Abstract
Background:
Vaccination against human papillomaviruses (HPV) prevents HPV infections and consequently, cervical lesions. However, the effect of vaccination on HPV transmission within couples is unknown.
Methods:
We used data from HITCH, a prospective cohort study of heterosexual couples (women aged 18-24y) in Montreal, 2005-2013. Vaccination history was self-reported. Genital samples were tested for HPV DNA by PCR (Linear Array). Type-specific viral loads were quantified using real-time PCR. Odds and hazard ratios (OR/HR) were estimated using multi-level mixed-effects logistic regression and a parametric model for interval-censored survival-time data, respectively. Differences in viral loads were evaluated using the Friedman’s ANOVA test.
Results:
Among 497 couples, 12, 16, and 35 women received 1, 2, or 3 vaccination doses at baseline, respectively. Median age at vaccination was 18y. Most women (92.1%) had their first coitus before vaccination. At baseline, partner concordance of persistent HPV6/11/16/18 infections was lower in vaccinated than unvaccinated women (adjusted OR=0.10, 95%CI: 0.01-0.65) but not for non α7/α9/α10-HPV types (adjusted OR=1.00, 95%CI: 0.44-2.29). Incidence of persistent α7/α9/α10 HPV types in women was inversely associated with vaccination status at baseline (adjusted HR=0.12, 95%CI: 0.03-0.47). Likewise, male partners of vaccinated women had a lower incidence of α7/α9/α10 HPV infections (adjusted OR=0.22, 95%CI: 0.05-0.95). Vaccinated women with HPV 6/11/16/18 infections had lower viral loads (p=0.001) relative to unvaccinated women.
Conclusion:
Vaccination of sexually active women significantly reduced transmission of α7/α9/α10 HPV types in heterosexual couples.
Impact:
These results underscore and quantify the positive effect of HPV vaccination on HPV transmission within heterosexual couples.
Keywords: human papillomaviruses, infection transmission, papillomavirus vaccines, viral load, HPV incidence
INTRODUCTION
Human papillomavirus (HPV) is the most common sexually transmitted viral infection (1). In the late 20th century, persistent infection with HPV was recognized as a necessary cause of cervical cancer and anogenital warts, and an important cause of other anogenital and head-and-neck cancers (2). Worldwide, HPV infections causally contribute to approximately 5% of all incident malignant neoplasms (3). The identification of HPV as a risk factor for cancer resulted in the successful development of prophylactic HPV vaccines. Three of these vaccines are commonly used in clinical practice: Cervarix, Gardasil, and Gardasil-9, which respectively target two (HPV16/18), four (HPV6/11/16/18) and nine (HPV6/11/16/18/31/33/45/52/58) HPV types (4-8). Vaccination significantly decreases the chance for HPV-related disease in vaccinated individuals (4-8). Vaccination coverage is expanding globally, as an increasing number of countries have implemented HPV vaccination in their national vaccine programs, and gender-neutral vaccination is becoming more common.
Studies have reported that the benefit of HPV vaccination may extend beyond vaccinated individuals; herd immunity occurs after implementation of vaccine programs, as HPV infections and HPV-related diseases are reduced in unvaccinated individuals too. Unvaccinated women have a reduced prevalence of vaccine-targeted HPV genotypes years after the introduction of vaccination, albeit to a lesser extent than that for fully vaccinated women (9,10). This could be explained by unvaccinated male partners being less likely to acquire HPV from vaccinated female partners, and therefore being less likely to transmit HPV to subsequent unvaccinated partners. However, the effect of vaccination on transmission dynamics between sexual partners remains largely unknown. Expectedly, a vaccinated woman is more likely than an unvaccinated woman to be negative for HPV types included in the vaccine, reducing female-to-male transmission of these HPV types. Other mechanisms may further reduce transmission. Being infected with one HPV type tends to be predictive of infection risk with other HPV types (1). Some girls/women may have acquired a genital HPV infection before vaccination, particularly if vaccinated at an older age. A key question is whether vaccination reduces HPV transmission between heterosexual partners when women are HPV-positive and vaccinated at an older age, possibly because vaccination might reduce the viral load of the infection (11). Furthermore, HPV-positive men may have improved clearance rates of HPV infections while in a relationship with a vaccinated woman, as these men are not re-infected by their female partner (9).
We analyzed data from the ‘HPV Infection and Transmission among Couples through Heterosexual Activity’ (HITCH) cohort study to evaluate the effect of HPV vaccination on HPV transmission dynamics between sexually active couples (12). Due to its recruitment period (2005-2011), HITCH participants had been vaccinated voluntarily in their late teens/early twenties, generally after their first sexual activity. Hence, we were able to study the effect of late vaccination on HPV transmission dynamics between young, newly formed, heterosexual couples.
METHODS
Study population
The HITCH cohort study has been described previously (12-15). In brief, we enrolled 502 heterosexual couples between May 2005 and February 2011, consisting of young female university or junior college students (primarily aged 18-24 years) in Montreal, Canada, and their male partners (≥18 years). Eligibility criteria were as follows: the couple had started their (sexual) relationship less than six months before recruitment, the woman was not pregnant or planning to become pregnant in the next two years, had an intact uterus, and no history of cervical lesions/cancer. Women had up to six clinical visits during two years of follow-up (at 0, 4, 8, 12, 18, and 24 months). Male partners were invited for clinical visits at 0 and 4 months, with the baseline visit being on the same date as the female’s baseline visit. If men were unable to have their baseline visit on the same date as their female partner (n=4), their visit was scheduled as closely to the female’s baseline visit as possible. During clinical visits, genital specimens were collected, either by self-sampling (vaginal samples) or by a nurse (penile samples). To minimize contamination of genital specimens through recent sexual contact, participants were asked to abstain from intercourse 24 hours prior to sample collection. At baseline and during follow-up, participants also filled out web-based questionnaires, which included questions on HPV vaccination, demographics, and sexual behavior/history.
All subjects provided written informed consent. HITCH follows all national and international laws regarding research with human data and materials, including the Declaration of Helsinki and Canadian laws. Ethical approval was obtained from the McGill University Institutional Review Board (Study Number A09-M77-04A), and is annually renewed.
Genital specimen processing
Specimens were tested by polymerase chain reaction (PCR) based on amplification of a 450 base-pair segment of the HPV L1 capsid gene and identification of 36 mucosal HPV genotypes using the Linear Array HPV genotyping assay (LA-HPV) (Roche Molecular Systems, Laval, Canada), as described previously (16). A β-globin DNA sequence was amplified to verify that specimens contained exfoliated cells for testing. For some analyses, we grouped HPV genotypes according to phylogenetic relatedness to the four types included in the HPV vaccine (17). Species α9 and α7 includes types related to HPV16 and HPV18, respectively, whereas species α10 includes types related to HPV6 and HPV11.
