRF1, and TFAM when treated with rNE (30nM) and NETs in TLR4 transfected cells. The blots shown are representatives of three independent experiments with similar results. E, Intracellular mitochondria staining was observed with Mitotracker (red) staining between the groups Magnification 40×, scale bar 50 µm. F, Flowcytometry analysis showing increased cancer cell proliferation when stained with proliferation dye 48h after treating with isolated NETs or rNE. PMA (250nM) group was added to serve as a control group in each experiment.
NE has been previously shown to bind to cell surface receptor Toll-like receptor (TLR)-4 (34,35). Since (TLR)-4 is expressed on both colorectal and hepatocellular cancer cell (36,37), we therefore investigated whether isolated NETs and endogenous NE stimulate this pathway to activate PGC-1α and induce mitochondrial biogenesis. MC38 and Hepa1-6 cells were transfected with TLR4 siRNA or treated with Eritoran, a TLR4 antagonist. Cells staining and protein lysate showed a marked decrease in the expression of PGC-1α in TLR4 siRNA transfected cells compared to control (Figure 7C and Supplementary Figure 3A). We then observed the activation of p38 mitogen-activated protein kinase (MAPK) pathway when rNE was added to MC38 cells (Figure 7D). Activation of the p38 pathway is dependent on upstream TLR4 stimulation and has been previously shown to promote PGC-1α expression (38-40). The effect of endogenous NE on the activation of p38 and PGC-1α was completely abolished in the absence of TLR4 or with the addition of Eritoran (Figure 7D and Supplementary Figure 3B). Similarly, the TLR4 siRNA transfected cells showed significantly diminished effects of NETs on the expression of PGC-1α and phosphorylation of p38 (Figure 7D). The increased expression of biogenesis related proteins coincided with an increase in the mitochondrial density as evident with Mitotracker staining (Figure 7E). Similarly, the depletion of TLR4 or addition of NE antibodies decreased the mitochondrial density in the cancer cells (Figure 7E and Supplementary Figure 3C). Coincident with the increase in mitochondrial biogenesis, the addition of NETs to cancer cells resulted in increased proliferation (Figure 7F and Supplementary Figure 3D). This rapid increase in proliferation was not observed in TLR4 KO cells or if NE inhibitors or Eritoran was added (Figure 7F and Supplementary Figure 3D and 3E).
DISCUSSION
In the past two decades, the conception that cancer is a purely cell-intrinsic disorder that stems from epigenetic or genetic alterations has required revision. Tumors are no longer viewed merely as genetic diseases. It is now clear that cancer growth is profoundly influenced by its complex cellular and humoral environment. Among other infiltrating innate and adaptive immune populations of cells, neutrophils have emerged as important players in the tumor microenvironment, given their heterogeneity and plasticity (41). Another view now revised is the concept that malignant cells satisfy their bioenergetic and anabolic needs solely via aerobic non-mitochondrial glycolysis. Currently, the fundamental influence of mitochondrial metabolism on all steps of oncogenesis, i.e., malignant transformation, tumor progression and response to treatment, is widely recognized (42). Now, we report a bidirectional interaction of the tumor cells with the host immune system in which tumor growth attracts infiltrating neutrophils to undergo NETosis; in turn, NETs directly alter the tumor’s metabolic profile to gain a survival advantage. The presence of NETs within human tumors confers a dismal outcome in patients with metastatic colorectal cancer. In our experimental model, NETs enhance tumor growth by increasing energy production in the cancer cell by increasing mitochondrial biogenesis by releasing NE and activating the TLR4-p38-PGC-1α axis in the cancer cell resulting in increased proliferation and survival (Supplementary Figure 4). In addition, NETs maintain mitochondrial homeostasis by affecting mitochondrial fission, fusion and mitophagy. Thus, our findings, provide further rationale for both targeting NETs or the mitochondria of cancer cells to modulate tumor progression. Further investigation will be required to determine which other interacting products of NETosis may participate.
In this study, increasing NET formation, as measured by IF and circulating MPO-DNA levels in clinical samples, was associated with poor survival in our patient population. This is the largest cohort and the first study to show evidence of NET formation and its clinical value in patients with colorectal metastases to the liver. Serum MPO-DNA testing is a simple and reliable test that could serve as an important biomarker of disease progression and prognosis and larger studies are needed to validate our findings. Our results are consistent with Zylinski et al. and Arelaki et. who showed that deposition of NETs in solid tumors such as Ewing sarcoma and primary colorectal cancer predicted development of early recurrence (43,44). In addition, similar findings were found in patients with diffuse large cell B-cell lymphoma where higher levels of NETs were correlated with dismal outcomes (45). However, the previous studies did not study the underlying mechanisms of NETosis in tumor microenvironment.
