Abstract
Background:
We aimed to investigate the association between lung cancer and tea-drinking habits of different subgroup populations.
Methods:
Systematic search of the PubMed, Web of Science, China National Knowledge Infrastructure (CNKI) and Sinomed databases from database construction until January 2017 for English and Chinese language articles on association of lung cancer and tea drinking. Meta-analysis was used to calculate the combined odds ratio (OR) value and its 95% confidence interval (95% CI). The Newcastle-Ottawa scale was used to evaluate the quality of the studies and Q-test and I2 was used for heterogeneity testing.
Results:
Forty two papers were included, 30 case-control studies included 14578 lung cancer patients and 180574 controls, 12 cohort studies included 543825 subjects, of which the outcome was 5085 with lung cancer. Tea drinkers were found to have a decreased OR of lung cancer compared with non-tea drinkers (OR 0. 80, 95% CI: 0. 73, 0. 87). Consumption of green, black or unspecified tea has a protective effect compared with not drinking tea at all. Increased intake of green tea to 7. 5 g per day can further reduce the OR of lung cancer (OR 0. 69, 95% CI: 0. 48–0. 98). Tea consumption had a protective effect against lung cancer in non-smokers, Further analysis found that drinking of one or more cups of tea a day has a protective effect on smokers (OR 0. 79, 95% CI: 0. 64–0. 96).
Conclusion:
Tea drinking could be a protective factor in lung cancer.
Keywords: Tea, Meta-analysis, Case-control studies, Cohort studies, Lung cancer
Introduction
Currently, lung cancer is one of the malignant cancers in the world with the highest incidence and mortality rates (1). Therefore, the prevention of lung cancer is of utmost importance. Many studies have investigated the risk of lung cancer and tea consumption, but the conclusions were not consistent (2–4). A meta-analysis in 2009 (5) found that drinking green tea has a protective effect on lung cancer statistically, while there was no association between drinking black tea and lung cancer. Either black or green tea consumption have a protective effect on lung cancer statistically. Hence there is a controversy between the results of these two studies (6).
Smoking is a major risk factor for lung cancer (7, 8). In vivo animal experiments have shown that tea polyphenols can decrease the probability of tumor formation and decrease the size and peak proliferation of tumors (9, 10). When smoking cessation is difficult, whether tea drinking can antagonize the effects of smoking on lung cancer risk is important in the prevention of lung cancer. Intake of green tea can decrease the lung cancer risk in smoking populations (11). However, two previous systematic meta-analyses did not find that tea drinking can decrease the risk of contracting lung cancer in smoking populations.
This study collected all local and overseas published articles up till January 2017 to carry out a meta-analysis to investigate the association between tea intake in different subgroup populations and lung cancer.
Methods
Tea, green tea, black tea, lung cancer, lung neoplasm, lung tumor, and lung carcinoma were used as keywords to search in the PubMed, Web of Science, the China National Knowledge Infrastructure (CNKI) and Sinomed databases. The keywords were used together or individually to search all databases from database construction until January 2017. The literature search was performed independently by two authors. All articles must fulfill the following inclusion criteria: 1) Lung cancer; 2) Case-control studies or cohort studies; 3) Exposure risk factors involves tea drinking, and study contains either OR or relative risk (RR), and its 95% CI, or these values can be computed.
Data extraction and quality assessment: The first author, publication year, study period, region, type of study, type of controls, sample size (number of cases and controls), tea drinking status, adjusted OR or RR and its 95% CI, were extracted from every article. The Newcastle-Ottawa scale (NOS) was used to evaluate the quality. Data extraction and quality assessment were also performed independently by two authors.
Statistical analysis: RevMan 5. 3 software was used for statistical analysis and the OR values and 95% CI comparing either tea drinking or highest tea intake with non-tea drinking were obtained from combining various studies. The amount of tea intake was shown by the weight of tea leaves (in grams). The intake amount in this study was readjusted and one cup of tea was defined as 2. 5 g of tea leaves (2).
The Q-test and I2 was used for heterogeneity testing, both P<0. 1 and I2>50% defined as the presence of heterogeneity (12). When heterogeneity presented, subgroup analysis was carried out to eliminate heterogeneity; and if heterogeneity still exists, sensitivity analysis was carried out and each study was omitted individually to see if there were studies with significant effects on heterogeneity. If heterogeneity was still presented, the random effects model was used for statistical analysis. A funnel plot was constructed to investigate publication bias (13), and an asymmetrical funnel plot shows that there is publication bias.