Viral loads of HPV types 6, 11, 16, 18, 31, 42, and 51 were measured using quantitative type-specific real-time PCR assays. Viral loads were determined in female genital samples for HPV types that tested positive in the Linear Array HPV genotyping assay. Samples and diluted samples free of inhibiting activity were tested in duplicate in a Light Cycler PCR and detection system (Roche Molecular Systems, Laval, Quebec) for quantification of HPV. Viral loads were calculated by dividing the number of HPV DNA copies by the total number of cells, which was estimated by quantitation of β-globin by real-time PCR, as described previously (18). If a participant was positive for a specific HPV type during multiple visits, we considered the maximum viral load measured during any visit as a surrogate measure for potential viral shedding. HPV-positive participants with a maximum viral load of 0 were excluded as these samples contained HPV DNA below the cut-off for quantitation.
Statistical analyses
We considered HPV prevalence, persistence, concordance, incidence, and clearance as study outcomes. Prevalence was defined as the number of HPV-positive individuals divided by the total number of individuals of the same sex tested for the same HPV type. When grouping results from multiple HPV types, one individual could have multiple observations. A persistent infection was defined as two consecutive positive measurements for the same HPV type. HPV concordance was defined as both members of the couple testing positive for the same HPV type during the same visit. Concordance of persistent infections was defined as an individual and his/her partner having the same type-specific HPV infection at both the first and second visits. Three definitions were used for HPV incidence and clearance. An individual had an incident infection when HPV was detected after ≥1 negative visit(s). In a more conservative definition, an individual needed to have ≥2 positive same-type episodes at consecutive visits after ≥1 negative visit(s) (incidence of a persistent infection). An even stricter definition of an incident persistent infection stipulated that an individual had ≥2 consecutive negative measurements followed by ≥2 consecutive positive visits. Using the same hierarchical approach, clearance of an infection was defined as a negative HPV test after ≥1 positive visit(s); an individual had persistent clearance of an infection when having ≥2 negative measurements after ≥1 positive visit(s); and an individual had persistent clearance of a persistent infection when having ≥2 consecutive negative measurements after ≥2 consecutive positive visit(s).
Both male and female participants self-reported condom use at the baseline visit. Condom use was divided into three categories: never, irregularly (rarely/sometimes/most of the time), and always. When partners’ answers differed, they were considered to have always worn condoms only if one answered ‘always’ and the other ‘always’ or ‘most of the time’. Similarly, we considered couples to never wear condoms if one of the partners stated ‘never’ and the other ‘rarely’ or ‘never’.
Differences in categorical baseline characteristics between vaccinated and unvaccinated couples were evaluated using Chi-square tests. Odds ratios (OR) and their respective 95% confidence intervals (95%CI) were calculated using univariable and multivariable logistic regression. Multilevel, mixed-effects logistic regression models that included a random intercept for each individual were used in repeated-measures analyses. In women, we calculated hazard ratios (HR) for the incidence and clearance of HPV infections using a parametric model for interval-censored survival-time data. Observations were clustered by participant to account for repeated measurements within individuals. Since men had only one follow-up visit, in general four months after the baseline visit, we limited the incidence and clearance analyses of HPV infections to multilevel mixed-effects logistic regression. Differences in viral loads between vaccinated and unvaccinated women were evaluated with the ANOVA Cochran–Mantel–Haenszel test using rank scores (Friedman’s ANOVA), stratifying the data by HPV type (19).
All statistical analyses were performed using Stata 15.1 (STATA Corp., College Station, TX).
RESULTS
In 5 of 502 couples (1.0%), baseline genital samples tested negative for β-globin; these couples were excluded from our analyses. Baseline characteristics of the remaining 497 couples are displayed in table 1, stratified by vaccination status. Sixty-three women (12.7%) reported that they had been vaccinated at the baseline visit, of whom 12 (19.0%), 16 (25.4%), and 35 (55.6%) had received 1, 2, and 3 vaccine doses, respectively. Five women (7.9%) reported that they had been vaccinated before their first sexual activity (coitus); we were able to determine that at least 35 women had been vaccinated before the first coitus with their HITCH partner (supplementary table S1). One woman received the bivalent vaccine while all other women received the quadrivalent vaccine. In general, characteristics were similar between vaccinated and unvaccinated couples. The median age of women was 21 and 20 years in unvaccinated and vaccinated women, respectively. Women reported a median number of 5 lifetime vaginal sex partners of the opposite sex in both groups, men reported a median number of 6 lifetime sex partners in both groups. The median age of first coitus was 17 years, irrespective of sex and vaccination status. Among both vaccinated and unvaccinated women, 10-15% reported having a concurrent sexual partner besides their HITCH partner at baseline. Unvaccinated women had had slightly more sex with same-sex partners relative to vaccinated women (15.7% versus 9.5%, p=0.21), the inverse was true for male partners of unvaccinated women (7.6% versus 9.5%, p=0.57). Most couples had irregular condom use, with 11.8% and 20.3% of unvaccinated couples never or always using condoms, respectively. In vaccinated couples, 20.6% and 15.9% never or always used condoms (p-value when comparing vaccinated to unvaccinated couples: 0.13).
Table 1.
Baseline characteristics of HITCH participants by vaccination status of the women at first visit.