The tumor microenvironment in vivo is complex, and neutrophils and NETS are known to interact with a variety of other infiltrating inflammatory cells and connective tissue. In fact, Albrengues et al. recently reported that NETs led to the awakening and proliferation of dormant cancer cells, acting via neutrophil elastase and MMP9 proteases (15). But these proteases did not directly act on the dormant malignant cells. Instead the proteases led to the remodeling of laminin in the matrigel matrix which then awakened the dormant tumor cells. Analogous complex and even contradictory interactions have been noted between neutrophils and NK cells (46,47). By contrast, in in our experimental model, we show that cancer cells can hijack neutrophils so that the neutrophil’s ability to destroy pathogens through NET formation aids cancer cell’s survival in the harsh TME. NETs were found to enable enhanced tumor growth by increasing energy production in the cancer cell by increasing mitochondrial biogenesis and activating the TLR4-p38-PGC-1α axis in the cancer cell in suspension and in the absence of other cell types or connective tissue components. In addition, NETs maintain mitochondrial homeostasis by affecting mitochondrial fission, fusion and mitophagy. These in vitro results are consistent with the laggard tumor growth in PAD4 KO mice or WT mice treated with DNAse which dissolves NETS as they form. Metabolic reprogramming of the immune cells themselves in the TME has been gaining more attention recently in the literature, however, this is the first study to show a direct role of immune cells in affecting the metabolism of cancer cells. This finding highlights promising targets to effectively halt tumor growth.
Although in vitro, under optimal growth conditions (which differ significantly from those encountered in the tumor microenvironment in vivo), cancer cells can obtain sufficient ATP from glycolysis, mitochondria are required for proliferation under physiological conditions. Different cancer cell types undergo different bioenergetic alterations, some to more glycolytic and others to more oxidative, depending in part on the developmental state of the cell undergoing neoplastic transformation. For example, migratory/invasive cancer cells specifically favor mitochondrial respiration and increased ATP production. Invasive cancer cells use the PGC-1α pathway to enhance oxidative phosphorylation, mitochondrial biogenesis and the oxygen consumption (48). One can speculate, given the findings of this paper, that cancer cells in the hypoxic areas of the tumor trigger the formation of NETs around them to increase their migratory ability and invasiveness through PGC-1α-mediated mitochondrial respiration. This allows the cancer cell to detach from the its environment to establish new foci of micrometastatic disease. Our clinical analysis of human colorectal metastatic tumors revealed a significant correlation between NET formation in invasive cancer cells and early recurrence of multiple new metastatic foci after ‘curative’ surgery. This may suggest that tumors with increased NETs may have potentiated or gave survival advantage to several cells to invade and migrate and form new clinical undetectable micrometastatic disease the eventually recurred. As micrometastatic disease present a challenge to detect and treat, it is important to develop therapeutic strategies that could potentially decrease the occurrence of such foci.
Our findings highlight a crosstalk between cancer cells and NETs to promote tumor growth and survival. This raises the potential to develop therapeutic strategies to target this crosstalk to halt tumor progression. Targeting neutrophils can be desirable, however, their depletion could lead to deleterious effects as they play a central role in host defense again pathogens and in repair. Therefore, selective targeting of NETs is a novel approach in cancer treatment. DNAse I treatment is approved by FDA for the treatment of cystic fibrosis, for which it is used to decrease mucus viscosity resulting from NET accumulation triggered by persistent infections (49). Our results with DNAse I serve as proof-of-principle that NETs are a drug target to reduce tumor progression. Another strategy may be to prevent NETs from forming, for example by targeting PAD4. Although we were able to prevent NET formation using PAD4 KO in vivo, PAD4 inhibitors could be of use although commercially available PAD4 inhibitors have serum very short half-lives of ∼15 min-4 h. NE inhibition has shown some promise in experimental models of breast cancer, skin malignancies and non-small lung cancer (31,50,51). NE inhibitors are currently undergoing clinical trials for treatment of cystic fibrosis and these results could also be useful in cancer research. On the other hand, metabolic reprogramming is a central feature of cancer cells that is intricately linked to mitochondria and provides unique opportunities for the development of drugs. The key consideration in targeting mitochondrial ATP production is that normal cells use mitochondrial ATP production for survival; thus, the therapeutic index may be limited. The only exception would be if cancer cells selectively uptake the inhibitors of mitochondrial ATP production compared to normal cells. On the basis of recent evidence, we suggest that the widely used antidiabetic drug metformin may be a viable anticancer agent that targets mitochondrial ATP production without invoking toxicity in normal tissues. In addition, one of the main problems with targeting mitochondria as a strategy to kill malignant cells or sensitize them to treatment is that multiple immune effector cells, and in particular CD8+ cytotoxic T lymphocytes, display remarkable metabolic similarities to cancer cells (52). This calls for the development of refined therapeutic approaches whereby malignant cells are selectively targeted while immune cells are spared from (or rendered insensitive to) the detrimental effects of treatment.