Results
Basic information
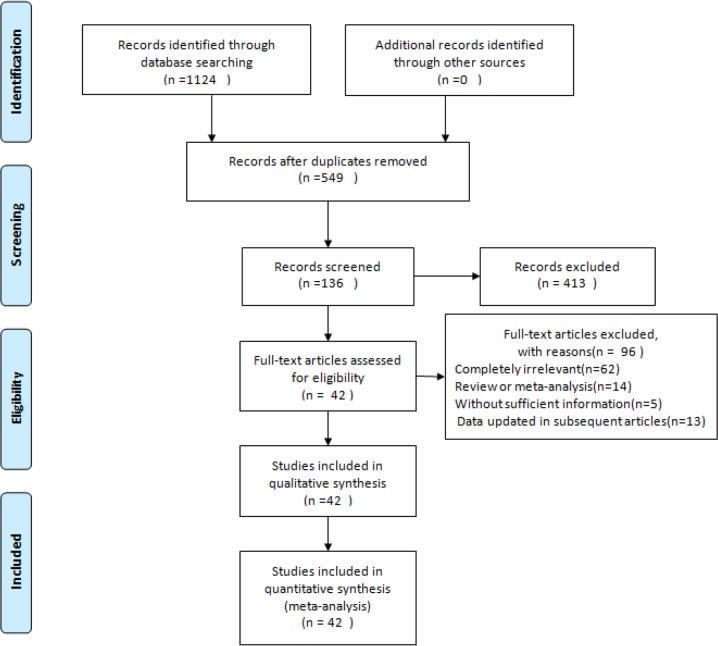
The initial search yielded 549 articles. Through screening of titles and abstracts, 413 articles were excluded and 60 articles were selected for data extraction after careful reading of the article. As the data from 13 articles were repeated in subsequent studies, these studies were excluded. Complete data could not be extracted from five studies and these studies were also excluded. Finally, 42 studies were included in the meta-analysis in this study (3–4, 14–53) (Fig. 1). There were 19, 433 lung cancer patients and 718, 854 controls. 30 case-control studies, with 17 population-based case-control studies, one mortality-based case-control study and the remainder were hospital-based case-control studies. Case-control studies included 14578 lung cancer patients and 180574 controls. Twelve cohort studies included 543825 subjects, of which the outcome was 5085 with lung cancer. Two studies investigated the association between lung cancer and black and green tea consumption, 12 studies for green tea and seven for black tea. The remaining 21 studies did not specify the type of tea (Table 1).
Fig. 1:
Process followed in the selection of studies
Table 1:
Characteristics of published studies on tea consumption and lung cancer risk
| Study | Study period | Country | Study design | Case-control or cohort | Tea type | OR (95%CI) | NOS score (stars) |
|---|---|---|---|---|---|---|---|
| Romain 2016(14) | 1996–2001 | Canada | PCC | 1111/1469 | Black | 0. 71[0. 61, 0. 83] | 8 |
| Wu 2015(15) | 2001–2010 | China | PCC | 117/1196 | Green | 0. 87[0. 70, 1. 07] | 7 |
| Wang 2015(16) | 2012–2014 | China | HCC | 88/84 | Tea | 0. 97[0. 53, 1. 76] | 7 |
| Mai 2015(17) | 1992–2011 | US | Cohort | 1137/94887 | Tea | 0. 83[0. 71, 0. 96] | 8 |
| Katarzyna 2015(18) | 2014 | Poland | PCC | 92/156 | Green | 0. 49[0. 26, 0. 93] | 7 |
| Bao 2014(18) | 2010–2013 | China | HCC | 50/50 | Green | 0. 22[0. 08, 0. 60] | 7 |
| Rup 2014(20) | 2009–2012 | India | PCC | 230/460 | Tea | 0. 95[0. 61, 1. 49] | 7 |
| P. gnagna 2013(22) | 2004–2005 | Italy | Cohort | 178/4158 | Tea | 0. 72[0. 52, 0. 99] | 8 |
| Xu 2013(21) | 2006–2012 | China | HCC | 1225/1234 | Tea | 0. 98[0. 84, 1. 15] | 7 |
| Yumie 2013(23) | 2002–2009 | China | Cohort | 359/60733 | Tea | 0. 66[0. 53, 0. 83] | 7 |
| Jin 2013(24) | 2003–2010 | China | PCC | 1424/4543 | Green | 1. 05[0. 92, 1. 20] | 8 |
| Yumiel 2013(25) | 2001–2011 | China | Cohort | 428/70839 | Tea | 1. 00[0. 81, 1. 23] | 7 |
| Lin 2012(26) | 2004–2008 | China | HCC | 170/340 | Green | 0. 34[0. 21, 0. 55] | 7 |
| Zhang 2012(27) | 1997–2009 | China | PCC | 900/133811 | Tea | 1. 16[1. 01, 1. 32] | 8 |
| Bganesh 2011(28) | 1997–1999 | India | HCC | 408/1383 | Tea | 0. 24[0. 11, 0. 55] | 6 |
| Jiang 2011(29) | 2009–2011 | China | HCC | 100/100 | Tea | 0. 92[0. 53, 1. 61] | 7 |
| Lu 2009(30) | 1992–1995 | US | Cohort | 201/38207 | Tea | 0. 81[0. 61, 1. 08] | 7 |
| Han 2008(31) | 2003–2008 | China | HCC | 523/1924 | Green | 0. 56[0. 44, 0. 73] | 7 |
| Zhang 2008(32) | 2002–2006 | China | PCC | 505/529 | Tea | 1. 16[0. 89, 1. 53] | 8 |