| Unvaccinated | Vaccinated | |
|---|---|---|
| Couples, n | 434 | 63 |
| Women | ||
| Age, median (range) | 21 (18-26) | 20 (18-24) |
| Born in Canada, n (%) | 295 (68.0%) | 77 (69.8%) |
| Born in Quebec, n (%) | 178 (60.3%) | 26 (59.1%) |
| Born in Ontario, n (%) | 64 (21.7%) | 7 (15.9%) |
| Had vaginal intercourse, % | 431 (99.3%) | 63 (100.0%) |
| Age at first coitus, median (range) | 17 (11-23) | 17 (12-23) |
| Lifetime sex partners, median (IQR) | 6 (3-11) | 5 (3-14) |
| Lifetime vaginal sex partners of opposite sex, median (IQR) | 5 (2-9) | 5 (2-11) |
| Had same-sex partners, n (%) | 68 (15.7%) | 6 (9.5%) |
| Days since first sexual activity with HITCH partner, median (IQR) | 124 (83-158) | 135 (81-172) |
| Days since first coitus with HITCH partner, median (IQR) | 118 (79-155) | 121 (75-149) |
| Concurrent sex partner during relationship with HITCH partner, n (%) | 58 (13.4%) | 8 (12.7%) |
| Number of vaccine doses received | ||
| 1 dose, n (%) | - | 12 (19.0%) |
| 2 doses, n (%) | - | 16 (25.4%) |
| 3 doses, n (%) | - | 35 (55.6%) |
| Type of vaccine received | ||
| - Bivalent vaccine | - | 1 (1.6%) |
| - Quadrivalent vaccine | - | 62 (98.4%) |
| Age at vaccination, median (range) | - | 18 (15-24) |
| - Vaccinated before first coitus, n (%) | - | 5 (7.9%) |
| Male partners | ||
| Age, median (range) | 22 (17-45) | 20 (18-28) |
| Born in Canada, n (%) | 276 (63.6%) | 39 (61.9%) |
| Born in Quebec, n (%) | 181 (65.6%) | 27 (69.2%) |
| Born in Ontario, n (%) | 52 (18.8%) | 5 (12.8%) |
| Had vaginal intercourse, n (%) | 434 (100%) | 63 (100%) |
| Age at first coitus, median (range) | 17 (12-26) | 17 (13-26) |
| Lifetime sex partners, median (IQR) | 6 (3-13) | 6 (3-8) |
| Lifetime vaginal sex partners of opposite sex, median (IQR) | 5 (3-11) | 4 (3-7) |
| Had same-sex partners, n (%) | 33 (7.6%) | 6 (9.5%) |
| Days since first sexual activity with HITCH partner, median (IQR) | 123 (83-158) | 135 (81-172) |
| Days since first coitus with HITCH partner, median (IQR) | 118 (79-155) | 121 (75-149) |
| Concurrent sex partner during relationship with HITCH partner, n (%) | 49 (11.3%) | 7 (11.1%) |
| Couples | ||
| Condom use | ||
| - Never, n (%) | 51 (11.8%) | 13 (20.6%) |
| - Irregular, n (%) | 269 (62.0%) | 36 (57.1%) |
| - Always, n (%) | 88 (20.3%) | 10 (15.9%) |
| - Unknown, n (%) | 26 (6.0%) | 4 (6.3%) |
We compared the prevalence of all HPV infections and persistent HPV infections at the baseline visit between couples in which the woman was or was not vaccinated (table 2, supplementary tables S2 and S3). In couples in which the woman had not been vaccinated at baseline, HPV16 was the most prevalent persistent infection in both men (14.0%) and women (14.7%). When the woman was vaccinated, HPV51 was the most prevalent persistent HPV infection in both women (10.3%) and their male partners (13.2%). Vaccinated women had significantly fewer HPV6/11/16/18 infections, also after adjusting for the partner’s type-specific genital HPV status at baseline (adjusted OR for persistent infection=0.14, 95%CI: 0.04-0.51). There were no significant differences in the risk of HPV types unrelated to the ones in the vaccine, but there was a reduction, albeit non-significant, in prevalence of infections of other α7, α9 or α10 types in vaccinated women (adjusted OR for persistent infection=0.75, 95%CI: 0.39-1.46). In men, there was a non-significant lower prevalence of HPV6/11/16/18 infections (adjusted OR for any infection=0.81, 95%CI 0.42-1.56). However, after adjusting for the partner’s type-specific genital HPV status at the baseline visit, this difference disappeared (adjusted OR for any infection=1.90, 95%CI: 0.82-4.39).
Table 2.
Sex-specific odds ratios for the prevalence of any HPV infection at baseline and a persistent HPV infection (type-specific HPV-positive samples at the baseline and first follow-up visit) in couples in which the woman reported being vaccinated prior to the visit, relative to unvaccinated couples.
| All infections | Persistent infection | ||||||
|---|---|---|---|---|---|---|---|
| Sex | Infection Outcome | Univariable | Multivariable 11 | Multivariable 22 | Univariable | Multivariable 11 | Multivariable 22 |
| Women | HPV types included in vaccine3 |
0.28 (0.11-0.69) |
0.19 (0.07-0.54) |
0.14 (0.04-0.51) |
0.17 (0.04-0.71) |
0.14 (0.03-0.58) |
0.13 (0.03-0.63) |
| Other HPV α7, α9 or α10 types4 | 0.72 (0.41-1.24) |
0.74 (0.43-1.28) |
0.75 (0.39-1.46) |
0.59 (0.29-1.21) |
0.63 (0.31-1.29) |
0.72 (0.31-1.68) |
|
| Non-α7, -α9 or -α10 HPV types5 | 0.96 (0.63-1.47) |
0.92 (0.60-1.41) |
0.85 (0.49-1.50) |
1.10 (0.65-1.85) |
1.08 (0.64-1.80) |
1.01 (0.53-1.93) |
|
| Men | HPV types included in vaccine3 | 0.65 (0.33-1.27) |
0.81 (0.42-1.56) |
1.89 (0.82-4.38) |
0.81 (0.39-1.67) |
1.02 (0.49-2.12) |
2.79 (1.04-7.51) |
| Other HPV α7, α9 or α10 types4 | 0.86 (0.48-1.52) |
1.19 (0.69-2.05) |
1.54 (0.83-2.85) |
0.72 (0.37-1.43) |
0.95 (0.50-1.83) |
1.32 (0.57-3.06) |
|
| Non-α7, -α9 or -α10 HPV types5 | 0.97 (0.64-1.48) |
1.35 (0.91-1.99) |
1.37 (0.86-2.17) |
1.14 (0.70-1.84) |
1.61 (1.02-2.56) |
1.68 (0.96-2.95) |
|
Adjusted for age, race, smoking status, age at first coitus, number of lifetime sex partners (coitus), and whether the individual had same-sex sex partners.
Adjusted for all variables in multivariable model 1 plus for the partner’s type-specific genital HPV status at baseline, concurrent sex partners at baseline, condom use, average frequency of coitus with HITCH partner per week, and duration of relationship.
HPV 6/11/16/18
HPV 31/33/35/39/44/45/52/58/59/67/68/70
HPV 26/34/40/42/51/53/54/56/61/62/66/69/71/72/73/81/82/83/84/89
Next, we evaluated HPV concordance between active sexual partners at the first two visits (table 3). When a male partner was positive for HPV 6/11/16/18, concordance at that visit was significantly lower in vaccinated women (adjusted OR=0.10, 95%CI: 0.01-0.65). Similarly, when evaluating persistent HPV 6/11/16/18 infections, the adjusted OR was below 0.01 (95%CI: 0.00-0.24). When comparing concordance between vaccinated and unvaccinated women for other α7, α9 or α10 HPV types, a non-significant decrease in concordance was observed (adjusted OR=0.76, 95%CI: 0.20-2.86). No significant difference in concordance was found between vaccinated and unvaccinated women for other HPV types, or when comparing concordance in male sexual partners of vaccinated and unvaccinated HPV-positive women.
Table 3.