In conclusion, we demonstrated that cancer cells can recruit and promote the formation of NETs to promote growth and survival. NETs This concept for how cancer cells exploit host cells represents a therapeutic opportunity to prevent tumor growth in an effort to decrease of cancer-associated morbidity and deaths.
Supplementary Material
STATEMENT OF SIGNIFICANCE.
Neutrophils through the release of NETs facilitate the growth of stressed cancer cells by altering their bioenergetics, the inhibition of which induces cell death.
ACKNOWLEDGEMENT
The authors thank Xinghua Liao, Heather Waring, Sakeena Badrane, Yara Zayout, and Megan Wang for technical assistance in preparing the manuscript. This work was supported by University of Pittsburgh Internal (S. Tohme) and National Cancer Institute (NIC) RO1 CA214865-01 (A. Tsung).
Footnotes
Conflict of Interest: No potential conflicts of interest were disclosed by the authors
REFEERENCES
- 1.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahlert C, Pecqueux M, Halama N, Dienemann H, Muley T, Pfannschmidt J, et al. Tumour-site-dependent expression profile of angiogenic factors in tumour-associated stroma of primary colorectal cancer and metastases. Br J Cancer. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid MD, Basturk O, Thirabanjasak D, Hruban RH, Klimstra DS, Bagci P, et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Mod Pathol. 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YW, Qiu SJ, Fan J, Zhou J, Gao Q, Xiao YS, et al. Intratumoral neutrophils: A poor prognostic factor for hepatocellular carcinoma following resection. J Hepatol. 2011; [DOI] [PubMed] [Google Scholar]
- 5.J TO, S H, M HJ, D F, H M, S P, et al. Intratumoral neutrophils, plasmacytoid dendritic cells, and pSTAT3 in AJCC stage I/II melanoma prognosis. J. Clin. Oncol. 2011. [Google Scholar]
- 6.Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coëlle C, Mouroux J, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012; [DOI] [PubMed] [Google Scholar]
- 7.Shen M, Hu P, Donskov F, Wang G, Liu Q, Du J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS One. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee W, Ko SY, Mohamed MS, Kenny HA, Lengyel E, Naora H. Neutrophils facilitate ovarian cancer premetastatic niche formation in the omentum. J Exp Med. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cools-Lartigue J, Spicer J, Najmeh S, Ferri L. Neutrophil extracellular traps in cancer progression. Cell. Mol. Life Sci. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papadaki G, Kambas K, Choulaki C, Vlachou K, Drakos E, Bertsias G, et al. Neutrophil extracellular traps exacerbate Th1-mediated autoimmune responses in rheumatoid arthritis by promoting DC maturation. Eur J Immunol. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dyer MR, Chen Q, Haldeman S, Yazdani H, Hoffman R, Loughran P, et al. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci Rep. 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albrengues J, Shields MA, Ng D, Park CG, Ambrico A, Poindexter ME, et al. Neutrophil extracellular traps produced during inflammation awaken dormant cancer cells in mice. Science (80- ). 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward PS, Thompson CB. Metabolic Reprogramming: A Cancer Hallmark Even Warburg Did Not Anticipate. Cancer Cell. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res [Internet]. 2016;76:1367–80. Available from: http://cancerres.aacrjournals.org/lookup/doi/10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y, et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razak NA, Elaskalani O, Metharom P. Pancreatic cancer-induced neutrophil extracellular traps: A potential contributor to cancer-associated thrombosis. Int J Mol Sci. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, et al. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosales C Neutrophil: A cell with many roles in inflammation or several cell types? Front. Physiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadie J-M, Bae H-B, Jiang S, Park DW, Bell CP, Yang H, et al. HMGB1 promotes neutrophil extracellular trap formation through interactions with Toll-like receptor 4. AJP Lung Cell Mol Physiol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tohme S, Yazdani HO, Liu Y, Loughran P, van der Windt DJ, Huang H, et al. Hypoxia mediates mitochondrial biogenesis in hepatocellular carcinoma to promote tumor growth through HMGB1 and TLR9 interaction. Hepatology. 2017;66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: The central role of PGC-1α. Cardiovasc. Res. 2008. [DOI] [PubMed] [Google Scholar]