| Wang 2008(24) | 2006 | China | HCC | 363/363 | Tea | 0. 60[0. 44, 0. 82] | 7 |
| Qli 2008(33) | 1994–2001 | Japan | cohort | 302/41138 | Green | 1. 29[0. 98, 1. 69] | 8 |
| Yan 2008(35) | 1999–2004 | US | PCC | 558/837 | Green&Black | 0. 52[0. 42, 0. 66] | 7 |
| Tao 2007(36) | 2002–2006 | China | HCC | 47/94 | Tea | 0. 72[0. 31, 1. 70] | 6 |
| Shinchi 2006(4) | 1995–2005 | Japan | Cohort | 222/16247 | Green | 1. 13[0. 82, 1. 56] | 8 |
| Hu 2002(39) | 1994–1997 | Canada | PCC | 161/483 | Tea | 0. 52[0. 34, 0. 81] | 8 |
| Mattew 2005(37) | 1995–1996 | China | PCC | 122/121 | Green | 0. 83[0. 44, 1. 54] | 7 |
| Ja 2005(38) | 1982–1998 | US | PCC | 993/986 | Black | 0. 95[0. 79, 1. 13] | 6 |
| Nagano 2001(41) | 1979–1994 | Japan | Cohort | 395/35930 | Green | 0. 86[0. 66, 1. 12] | 9 |
| Zhong 2001(40) | 1992–1994 | China | PCC | 649/675 | Green | 0. 97[0. 74, 1. 26] | 7 |
| Hivonen 2001(42) | 1995–1998 | Finland | PCC | 791/25643 | Tea | 0. 66[0. 53, 0. 81] | 7 |
| Kei 2000(3) | 1986–1997 | Japan | Cohort | 69/9483 | Green | 1. 01[0. 62, 1. 63] | 6 |
| Ki 1997(45) | 1992–1993 | China | HCC | 105/105 | Tea | 0. 50[0. 23, 1. 10] | 7 |
| Fredrik 1998(43) | 1989–1995 | Sweden | PCC | 124/235 | Black | 1. 23[0. 78, 1. 96] | 8 |
| Maria 1998(44) | 1994–1996 | Uruguay | HCC | 427/428 | Black | 0. 78[0. 60, 1. 02] | 7 |
| Alexandra 1996(46) | 1986–1990 | Netherlands | Cohort | 764/120088 | Black | 0. 58[0. 49, 0. 70] | 8 |
| Zheng 1996(47) | 1986–1993 | US | Cohort | 312/35057 | Black | 0. 78[0. 62, 0. 99] | 7 |
| Gosta 1996(48) | 1989–1993 | Sweden | PCC | 308/504 | Black | 0. 71[0. 53, 0. 94] | 7 |
| Xu 1996(49) | 1987–1993 | China | PCC | 598/926 | Tea | 0. 84[0. 68, 1. 03] | 9 |
| Ohno 1995(50) | 1988–1991 | Japan | PCC | 333/666 | Tea | 0. 57[0. 39, 0. 83] | 9 |
| Tewes 1990(51) | 1981–1983 | China | PCC | 200/200 | Green&Black | 0. 98[0. 66, 1. 45] | 6 |
| Mettlin 1989(52) | 1982–1987 | US | HCC | 569/569 | Tea | 0. 71[0. 56, 0. 91] | 6 |
| Kinlen 1988(53) | 1969–1986 | UK | Cohort | 718/12868 | Tea | 1. 67[1. 31, 2. 13] | 7 |
PCC, population-based case-control study; HCC, hospital-based case-control study; US, United States; UK, United Kingdom
The quality evaluation scores of every article ranged from 6 to 9 points. Among these articles, 36 were high-quality articles (NOS 7–9) and the remaining articles were medium-quality articles (NOS 6) (Table 1).
Association of tea drinking and lung cancer
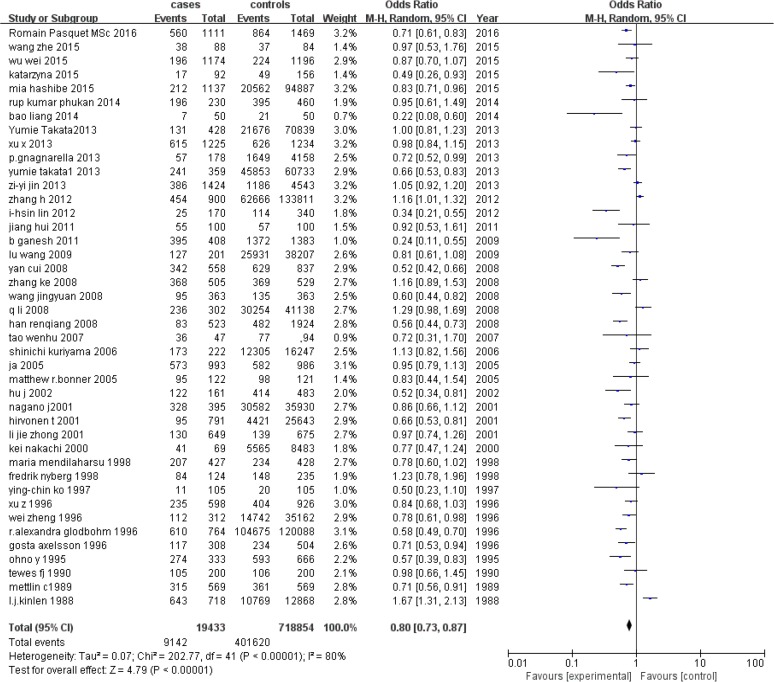
When compared with non-tea drinking populations, tea drinking was found to have a protective effect against lung cancer (OR 0. 80, 95% CI: 0. 73–0. 87) (Fig. 2). Statistically significant heterogeneity was observed (I2=80%, P<0. 01) (Fig. 3). Subgroup analyses were done in order to identify sources of heterogeneity. As shown in Table 2, the heterogeneity was not reduced by subgroup analysis of Tea types, Study design, Geographical region, Sex, Smoking status and Study period. When stratified analysis was conducted by study design. It was found to have a decreased OR in the case-control studies (OR 0. 76, 95% CI: 0. 68, 0. 85), but no statistically significant association in cohort studies (OR 0. 88, 95% CI: 0. 74, 1. 05).