Concordance of HPV infections at baseline. Percentages display the fraction of female or male participants that tested positive for a genital HPV infection when their sexual partner had a genital infection for that particular HPV type. We evaluated both all HPV infections at baseline (top) and persistent HPV infections (i.e., couples who were positive at baseline and the first follow-up visit; bottom). Odds ratios were calculated using multilevel mixed-effects logistic regression to adjust for repeated measures within couples (multiple HPV types are evaluated in each couple).
| HPV 6/11/16/18 | Any other α7, α9 or α10 HPV type | Any non-α7, -α9 or α10 HPV type | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Outcome | Un- vaccinated |
Vaccinated | Unadjusted odds ratio (95% CI) |
Adjusted1 odds ratio (95%CI) |
Un- vaccinated |
Vaccinated | Unadjusted odds ratio (95% CI) |
Adjusted1 odds ratio (95%CI) |
Un- vaccinated |
Vaccinated | Unadjusted odds ratio (95% CI) |
Adjusted1 odds ratio (95%CI) |
| Man is HPV-positive | Female partner’s HPV-positivity | 74/111 (66.7%) |
3/11 (27.3%) |
0.19 (0.05-0.75) |
0.10 (0.01-0.65) |
104/174 (59.8%) |
13/23 (56.5%) |
0.89 (0.35-2.26) |
0.76 (0.20-2.86) |
231/413 (55.9%) |
38/62 (61.3%) |
1.16 (0.50-2.71) |
1.00 (0.44-2.29) |
| Woman is HPV-positive | Male partner’s HPV-positivity | 74/115 (64.3%) |
3/5 (60.0%) |
0.60 (0.01-40.80) |
ND | 104/195 (53.3%) |
13/21 (61.9%) |
1.50 (0.37-6.05) |
1.75 (0.36-8.60) |
231/365 (63.3%) |
38/55 (69.1%) |
1.08 (0.28-4.08) |
2.44 (0.62-9.59) |
| Man has a persistent HPV infection | Female partner has a persistent HPV infection | 35/65 (53.8%) |
2/8 (25.0%) |
0.13 (0.01-3.28) |
0.00 (0.00-0.24) |
55/105 (52.4%) |
9/13 (69.2%) |
2.90 (0.47-17.78) |
34.37 (1.34-880.7) |
102/224 (45.5%) |
26/45 (57.8%) |
1.61 (0.67-3.84) |
1.19 (0.47-3.05) |
| Woman has a persistent HPV infection | Male partner has a persistent HPV infection | 35/60 (58.3%) |
2/2 (100.0%) |
∞ | ND | 55/103 (53.4%) |
9/10 (90.0%) |
7.83 (0.96-63.97) |
4.70 (0.25-88.35) |
102/170 (60.0%) |
26/35 (74.3%) |
1.87 (0.62-5.65) |
1.93 (0.46-8.12) |
| Man has a persistent HPV infection | Female partner’s HPV-positivity | 49/66 (74.2%) |
3/9 (33.3%) |
0.14 (0.01-1.43) |
0.00 (0.00-0.19) |
75/105 (71.4%) |
10/13 (76.9%) |
1.52 (0.29-8.10) |
9.57 (0.31-294.1) |
145/231 (62.8%) |
34/45 (75.6%) |
2.03 (0.65-6.36) |
1.41 (0.48-4.17) |
| Woman has a persistent HPV infection | Male partner’s HPV-positivity | 48/74 (64.9%) |
2/2 (100.0%) |
∞ | ND | 68/118 (57.6%) |
11/11 (100.0%) |
∞ | ND | 142/211 (67.3%) |
28/36 (77.8%) |
1.66 (0.43-6.37) |
1.46 (0.32-6.73) |
Adjusted for age, race, smoking status, age at first coitus, number of lifetime sex partners (coitus), whether the individual had same-sex partners, concurrent sex partners, condom use, average frequency of coitus with HITCH partner per week, and duration of the sexual relationship.
ND, not determined.
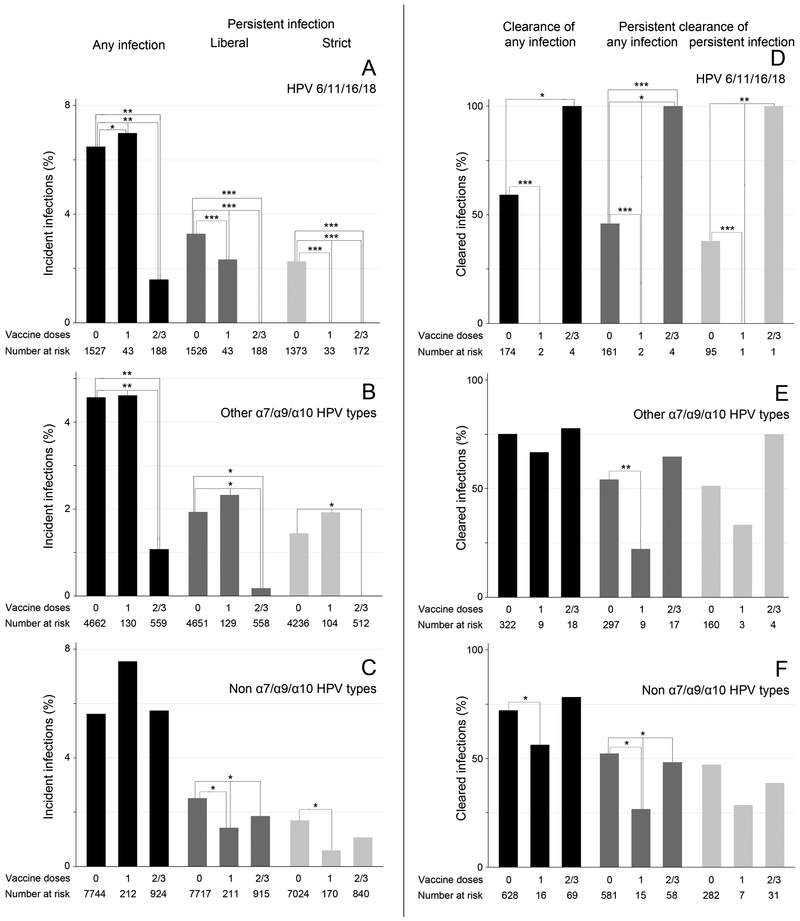
The incidence of any HPV6/11/16/18 infection was significantly decreased in women who had received two or three HPV vaccine doses before their first visit (adjusted HR=0.43, 95%CI: 0.23-0.81) (figure 1A, supplementary table S4). Using the strictest definition of an incident infection (≥2 negative measurements followed by ≥2 positive measurements), none of the vaccinated women had an incident infection with HPV6/11/16/18. Women who had received two or three vaccine doses also had a decreased incidence of other α7, α9, and α10 HPV types (adjusted HR for any incident infection=0.47, 95%CI: 0.30-0.75; figure 1B). Between vaccinated and unvaccinated women, no consistent differences were observed for the incidence of HPV types that were not part of the α7, α9, and α10 species (figure 1C).
Figure 1.