- 28.Bordi M, Nazio F, Campello S. The Close Interconnection between Mitochondrial Dynamics and Mitophagy in Cancer. Front Oncol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rilke F, Colnaghi MI, Cascinelli N, Andreola S, Baldini MT, Bufalino R, et al. Prognostic significance of her‐2/neu expression in breast cancer and its relationship to other prognostic factors. Int J Cancer. 1991; [DOI] [PubMed] [Google Scholar]
- 30.Sato T, Takahashi S, Mizumoto T, Harao M, Akizuki M, Takasugi M, et al. Neutrophil elastase and cancer. Surg. Oncol. 2006. [DOI] [PubMed] [Google Scholar]
- 31.Houghton AMG, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med. 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akizuki M, Fukutomi T, Takasugi M, Takahashi S, Sato T, Harao M, et al. Prognostic significance of immunoreactive neutrophil elastase in human breast cancer: long-term follow-up results in 313 patients. Neoplasia. 2007; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita JI, Ogawa M, Abe M, Hayashi N, Kurusu Y, Kawahara K, et al. Tumor neutrophil elastase is closely associated with the direct extension of non-small cell lung cancer into the aorta. Chest. 1997; [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro-Gomes FL, Moniz-de-Souza MCA, Alexandre-Moreira MS, Dias WB, Lopes MF, Nunes MP, et al. Neutrophils Activate Macrophages for Intracellular Killing of Leishmania major through Recruitment of TLR4 by Neutrophil Elastase. J Immunol. 2007; [DOI] [PubMed] [Google Scholar]
- 35.Faria MS, Reis FCG, Lima APCA. Toll-like receptors in Leishmania infections: Guardians or promoters? J. Parasitol. Res. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Earl TM, Nicoud IB, Pierce JM, Wright JP, Majoras NE, Rubin JE, et al. Silencing of TLR4 decreases liver tumor burden in a murine model of colorectal metastasis and hepatic steatosis. Ann Surg Oncol. 2009; [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Xie Y, Zhong X, Fu Y, Huang Y, Zhen Y, et al. Novel chemokine-like activities of histones in tumor metastasis. Oncotarget. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang G, Liu Z, Ding H, Miao H, Garcia JM, Li YP. Toll-like receptor 4 mediates Lewis lung carcinoma-induced muscle wasting via coordinate activation of protein degradation pathways. Sci Rep. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Z-F H, R J-M, D L, R P, F M. TLR2 and TLR4 activate p38 MAPK and JNK during endurance exercise in skeletal muscle. Med Sci Sports Exerc. 2012; [DOI] [PubMed] [Google Scholar]
- 40.Tan Z, Luo X, Xiao L, Tang M, Bode AM, Dong Z, et al. The Role of PGC1 in Cancer Metabolism and its Therapeutic Implications. Mol Cancer Ther. 2016; [DOI] [PubMed] [Google Scholar]
- 41.Coffelt SB, Wellenstein MD, De Visser KE. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer. 2016. [DOI] [PubMed] [Google Scholar]
- 42.Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berger-Achituv S, Brinkmann V, Abed UA, Kühn LI, Ben-Ezra J, Elhasid R, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.A S, A A, K K, P C, M P, A I, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, et al. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin Cancer Res [Internet]. 2018; Available from: http://clincancerres.aacrjournals.org/content/early/2019/01/28/1078-0432.CCR-18-1226.abstract [DOI] [PubMed] [Google Scholar]
- 46.Ogura K, Sato-Matsushita M, Yamamoto S, Hori T, Sasahara M, Iwakura Y, et al. NK cells control tumor-promoting function of neutrophils in mice. Cancer Immunol Res. 2018; [DOI] [PubMed] [Google Scholar]
- 47.Jaeger BN, Donadieu J, Cognet C, Bernat C, Ordoñez-Rueda D, Barlogis V, et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J Exp Med. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lebleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez-Alemán SR, Campos-García L, Palma-Nicolas JP, Hernández-Bello R, González GM, Sánchez-González A. Understanding the Entanglement: Neutrophil Extracellular Traps (NETs) in Cystic Fibrosis. Front Cell Infect Microbiol. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caruso JA, Akli S, Pageon L, Hunt KK, Keyomarsi K. The serine protease inhibitor elafin maintains normal growth control by opposing the mitogenic effects of neutrophil elastase. Oncogene. 2015; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lerman I, Garcia-Hernandez M de la L, Rangel-Moreno J, Chiriboga L, Pan C, Nastiuk KL, et al. Infiltrating Myeloid Cells Exert Protumorigenic Actions via Neutrophil Elastase. Mol Cancer Res. 2017; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharping NE, Menk A V., Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The Tumor Microenvironment Represses T Cell Mitochondrial Biogenesis to Drive Intratumoral T Cell Metabolic Insufficiency and Dysfunction. Immunity. 2016; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.