Fig. 2:
Association between tea consumption and OR for lung cancer
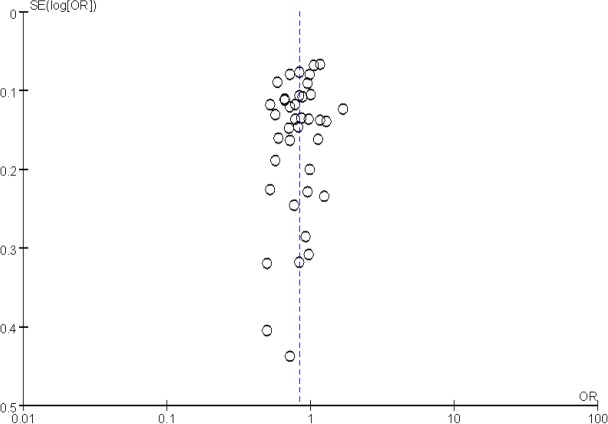
Fig. 3:
Funnel plot of studies on tea consumption and lung cancer
Table 2:
Subgroup analyses of tea intake and lung cancer risk
| Study | Number (n) | OR (95%CI) | Case-control or cohort(n) | Heterogeneity test | |
|---|---|---|---|---|---|
| I2(%) | P-value(%) | ||||
| All studies | 42 | 0. 80[0. 73, 0. 87] | 19433/718854 | 80 | <0. 01 |
| 2. 5g/day | 25 | 0. 79[0. 68, 0. 91] | 10932/404166 | 82 | <0. 01 |
| Cohort | 10 | 0. 89[0. 71, 1. 11] | 4888/394604 | 87 | <0. 01 |
| CC | 15 | 0. 71[0. 58, 0. 87] | 6044/9562 | 79 | <0. 01 |
| 7. 5g/day | 16 | 0. 82[0. 67, 1. 01] | 7652/277373 | 86 | <0. 01 |
| Cohort | 6 | 0. 87[0. 60, 1. 28] | 2470/234754 | 93 | <0. 01 |
| CC | 10 | 0. 91[0. 70, 1. 18] | 4904/54490 | 80 | <0. 01 |
| Tea types | |||||
| Green tea | 14 | 0. 75[0. 61, 0. 92] | 5750/111640 | 84 | <0. 01 |
| Cohort | 4 | 1. 02[0. 81, 1. 28] | 988/101798 | 51 | 0. 1 |
| CC | 10 | 0. 79[0. 73, 0. 86] | 4762/9842 | 86 | <0. 01 |
| 2. 5g/day | 9 | 0. 73[0. 54, 0. 98] | 1959/104387 | 76 | <0. 01 |
| Cohort | 4 | 1. 00[0. 87, 1. 15] | 1511/103722 | 49 | 0. 1 |
| CC | 5 | 0. 41[0. 21, 0. 80] | 971/2589 | 73 | <0. 01 |
| 7. 5g/day | 7 | 0. 69[0. 48, 0. 98] | 1667/103926 | 84 | <0. 01 |
| Cohort | 4 | 0. 86[0. 74, 0. 98] | 988/101798 | 90 | <0. 01 |
| CC | 3 | 0. 61[0. 44, 0. 85] | 679/2128 | 59 | 0. 09 |
| Black tea | 9 | 0. 80[0. 70, 0. 91] | 4797/159909 | 65 | <0. 01 |
| Cohort | 2 | 0. 78[0. 72, 0. 84] | 1076/155250 | 72 | 0. 05 |
| CC | 7 | 0. 82[0. 76, 0. 90] | 3721/4659 | 45 | 0. 09 |
| 2. 5g/day | 7 | 0. 88[0. 68, 1. 14] | 4039/158872 | 87 | <0. 01 |
| Cohort | 2 | 0. 76[0. 44, 1. 29] | 1076/155250 | 90 | <0. 01 |
| CC | 5 | 0. 94[0. 72, 1. 23] | 2963/3622 | 79 | <0. 01 |
| 7. 5g/day | 5 | 0. 75[0. 56, 1. 02] | 2805/157168 | 78 | <0. 01 |
| Cohort | 2 | 0. 81[0. 49, 1. 36] | 1077/155250 | 67 | 0. 08 |
| CC | 3 | 0. 68[0. 40, 1. 16] | 1728/1918 | 73 | 0. 02 |
| Tea unknown | 21 | 0. 84[0. 73, 0. 96] | 8627/316770 | 78 | <0. 01 |
| Cohort | 4 | 0. 77[0. 70, 0. 86] | 2056/254560 | 0 | 0. 39 |
| CC | 17 | 0. 89[0. 83, 0. 95] | 7526/119181 | 76 | <0. 01 |
| 2. 5g/day | 9 | 0. 75[0. 59, 0. 96] | 4934/140907 | 84 | <0. 01 |
| Cohort | 4 | 0. 86[0. 56, 1. 32] | 2824/137556 | 92 | <0. 01 |
| CC | 6 | 0. 67[0. 58, 0. 78] | 2110/3351 | 20 | 0. 29 |
| 7. 5g/day | 5 | 1. 13[0. 81, 1. 57] | 4258/27690 | 83 | <0. 01 |
| Cohort | 1 | 1. 67[1. 31, 2. 13] | 718/12868 | - | - |
| CC | 4 | 1. 01[0. 71, 1. 43] | 2462/3411 | 79 | <0. 01 |
| Study design | |||||
| Cohort | 12 | 0. 88[0. 74, 1. 05] | 5085/538740 | 84 | <0. 01 |
| CC | 30 | 0. 76[0. 68, 0. 85] | 14578/180574 | 78 | <0. 01 |
| Geographical region | |||||
| Western population | 15 | 0. 81[0. 70, 0. 94] | 7325/329216 | 79 | <0. 01 |
| Cohort | 6 | 0. 93[0. 74, 1. 16] | 3310/299387 | 83 | <0. 01 |
| CC | 9 | 0. 73[0. 61, 0. 88] | 4015/29829 | 73 | <0. 01 |