Incidence (A-C) and clearance (D-F) of type-specific genital HPV infections in women, stratified by the number of vaccine doses received at baseline. Top row: incidence (A) and clearance (D) of HPV types included in the quadrivalent vaccine (HPV6/11/16/18). Middle row: incidence (B) and clearance (E) of other HPV types within the α7/α9/α10 species (HPV31/33/35/39/44/45/52/58/59/67/68/70). Bottom row: incidence (C) and clearance (F) of non-α7/α9/α10 HPV types (HPV26/34/40/42/51/53/54/56/61 /62/66/69/71/72/73/81/82/83/84/89). Three definitions of incidence were used: any infection (black bars): HPV detection after ≥1 negative visit(s); any incident persistent infection (liberal; dark grey bars): HPV detection in ≥2 consecutive visits after ≥1 negative measurement(s); strict definition of an incident persistent infection (light grey bars): ≥2 consecutive negative measurements followed by ≥2 consecutive HPV-positive visits. Similarly, three definitions of clearance were used: any infection (black bars): ≥1 positive visit(s) followed by ≥1 negative measurement(s); persistent clearance of any infection (dark grey bars): ≥2 negative measurements at consecutive visits after ≥1 positive HPV detection(s); persistent clearance of persistent infections (light grey bars): ≥2 consecutive positive measurements followed by ≥2 consecutive negative visits. P-values were calculated using a multivariable parametric model for interval-censored survival-time data. We adjusted for age, race, smoking status, age at first coitus, number of lifetime sex partners (coitus), whether the individual had same-sex partners and/or concurrent sex partners, condom use, average frequency of coitus with HITCH partner per week, and duration of the sexual relationship. All significant p-values are displayed: *, p<0.05; **, p<0.01; ***, p<0.001. Exact hazard ratio values are reported in supplementary table S4-S5.
In terms of clearance, women who received two or three vaccine doses before the baseline visit had increased clearance rates of HPV6/11/16/18 infections (adjusted HR=1.64, 95%CI: 1.09-2.46, supplementary table S5), but interpretation of clearance analyses was limited due to the low numbers of vaccinated women at risk, i.e., few vaccinated women had HPV6/11/16/18 infections (figure 1D). Generally, clearance rates did not differ significantly between vaccinated and unvaccinated women for other HPV types (figures 1E-F).
Male partners of vaccinated women had a decreased incidence of α7, α9, and α10 HPV types at their follow-up visit (adjusted OR=0.22, 95%CI: 0.05-0.95, table 4). We also calculated incidence limited to men ‘at risk’ of an incident HPV infection, i.e., men who were negative for an HPV type at baseline, while their female partner had a type-specific HPV infection at the baseline visit. While 25.0% of ‘at risk’ men with an unvaccinated female partner had an incident α7, α9 or α10 HPV infection, none of the five men with a vaccinated female partner had an incident HPV infection with these species. Clearance rates did not differ significantly between men with vaccinated and unvaccinated female partners.
Table 4.
Incidence and clearance of type-specific HPV infections in men. Incidence was calculated for all men, and for men whose sexual partner had tested positive for that particular HPV type at baseline (i.e., men ‘at risk’ for an incident infection). Data were pooled over HPV types; odds ratios were adjusted for repeated measures within individuals.
| Outcome | HPV infection category |
Female partner had not received HPV vaccine before HITCH |
Female partner had received HPV vaccine before HITCH |
Odds ratio (95% CI) |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted1 | ||||
| Incidence, all men | All HPV types | 1.6% (173/11043) |
1.0% (19/1818) |
0.68 (0.37-1.26) |
0.80 (0.44-1.47) |
| α7, α9 & α10 HPV types | 1.4% (68/4923) |
0.2% (2/818) |
0.17 (0.04-0.75) |
0.22 (0.05-0.95) |
|
| - HPV 6/11/16/18 | 1.7% (20/1200) |
0.5% (1/201) |
0.29 (0.04-2.25) |
0.32 (0.04-2.53) |
|
| - Other α7, α9 & α10 HPV types | 1.3% (48/3723) |
0.2% (1/617) |
0.12 (0.02-0.92) |
0.17 (0.02-1.27) |
|
| Non-α7, -α9 or -α10 HPV types | 1.7% (105/6120) |
1.7% (17/1000) |
1.01 (0.54-1.89) |
1.23 (0.68-2.26) |
|
| Incidence, men with type-specific HPV-positive female partner | All HPV types | 30.2% (38/126) |
31.3% (5/16) |
1.05 (0.27-4.12) |
0.39 (0.09-1.80) |
| α7, α9 & α10 HPV types | 25.0% (17/68) |
0.0% (0/5) |
0 | ND | |
| - HPV 6/11/16/18 | 33.3% (7/21) |
0.0% (0/2) |
0 | ND | |
| - Other α7, α9 & α10 HPV types | 21.3% (10/47) |
0.0% (0/3) |
0 | ND | |
| Non-α7, -α9 or -α10 HPV types | 36.2% (21/58) |
45.5% (5/11) |
1.57 (0.27-9.00) |
0.51 (0.06-4.17) |
|
| Clearance, all men | All HPV types | 26.2% (144/549) |
25.6% (23/90) |
0.99 (0.54-1.80) |
0.77 (0.39-1.49) |
| α7, α9 & α10 HPV types | 24.9% (57/229) |
26.7% (8/30) |
1.10 (0.46-2.60) |
0.96 (0.34-2.75) |
|
| - HPV 6/11/16/18 | 23.9% (21/88) |
18.2% (2/11) |
0.71 (0.14-3.54) |
0.39 (0.03-4.54) |
|
| - Other α7, α9 & α10 HPV types | 25.5% (36/141) |
31.6% (6/19) |
1.35 (0.48-3.80) |
1.42 (0.41-4.94) |
|
| Non-α7, -α9 or -α10 HPV types | 27.2% (87/320) |
25.0% (15/60) |
0.89 (0.45-1.78) |
0.69 (0.30-1.57) |
|
Adjusted for age, race, smoking status, age at first coitus, number of lifetime sex partners (coitus), whether the individual had same-sex partners and/or concurrent sex partners at the baseline visit, condom use, average frequency of coitus with HITCH partner per week, and duration of the sexual relationship.
Vaccinated women who tested positive for HPV6, 11, 16, or 18 despite being vaccinated, had lower viral loads than unvaccinated women (p=0.001, table 5). This difference was primarily driven by HPV16. However, viral load distribution for HPV31, 42, and 51 was similar between vaccinated and unvaccinated women (p=0.93).
Table 5.
Type-specific viral loads (copies/cell) in HPV-positive women, stratified by baseline vaccination status.
| HPV type |
Unvaccinated women | Vaccinated women | P-value1 | |||||
|---|---|---|---|---|---|---|---|---|
| n | Median (IQR) | Geometric mean (95% CI) |
n | Median (IQR) | Geometric mean (95% CI) |
|||
| HPV6 | 56 | 0.19 (0.007-4.13) |
0.19 (0.07-0.51) |
2 | 4.21 (0.0008-8.42) |
0.08 (0.00-3.4·1024) |
0.77 | 0.001 |
| HPV11 | 7 | 0.02 (0.0002-0.22) |
0.01 (0.0005-0.23) |
0 | - | - | - | |
| HPV16 | 106 | 3.09 (0.20-15.8) |
1.44 (0.73-2.84) |
8 | 0.006 (0.0004-0.43) |
0.008 (0.0004-0.16) |
0.001 | |
| HPV18 | 29 | 1.21 (0.04-9.83) |
0.60 (0.11-3.21) |
1 | 0.36 | 0.36 | 0.86 | |
| HPV31 | 46 | 0.47 (0.03-10.9) |
0.34 (0.10-1.21) |
3 | 0.13 (0.04-6.40) |
0.32 (0.0004-238.5) |
0.77 | 0.93 |
| HPV42 | 74 | 26.3 (1.70-179.5) |
12.9 (6.02-27.8) |
14 | 24.8 (0.87-261.1) |
18.0 (2.48-130.8) |
0.86 | |
| HPV51 | 73 | 7.36 (0.27-107.4) |
3.81 (1.38-10.53) |
12 | 4.77 (0.16-72.7) |
3.87 (0.38-39.1) |
0.81 | |
P-values for individual HPV types were calculated using Wilcoxon rank-sum tests. When HPV types were grouped based on whether the type is included in the quadrivalent vaccine, p-values were calculated using the Friedman test, stratifying by HPV type.
DISCUSSION
In this study, we present evidence of changes in HPV transmission dynamics following HPV vaccination in a cohort of young, recently formed, sexually active, heterosexual couples. We showed how epidemiologic measures such as HPV type-concordance, type-specific prevalence, incidence, clearance, and viral loads varied in couples in which the woman had been vaccinated against HPV before the first study visit, relative to those in which the woman was not vaccinated. Our findings provide empirical support to the expectation that vaccination of sexually active women benefits not only the women themselves, but also their sexual partners, even in a population with late vaccination (after the onset of sexual activity) and in which men have had multiple sexual partners in the past.
As expected, vaccinated women had a lower incidence, prevalence and persistence of HPV6/11/16/18 infections. Interestingly, they also had a higher clearance rate of HPV 6/11/16/18 infections, regardless of the clearance definition used. This may have been a chance finding, due to the low number of vaccinated women who tested HPV 6/11/16/18 positive. It may also indicate that vaccination prevents persistence of HPV infections, and/or that such short-lasting, clearing HPV detections are not ‘true’ infections of epithelial cells, but for example deposition from the sexual partner (20,21). Vaccinated women who tested positive for HPV 6, 11, 16, or 18 also had lower type-specific viral loads than unvaccinated women who were positive for these types. There could be various explanations for this decrease in viral loads in vaccinated women. First, previous studies have shown an inverse correlation between viral loads and clearance rates (22,23). Indeed, women who had received 2 or 3 HPV vaccine doses at baseline, but were HPV6/11/16/18-positive, cleared these infections during the course of HITCH. Second, incident HPV infections may be rapidly cleared by the immune system in vaccinated women. Third, it is also possible that these low viral load infections in vaccinated women do not represent true infections, but HPV DNA deposition from recent sexual activity with male partners (20).
Some evidence for cross-type protection was found. For example, the prevalence and incidence of HPV types within the α7, α9, and α10 species not included in the quadrivalent vaccine were decreased in vaccinated women as compared to unvaccinated women. Similarly, the incidence of α7, α9, and α10 HPV types was significantly lower in male partners of vaccinated women. Previous studies have reported cross-type protection from HPV vaccines via induction of cross-neutralizing antibodies (24-28). It is conceivable that this effect on the humoral immune response would ultimately alter the transmission dynamics of HPV types of the α7, α9, and α10 species not included in the vaccines. As previously reported by our research group (29), we found no evidence of type replacement in vaccinated individuals in our cohort.
While women in HITCH who had received two or three vaccine doses before their first visit, had a lower incidence of α7, α9, and α10 HPV types, those who had received only one HPV vaccine dose generally did not have a reduced incidence of these HPV types as compared to unvaccinated women. Most of these women were vaccinated days to weeks before the first visit; a woman infected before her first vaccine dose may seem to have an incident infection post-vaccination due to a lag period between the start of HPV invading the basal cells and HPV detectable in a vaginal swab. This reasoning is confirmed when using a more conservative definition of HPV incidence: when limiting incidence to ≥2 negative measurements followed by ≥2 positive measurements, as opposed to only one negative measurement followed by positive measurement(s), women who had received one dose had a significantly lower incidence of HPV6/11/16/18 infections. An alternative explanation would be that women who had received only one dose had insufficient protection against new HPV infections. Although studies report high efficacy of a single dose, vaccine efficacy may be lower when women are vaccinated at a higher age as in HITCH (30,31). Finally, misclassification may have occurred due to women misreporting their vaccination status in the questionnaire, or the analyses may have been underpowered due to the low number of women with one vaccine dose at baseline (n=12).
Interestingly, we found an effect of vaccination in male partners of vaccinated women. It seems that the reduced HPV prevalence and incidence in male partners of vaccinated women is completely caused by the reduced prevalence of HPV infections in the female sex partner: adjusting for type-specific HPV prevalence in the female partner eliminated the observed benefit in male partners. As far as we know, this is the first study directly measuring the effect of female vaccination in heterosexual male partners. While various studies have reported herd immunity in vaccinated populations (10,32-35), our findings are related to transmission at the individual level. Men had a median of six lifetime sex partners with whom genital HPV transmission had been possible. Given the timing of recruitment of HITCH, it is unlikely that these men had had (all) vaccinated sex partners before their current HITCH partner. Furthermore, HITCH couples had been in a sexual relationship with their current partner for less than six months. Despite this short duration and the sexual history with (probably) unvaccinated women, we already observed an effect in male partners of vaccinated women. Considering our findings, and since men remain equally prone to HPV (re)infections later in life, it will be interesting to assess whether our observed difference translate to a decreased chance for long-term persistent genital infections, and subsequently, less HPV-related genital diseases and decreased transmission to new sexual partners. This would corroborate findings of herd immunity in populations (32). Similarly, future studies will need to evaluate whether vaccination of boys significantly reduces HPV prevalence/incidence in their female partners, as this would further encourage gender neutral vaccination.
Since our cohort was recruited before HPV vaccination was implemented in Canada, most if not all vaccinated women received their vaccine after becoming sexually active and in their late teens/early twenties. Vaccine efficacy might be lower in this population, primarily as they can already be infected before vaccination, and it is generally thought that vaccination does not affect infection clearance (30,36-38). Conversely, alterations in transmission dynamics might be more pronounced when evaluating couples whose members were vaccinated at a young age in established vaccine programs.
General limitations of the HITCH study have been discussed previously, including false-positive and false-negative HPV measurements, and the risk of contamination due to recent sexual activity (20,39). Since patients had not been randomized for vaccination, baseline characteristics were not completely equal between vaccinated and unvaccinated women, and residual confounding is possible. Since men attended only two study visits according to the study protocol, we were unable to study the incidence and clearance of persistent infections in men, which would require at least three visits. Because the time between visits generally lasted 4-6 months, we conducted interval analyses for time to events, as the timing of an incident or clearing infection could not be determined with precision. We were unable to determine whether an HPV infection had been transmitted from the HITCH partner, or from a concurrent, previous or new sex partner. Importantly, vaccination history was self-reported and therefore prone to recall bias. Nevertheless, we previously showed that serum antibody levels strongly correlated to self-reported number of vaccine doses, suggesting that this reporting was accurate (40). Based on antibody levels, we also previously determined that only one woman had received the bivalent vaccine against HPV 16 and 18, while all other women had received the quadrivalent vaccine. The woman who received the bivalent vaccine did not have HPV6/11 detection during any visit. Also, we only asked women when they received their last vaccination dose. Therefore, we were unable to determine for some women whether they had already received a vaccine dose before the first sexual activity with their HITCH partner. Finally, we did not verify that the self-sampling by the participating women was as reliable as provider-sampling in HITCH. However, women received detailed instructions on how to conduct self-sampling from the nurse, and self-sampling of vaginal samples is generally considered a reliable method and comparable to provider-sampling (41).