| Asian population | 25 | 0. 80[0. 70, 0. 92] | 10630/321441 | 80 | <0. 01 |
| Cohort | 5 | 0. 94[0. 91, 0. 98] | 1416/172637 | 91 | <0. 01 |
| CC | 20 | 0. 96[0. 94, 0. 99] | 9214/148804 | 75 | <0. 01 |
| Sex | |||||
| Male | 11 | 0. 82[0. 64, 1. 05] | 5183/240914 | 90 | <0. 01 |
| Cohort | 4 | 1. 00[0. 61, 1. 61] | 1980/150566 | 94 | <0. 01 |
| CC | 7 | 0. 73[0. 55, 0. 98] | 3203/90348 | 87 | <0. 01 |
| Female | 14 | 0. 80[0. 67, 0. 95] | 4447/304808 | 64 | <0. 01 |
| Cohort | 5 | 0. 93[0. 82, 1. 06] | 1105/228461 | 19 | 0. 29 |
| CC | 8 | 0. 90[0. 82, 0. 97] | 3073/76121 | 28 | 0. 21 |
| Smoking status | |||||
| Smoking | 8 | 0. 80[0. 63, 1. 01] | 3663/32347 | 79 | <0. 01 |
| Cohort | 2 | 0. 67[0. 56, 0. 81] | 969/29801 | 0 | 0. 65 |
| CC | 5 | 0. 85[0. 63, 1. 15] | 2694/2546 | 80 | <0. 01 |
| No-smoking | 8 | 0. 67[0. 51, 0. 89] | 2973/74512 | 81 | <0. 01 |
| Cohort | 1 | 0. 66[0. 53, 0. 83] | 359/60733 | - | - |
| CC | 7 | 0. 63[0. 46, 0. 85] | 2545/3673 | 77 | <0. 01 |
| Study period | |||||
| Before 2000 | 22 | 0. 80[0. 70, 0. 91] | 9660/326269 | 77 | <0. 01 |
| Cohort | 7 | 0. 91[0. 67, 1. 23] | 2761/291876 | 89 | <0. 01 |
| CC | 15 | 0. 76[0. 67, 0. 85] | 6899/34393 | 57 | <0. 01 |
| After 2000 | 15 | 0. 75[0. 64, 0. 89] | 7422/147484 | 81 | <0. 01 |
| Cohorts | 2 | 0. 87[0. 63, 1. 20] | 606/74997 | 66 | <0. 01 |
| CC | 13 | 0. 74[0. 61, 0. 91] | 6457/11754 | 83 | <0. 01 |
CC, case-control study
All subgroup analysis by study design
Type of tea
Green, black or unspecified tea were correlated with protection against lung cancer in the case-control studies. Black tea and tea unknow also showed protective effect in cohort studies (Table 2).
There were no statistical significances in consumption of more than one cup/day black tea and lung cancer. Increasing daily intake of green tea to 7. 5 g increased the protective effect against lung cancer both in case-control studies and Cohort studies (Table 2).
Geographical region
There were obvious differences in the protective effect of tea drinking on lung cancer of Western and Asian countries in different study designs (Table 2).
Gender
Both females and males, tea drinking had a protective effect against lung cancer the case-control studies (Table 2). But no statistically significant association was found in cohort studies.
Study period
In both time periods of studies conducted before 2000 and after 2000, tea drinking showed a protective effect against lung cancer in the case-control studies. But no statistically significant association in cohort studies (Table 2).
Smoking status
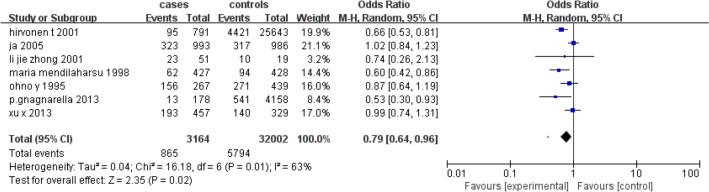
Tea consumption has a protective effect against lung cancer in non-smoking populations. When daily tea intake was greater than 2. 5 g, there was a protective effect of tea drinking on lung cancer in smoking populations (Fig. 4). All studies showed heterogeneity but no publication bias (I2=63%, P=0. 01).
Fig. 4:
Association of between 2. 5g/day tea consumption and risk for lung cancer on smoking status
Discussion
This study showed that tea drinking had some protective effect against lung cancer. Increasing amounts of green tea intake showing a further decrease in lung cancer OR. Black tea also showed a protective effect of against lung cancer, but it didn’t further decrease the OR of lung cancer by increasing the amount. This can be attributed to the differences in the production of the two tea (49). The main active component in green tea, EGCG was present in higher amounts in green tea than black tea. This could explain why increasing black tea consumption didn’t increase its protective effect against lung cancer.
In smoking populations, when increased tea consumption to 2. 5 g/day, it showed a protective effect against lung cancer, which was consistent with previous studies (9). The preventive effect on lung cancer by tea could be due to the presence of polyphenols in tea. Evidence has shown that EGCG can prevent the formation of mutated cells and that EGCG can increase the activity of phase II enzymesin vivo animal studies (54–57). Phase II enzymes are involved in the detoxification of carcinogens that will be subsequently excreted (58). EGCG could induce apoptosis in cells that were damaged by carcinogens in cigarette smoke (59–61). However, smoking is considered as chronic exposure and long-term smoking has a much greater effect on lung cancer risk than just cumulative effects of daily smoking (62). Hence, long-term intake of high EGCG doses is required to reduce the damage caused by tobacco carcinogens. The types of tea involved in this study are complex, and there was no adjustment for amount of smoking, period of smoking, period of tea drinking, etc. Hence, It need for well-designed studies with larger sample sizes and better control of various confounding factors, and the inclusion of intervention and mechanistic studies, in order to more accurately verify the association of lung cancer and different amounts of different tea in smoking populations.
It showed heterogeneity in this study. Subgroup analysis of sex, smoking status, type of tea, intake amounts and other adjustment factors could not reduce the heterogeneity. The study by Kinlen et al. (53) is the source of heterogeneity when study type, region, sex and study period were used as subgroups. This study had a NOS score of 7, with large number of cases and low sensitivity, and removing it from inclusion did not cause any obvious differences in results. Therefore, the random effects model was used for data analysis in this study.
In addition, The combination of results of studies with different designs (case-control and cohort) lead to biased results, the subgroup analysis by study design of tea types (green tea, black tea and tea un-know), geographical region, sex, smoking status, study period and the amount of tea also have shown different. However, cohort study reveals a causal relationship, and case-control cannot, cohort studies are considered preferable to case-control studies in the hierarchy of scientific evidence, and Cohort studies results should play as the standard. Our results showed that significant association existed in case-control studies, but not in cohort studies. The results may be related to the difference of study design types and sample size. Participants in case-control studies were greatly less than participants included in cohort studies.
The results of this meta-analysis were limited by some factors. Firstly, some articles did not specify the type of tea. Secondly, the data from included studies were raw primary data and most studies were retrospective case-control studies that could have possible bias and confounding factors. Lastly, this study included a small number of countries such as China, Japan and the USA, etc., and the representation by these countries requires further verification. Despite these limitations, our study collected all studies published to date on the association of tea drinking and lung cancer for a meta-analysis, and results showed that tea drinking could have protective effect against lung cancer. Increasing the amount of green tea intake to 7. 5 g a day showed an increased protective effect of green tea against lung cancer. Regular intake of one cup of tea or more could antagonize the effects of smoking on lung cancer in smokers. However, larger sample sizes or prospective cohort studies are required for verification of these results and for further mechanistic studies.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
No fund was received for this study.
Footnotes
Conflict of interests
The authors declare that there is no conflict of interests.
References
- 1.Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin, 61(2):69–90. [DOI] [PubMed] [Google Scholar]
- 2.Le Marchand L, Murphy SP, Hankin JH, et al. (2000). Intake off lavonoidsand lung cancer. J Natl Cancer Inst, 92(2):154–60. [DOI] [PubMed] [Google Scholar]
- 3.Nakachi K, Matsuyama S, Miyake S, et al. (2000). Preventive effects of drinking green tea on cancer and cardiovascular disease:epidemiological evidence for multiple targeting prevention. Biofactors, 13(1–4):49–54. [DOI] [PubMed] [Google Scholar]
- 4.Kuriyama S, Shimazu T, Ohmori K, et al. (2006). Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan:the Ohsaki study. JAMA, 296(10):1255–65. [DOI] [PubMed] [Google Scholar]