In summary, vaccination affected the prevalence, incidence, persistence, clearance, and viral loads of HPV6, 11, 16 and 18 in our longitudinal cohort of young, recently formed, sexually active, heterosexual couples, in which vaccination had generally occurred after an individual’s first sexual intercourse. The protective effect of vaccination was most evident in vaccinated women, but to a lesser extent in (unvaccinated) male partners of vaccinated women too. Considering the short duration of the sexual relationship of HITCH couples at the time of enrolment, this may indicate rapid herd protective effects. Changes in HPV transmission dynamics also suggested cross-protective effects against other HPV types of the α7, α9, and α10 species.
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank the volunteering participants. We would also like to thank Emilie Comète and Julie Guenoun for the processing and laboratory testing of samples, and the additional members of the HITCH Cohort Study group: Allita Rodrigues (study coordinator); Hélène Voyer and Véronique Legault (laboratory staff); Gail Kelsall, Suzanne Dumais, Natalia Morykon, and Amelia Rocamora (management of subject participation and specimen collection); Nathalie Slavtcheva (study management); Veronika Moravan (data management); Michel Roger (collaborator); Vicky D’Anjou-Pomerleau, Jennifer Selinger, Elizabeth Montpetit-Dubrule, Jessica Sammut, Emilie Lapointe, Johanna Bleecker, and Shady Rahayel (study promotion). The authors also thank Melanie Drew (Student Health Services Clinic, Concordia University) and the staff of the Student Health Services Clinics at McGill and Concordia universities for their collaboration with HITCH research nurses.
Financial support: The HITCH Cohort Study was supported by the Canadian Institutes of Health Research (grant numbers MOP-68893, CRN-83320 to ELF); and the U.S. National Institutes of Health (grant number RO1AI073889 to ELF). Supplementary and unconditional funding support was provided by Merck-Frosst Canada Ltd. and Merck & Co. Ltd. Validation of viral load assays was supported by the Réseau sida et maladies infectieuses (SIDA /MI) du Fonds de recherche du Québec - Santé (FRQS). MDW is funded by a Canadian Institutes of Health Research Postdoctoral Fellowship Award.
Abbreviation list:
- 95%CI
95% confidence interval
- ANOVA
Analysis of variance
- DNA
Deoxyribonucleic acid
- HITCH
HPV Infection and Transmission among Couples through Heterosexual Activity
- HPV
Human papillomavirus
- HR
Hazard ratio
- IQR
Interquartile range
- LA
Linear array
- ND
Not determined
- OR
Odds ratio
- PCR
Polymerase chain reaction
Footnotes
Disclosures: ELF has served as an occasional advisor to companies involved with HPV diagnostics (Roche, BD) and HPV vaccines (Merck, GSK). His institution has received unconditional grants from Merck in aid of investigator-initiated studies. FC has received grants through his institution from Merck and Roche, as well as honoraria from Merck and Roche for lectures on HPV. All other authors have no potential conflicts of interest to declare.
REFERENCES
- 1.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine 2006;30(24):S1–15. [DOI] [PubMed] [Google Scholar]
- 2.Franco EL, de Sanjose S, Broker TR, Stanley MA, Chevarie-Davis M, Isidean SD, et al. Human papillomavirus and cancer prevention: gaps in knowledge and prospects for research, policy, and advocacy. Vaccine 2012;30 Suppl 5:F175–82 doi 10.1016/j.vaccine.2012.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S. Global burden of cancers attributable to infections in 2012: a synthetic analysis. The Lancet Global health 2016;4(9):e609–16 doi 10.1016/s2214-109x(16)30143-7. [DOI] [PubMed] [Google Scholar]
- 4.FUTUREIIStudyGroup. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007;356(19):1915–27 doi 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 5.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007;356(19):1928–43 doi 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 6.Herrero R, Wacholder S, Rodriguez AC, Solomon D, Gonzalez P, Kreimer AR, et al. Prevention of persistent human papillomavirus infection by an HPV16/18 vaccine: a community-based randomized clinical trial in Guanacaste, Costa Rica. Cancer discovery 2011;1(5):408–19 doi 10.1158/2159-8290.cd-11-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. The lancet oncology 2012;13(1):89–99 doi 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- 8.Joura EA, Giuliano AR, Iversen OE, Bouchard C, Mao C, Mehlsen J, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015;372(8):711–23 doi 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 9.Moscicki AB, Schiffman M, Burchell A, Albero G, Giuliano AR, Goodman MT, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine 2012;30 Suppl 5:F24–33 doi 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow EPF, Machalek DA, Tabrizi SN, Danielewski JA, Fehler G, Bradshaw CS, et al. Quadrivalent vaccine-targeted human papillomavirus genotypes in heterosexual men after the Australian female human papillomavirus vaccination programme: a retrospective observational study. The Lancet infectious diseases 2017;17(1):68–77 doi 10.1016/s1473-3099(16)30116-5. [DOI] [PubMed] [Google Scholar]
- 11.Wissing MD, Louvanto K, Comete E, Burchell AN, El-Zein M, Rodrigues A, et al. Human papillomavirus viral load and transmission in young, recently-formed heterosexual couples. J Infect Dis 2019. doi 10.1093/infdis/jiz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Zein M, Coutlee F, Tellier PP, Roger M, Franco EL, Burchell AN. Human Papillomavirus Infection and Transmission Among Couples Through Heterosexual Activity (HITCH) Cohort Study: Protocol Describing Design, Methods, and Research Goals. JMIR research protocols 2019;8(1):e11284 doi 10.2196/11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchell AN, Rodrigues A, Moravan V, Tellier PP, Hanley J, Coutlee F, et al. Determinants of prevalent human papillomavirus in recently-formed heterosexual partnerships: A dyadic-level analysis. J Infect Dis 2014. doi 10.1093/infdis/jiu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology (Cambridge, Mass) 2010;21(1):31–7 doi 10.1097/EDE.0b013e3181c1e70b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burchell AN, Tellier PP, Hanley J, Coutlee F, Franco EL. Influence of partner’s infection status on prevalent human papillomavirus among persons with a new sex partner. Sexually transmitted diseases 2010;37(1):34–40 doi 10.1097/OLQ.0b013e3181b35693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutlee F, Rouleau D, Petignat P, Ghattas G, Kornegay JR, Schlag P, et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. Journal of clinical microbiology 2006;44(6):1998–2006 doi 10.1128/jcm.00104-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Villiers EM. Cross-roads in the classification of papillomaviruses. Virology 2013;445(1-2):2–10 doi 10.1016/j.virol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Azizi N, Brazete J, Hankins C, Money D, Fontaine J, Koushik A, et al. Influence of human papillomavirus type 16 (HPV-16) E2 polymorphism on quantification of HPV-16 episomal and integrated DNA in cervicovaginal lavages from women with cervical intraepithelial neoplasia. The Journal of general virology 2008;89(Pt 7):1716–28 doi 10.1099/vir.0.83579-0. [DOI] [PubMed] [Google Scholar]