- 5.Tang N, Wu Y, Zhou B, et al. (2009). Green tea, black tea consumption and risk of lung cancer:a meta-analysis. Lung Cancer, 65(3):274–83. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Zhang X, Liu J, et al. (2014). Tea consumption and lung cancer risk:a meta-analysis of case-control and cohort studies. Nutrition, 30(10):1122–7. [DOI] [PubMed] [Google Scholar]
- 7.Thun MJ, Carter BD, Feskanich D, et al. (2013). 50-year trends in smoking-related mortality in the United States. N Engl J Med, 368(4):351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prescott E, Hippe M, Schnohr P, et al. (1998). Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ, 316(7137):1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.No authors lists (1991). Coffee, tea, mate, methylxanthines and methylglyoxal. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 27 February to 6 March 1990. IARC Monogr Eval Carcinog Risks Hum, 51:1–513. [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrera C, Giménez R, López MC. (2003). Determination of tea components with antioxidant activity. J Agric Food Chem, 51(15):4427–35. [DOI] [PubMed] [Google Scholar]
- 11.Liang W, Binns CW, Jian L, Lee AH. (2007). Does the consumption of green tea reduce the risk of lung cancer among smokers? Evid Based Complement Alternat Med, 4(1):17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedges LV, Pigott TD. (2001). The power of statistical tests in meta-analysis. Psychol Methods, 6(3):203–17. [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ, 315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasquet R, Karp I, Siemiatycki J, et al. (2016). The consumption of coffee and black tea and the risk of lung cancer. Ann Epidemiol, 26(11):757–763. e2. [DOI] [PubMed] [Google Scholar]
- 15.Wu W. (2015). Association of endogenous, exogenous estrogen factors and single nucleotide polymorphisms in estrogen related genes with risk of lung cancer in non-smoking females. China medical university. [Google Scholar]
- 16.Wang Z, Yang K, Wan C, et al. (2015). Association of urine isothiocyanate ester levels with lung cancer: a case-control study. Chin J Public Health, 31(9). [Google Scholar]
- 17.Hashibe M, Galeone C, Buys SS, et al. (2015). Coffee, tea, caffeine intake, and the risk of cancer in the PLCO cohort. Br J Cancer, 113(5):809–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zabłocka-Słowińska K, Porębska I, Gołecki M, et al. (2015). Dietary habits of lung cancer patients from the Lower Silesia region of Poland. Contemp Oncol (Pozn), 19(5):391–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bao L. (2014). Clinical analysis of 50 cases of lung cancer. Chinese Journal of Practical Medicine, 41(24) [In Chinese). [Google Scholar]
- 20.Phukan RK, Saikia BJ, Borah PK, et al. (2014). Role of household exposure, dietary habits and glutathione S-Transferases M1, T1 polymorphisms in susceptibility to lung cancer among women in Mizoram India. Asian Pac J Cancer Prev, 15(7):3253–60. [DOI] [PubMed] [Google Scholar]
- 21.Xu X. (2013). A case-control study on tea consumption and the risk of lung cancer. Wei Sheng Yan Jiu, 42:211–6. [PubMed] [Google Scholar]
- 22.Gnagnarella P, Maisonneuve P, Bellomi M, et al. (2013). Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol, 24(10):2606–11. [DOI] [PubMed] [Google Scholar]
- 23.Takata Y, Xiang YB, Yang G, et al. (2013). Intakes of fruits, vegetables, and related vitamins and lung cancer risk: results from the Shanghai Men’s Health Study (2002–2009). Nutr Cancer, 65(1):51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin ZY, Wu M, Han RQ, et al. (2013). Raw garlic consumption as a protective factor for lung cancer, a population-based case-control study in a Chinese population. Cancer Prev Res (Phila), 6(7):711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takata Y, Shu XO, Yang G, et al. (2013). Calcium intake and lung cancer risk among female nonsmokers: a report from the Shanghai Women’s Health Study. Cancer Epidemiol Biomarkers Prev, 22(1):50–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin IH, Ho ML, Chen HY, et al. (2012). Smoking, green tea consumption, genetic polymorphisms in the insulin-like growth factors and lung cancer risk. PLoS One, 7:e30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H. (2012). Population attributable risk estimation of risk factors for lung cancer in urban Shanghai. Unpublished master’s thesis. Shanghai, China: Fudan University. [Google Scholar]
- 28.Ganesh B, Sushama S, Monika S, Suvarna P. (2011). A case-control study of risk factors for lung cancer in Mumbai, India. Asian Pac J Cancer Prev, 12(2):357–62. [PubMed] [Google Scholar]
- 29.Wang L, Lee IM, Zhang SM, et al. (2009). Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am J Clin Nutr, 89:905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han RQ, Zhao JK, Liu AM. (2008). The effect of green tea and its possible interactions with relevant factors on lung cancer in Dafeng county, Jiangsu province, China. Acta Universitatis Medicinalis Nanjing, 3:354–9. [Google Scholar]
- 31.Zhang K, Wang J, Qu Z, et al. (2008). A case-control study on risk factors of lung cancer in Tianning District. Chin Cancer, 17:567–9. [Google Scholar]
- 32.Li Q, Kakizaki M, Kuriyama S, et al. (2008). Green tea consumption and lung cancer risk:the Ohsaki study. Br J Cancer, 99:1179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JY, Zhu L, Wang XS. (2008). A case-control study on risk factors for common cancer in low incidence area of Jiangsu province, China. Chin Cancer, 17(1):3–5. [Google Scholar]
- 34.Cui Y, Morgenstern H, Greenland S, et al. (2008). Dietary flavonoid intake and lung cancerda population-based case-control study. Cancer, 112:2241–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao WH, Jin YT, Yu ZC, et al. (2007). The effects of CYP1A1 gene polymorphism and Pl-6 gene methylation on the risk of lung cancer. Acta Univ Med Anhui, 42(1):62–66. [Google Scholar]
- 36.Bonner MR, Rothman N, Mumford JL, et al. (2005). Green tea consumption, genetic susceptibility, PAH-rich smoky coal, and the risk of lung cancer. Mutat Res, 582(1–2):53–60. [DOI] [PubMed] [Google Scholar]
- 37.Baker JA, McCann SE, Reid ME, et al. (2005). Associations between black tea and coffee consumption and risk of lung cancer among current and former smokers. Nutr Cancer, 52:15–21. [DOI] [PubMed] [Google Scholar]
- 38.Khan MM, Goto R, Kobayashi K, et al. (2004). Dietary habits and cancer mortality among middle aged and older Japanese living in hokkaido, Japan by cancer site and sex. Asian Pac J Cancer Prev, 5:58–65. [PubMed] [Google Scholar]
- 39.Hu JF, Mao Y, Dryer D, et al. (2002). Canadian Cancer Registries Epidemiology Research Group. Risk factors for lung cancer among Canadian women who have never smoked. Cancer Detect Prev, 26:129–38. [DOI] [PubMed] [Google Scholar]
- 40.Zhong L, Goldberg MS, Gao YT, et al. (2001). A populationbased case-control study of lung cancer and green tea consumption among women living in Shanghai, China. Epidemiology, 12:695–700. [DOI] [PubMed] [Google Scholar]
- 41.Nagano J, Kono S, Preston DL, et al. (2001). A prospective study of green tea consumption and cancer incidence, Hiroshima and Nagasaki. Cancer Causes Control, 12:501–8. [DOI] [PubMed] [Google Scholar]
- 42.Hirvonen T, Virtamo J, Korhonen P, et al. (2001). Flavonol and flavone intake and the risk of cancer in male smokers. Cancer Causes Control, 12:789–96. [DOI] [PubMed] [Google Scholar]
- 43.Nyberg F, Agrenius V, Svartengren K. (1998). Dietary factors and risk of lung cancer in never-smokers. Int J Cancer, 78:430–6. [DOI] [PubMed] [Google Scholar]
- 44.Mendilaharsu M, De Stefani E, Deneo-Pellegrini H. (1998). Consumption of tea and coffee and the risk of lung cancer in cigarettesmoking men: a case-control study in Uruguay. Lung Cancer, 19:101–7. [DOI] [PubMed] [Google Scholar]
- 45.Ko YC, Lee CH, Chen MJ, et al. (1997). Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol, 26:24–31. [DOI] [PubMed] [Google Scholar]
- 46.Goldbohm RA, Hertog MG, Brants HA, et al. (1996). Consumption of black tea and cancer risk: a prospective cohort study. J Natl Cancer Inst, 88:93–100. [DOI] [PubMed] [Google Scholar]
- 47.Axelsson G, Liljeqvist T, Andersson L, et al. (1996). Dietary factors and lung cancer among men in west Sweden. Int J Epidemiol, 25:32–9. [DOI] [PubMed] [Google Scholar]
- 48.Zheng W, Doyle TJ, Kushi LH, et al. (1996). Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol, 144:175–82. [DOI] [PubMed] [Google Scholar]
- 49.Xu ZY, Brown LM, Pan GW, et al. (1996). Cancer risks among iron and steel workers in Anshan:Case-control studies of lung and stomach cancer. Am J Ind Med, 30:7–15. [DOI] [PubMed] [Google Scholar]
- 50.Ohno Y, Wakai K, Genka K, et al. (1995). Tea consumption and lung cancer risk:a case-control study in Okinawa, Japan. Jpn J Cancer Res, 86:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tewes FJ, Koo LC, Meisgen TJ, Rylander R. (1990). Lung cancer risk and mutagenicity of tea. Environ Res, 52:23–33. [DOI] [PubMed] [Google Scholar]
- 52.Mettlin C. (1989). Milk drinking, other beverage habits, and lung cancer risk. Int J Cancer, 43:608–12. [DOI] [PubMed] [Google Scholar]
- 53.Kinlen LJ, Willows AN, Goldblatt P, Yudkin J. (1988). Tea consumption and cancer. Br J Cancer, 58:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hecht SS. (1999). Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst, 91:1194–210. [DOI] [PubMed] [Google Scholar]
- 55.Katiyar SK, Mukhtar H. (1997). Tea antioxidants in cancer chemoprevention. J Cell Biochem Suppl, 27:59–67. [PubMed] [Google Scholar]
- 56.Khan SG, Katiyar SK, Agarwal R, et al. (1992). Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice:possible role in cancer chemoprevention. Cancer Res, 52:4050–2. [PubMed] [Google Scholar]
- 57.Ahmad N, Mukhtar H. (1999). Green tea polyphenols and cancer: biologic mechanisms and practical implications. Nutr Rev, 57:78–83. [DOI] [PubMed] [Google Scholar]
- 58.Hakim IA, Harris RB, Brown S, et al. (2003). Effect of increased tea consumption on oxidative DNA damage among smokers:a randomized controlled study. J Nutr, 133:3303S–3309S. [DOI] [PubMed] [Google Scholar]
- 59.Schwartz JL, Baker V, Larios E, et al. (2005). Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res, 49:43–51. [DOI] [PubMed] [Google Scholar]
- 60.Liao J, Yang GY, Park ES, et al. (2004). Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer, 48:44–53. [DOI] [PubMed] [Google Scholar]
- 61.Yang CS, Chung JY, Yang G, et al. (2000). Tea and tea polyphenols in cancer prevention. J Nutr, 130(2S Suppl):472S–478S. [DOI] [PubMed] [Google Scholar]
- 62.Alberg AJ, Samet JM. (2003). Epidemiology of lung cancer. Chest, 123(1 Suppl):21S–49S. [DOI] [PubMed] [Google Scholar]