- 19.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22(4):719–48. [PubMed] [Google Scholar]
- 20.Malagon T, Burchell AN, El-Zein M, Guenoun J, Tellier PP, Coutlee F, et al. Estimating HPV DNA Deposition Between Sexual Partners Using HPV Concordance, Y Chromosome DNA Detection, and Self-reported Sexual Behaviors. J Infect Dis 2017;216(10):1210–8 doi 10.1093/infdis/jix477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naud PS, Roteli-Martins CM, De Carvalho NS, Teixeira JC, de Borba PC, Sanchez N, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother 2014;10(8):2147–62 doi 10.4161/hv.29532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu M, Liu F, Pan Y, He Z, Guo C, Zhang C, et al. Viral Load in the Natural History of Human Papillomavirus Infection Among Men in Rural China: A Population-based Prospective Study. Clin Infect Dis 2018. doi 10.1093/cid/ciy376. [DOI] [PubMed] [Google Scholar]
- 23.Trevisan A, Schlecht NF, Ramanakumar AV, Villa LL, Franco EL. Human papillomavirus type 16 viral load measurement as a predictor of infection clearance. The Journal of general virology 2013;94(Pt 8):1850–7 doi 10.1099/vir.0.051722-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barzon L, Squarzon L, Masiero S, Pacenti M, Marcati G, Mantelli B, et al. Neutralizing and cross-neutralizing antibody titres induced by bivalent and quadrivalent human papillomavirus vaccines in the target population of organized vaccination programmes. Vaccine 2014;32(41):5357–62 doi 10.1016/j.vaccine.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Toft L, Tolstrup M, Muller M, Sehr P, Bonde J, Storgaard M, et al. Comparison of the immunogenicity of Cervarix(R) and Gardasil(R) human papillomavirus vaccines for oncogenic non-vaccine serotypes HPV-31, HPV-33, and HPV-45 in HIV-infected adults. Hum Vaccin Immunother 2014;10(5):1147–54 doi 10.4161/hv.27925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown DR, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in generally HPV-naive women aged 16-26 years. J Infect Dis 2009;199(7):926–35 doi 10.1086/597307. [DOI] [PubMed] [Google Scholar]
- 27.Wheeler CM, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Perez G, et al. The impact of quadrivalent human papillomavirus (HPV; types 6, 11, 16, and 18) L1 virus-like particle vaccine on infection and disease due to oncogenic nonvaccine HPV types in sexually active women aged 16-26 years. J Infect Dis 2009;199(7):936–44 doi 10.1086/597309. [DOI] [PubMed] [Google Scholar]
- 28.Ryser M, Berlaimont V, Karkada N, Mihalyi A, Rappuoli R, van der Most R. Post-hoc analysis from phase III trials of human papillomavirus vaccines: considerations on impact on non-vaccine types. Expert review of vaccines 2019;18(3):309–22 doi 10.1080/14760584.2019.1579647. [DOI] [PubMed] [Google Scholar]
- 29.Tota JE, Jiang M, Ramanakumar AV, Walter SD, Kaufman JS, Coutlee F, et al. Epidemiologic Evaluation of Human Papillomavirus Type Competition and the Potential for Type Replacement Post-Vaccination. PloS one 2016;11(12):e0166329 doi 10.1371/journal.pone.0166329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreimer AR, Struyf F, Del Rosario-Raymundo MR, Hildesheim A, Skinner SR, Wacholder S, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. The lancet oncology 2015;16(7):775–86 doi 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safaeian M, Porras C, Pan Y, Kreimer A, Schiller JT, Gonzalez P, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus-like particle vaccine in the Costa Rica Vaccine Trial. Cancer prevention research (Philadelphia, Pa) 2013;6(11):1242–50 doi 10.1158/1940-6207.capr-13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brisson M, Benard E, Drolet M, Bogaards JA, Baussano I, Vanska S, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. The Lancet Public health 2016;1(1):e8–e17 doi 10.1016/s2468-2667(16)30001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kavanagh K, Pollock KG, Cuschieri K, Palmer T, Cameron RL, Watt C, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. The Lancet infectious diseases 2017;17(12):1293–302 doi 10.1016/s1473-3099(17)30468-1. [DOI] [PubMed] [Google Scholar]
- 34.Tabrizi SN, Brotherton JM, Kaldor JM, Skinner SR, Liu B, Bateson D, et al. Assessment of herd immunity and cross-protection after a human papillomavirus vaccination programme in Australia: a repeat cross-sectional study. The Lancet infectious diseases 2014;14(10):958–66 doi 10.1016/s1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 35.Malagon T, Laurie C, Franco EL. Human papillomavirus vaccination and the role of herd effects in future cancer control planning: a review. Expert review of vaccines 2018;17(5):395–409 doi 10.1080/14760584.2018.1471986. [DOI] [PubMed] [Google Scholar]
- 36.Schlecht NF, Diaz A, Shankar V, Szporn AH, Wu M, Nucci-Sack A, et al. Risk of Delayed Human Papillomavirus Vaccination in Inner-City Adolescent Women. J Infect Dis 2016;214(12):1952–60 doi 10.1093/infdis/jiw486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Westra TA, Rozenbaum MH, Rogoza RM, Nijman HW, Daemen T, Postma MJ, et al. Until which age should women be vaccinated against HPV infection? Recommendation based on cost-effectiveness analyses. J Infect Dis 2011;204(3):377–84 doi 10.1093/infdis/jir281. [DOI] [PubMed] [Google Scholar]
- 38.Munoz N, Manalastas R Jr., Pitisuttithum P, Tresukosol D, Monsonego J, Ault K, et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet 2009;373(9679):1949–57 doi 10.1016/s0140-6736(09)60691-7. [DOI] [PubMed] [Google Scholar]
- 39.Burchell AN, Coutlee F, Tellier PP, Hanley J, Franco EL. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis 2011;204(11):1723–9 doi 10.1093/infdis/jir644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wissing MD, Trevisan A, Burchell AN, El-Zein M, Tellier PP, Coutlee F, et al. Time-dependent dose-response relationship between vaccination dose and serum HPV antibody titers. At: HPV2017, the 31st international papillomavirus conference 2017;February 28 - March 4, 2017; Cape Town, South Africa. Abstract HPV17-0974. [Google Scholar]
- 41.Cerigo H, Coutlee F, Franco EL, Brassard P. Dry self-sampling versus provider-sampling of cervicovaginal specimens for human papillomavirus detection in the Inuit population of Nunavik, Quebec. Journal of medical screening 2012;19(1):42–8 doi 10.1258/jms.2012.012011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.