Abstract
Background:
Clostridium difficile is the most common causes of hospital-acquired diarrhea affecting particularly hospitalized patients globally. This organism has re-emerged in recent years with significant morbidity and mortality. The present study aimed to estimate the burden of C. difficile infection (CDI) and to acquire information on the overall rates of community- and hospital-acquired CDI in western Asia.
Methods:
A systematic literature search was performed to identify articles published from the eight Persian Gulf countries in western Asia including Iran, Iraq, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates in the electronic databases within Jan of 2000 to Dec of 2017. Then, 20 publications which met our inclusion criteria were selected for data extraction and analysis by Comprehensive Meta-Analysis Software.
Results:
Twenty studies reported the prevalence of toxigenic strains of C. difficile among patients from Persian Gulf countries, of these the pooled prevalence of CDI was 9% (95% CI: 6.5%–12.5%). Totally, 8 studies showed the prevalence of hospital-acquired CDI, from those studies the prevalence of CDI was estimated 8.4% (95% CI: 4.9%–14.1%). Moreover, 7 studies reported the prevalence of community-acquired CDI, from those studies the prevalence of CDI was estimated 1.8% (95% CI: 1.2%–2.9%).
Conclusion:
The prevalence of CDI in western Asia is lower than southern and eastern region. Moreover, the lower prevalence of community-acquired CDI compared to hospital-acquired CDI, indicate that the source of infection in western Asia is more likely in the hospitals.
Keywords: Clostridium difficile infection (CDI), Western Asia, Infection control, Meta-analysis
Introduction
Hospital-acquired infections (HAIs) are a serious public health concern resulting in prolonged hospitalization and risk of death (1). The occurrence of HAIs according to the WHO estimates is around 7.1 million cases every year (2). Among the wide range of bacteria has been reported as a cause of HAIs, vancomycin-resistant enterococci (VRE), methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacteriaceae are the most prevalent pathogens (3, 4).
Clostridium difficile is the most common cause of hospital-acquired diarrhea affecting particularly hospitalized patients globally (5). This organism has re-emerged in recent years with apparent greater morbidity and mortality and also associated with increased health care costs (6). Factors contributing to the development of this phenomenon include usage of broad-spectrum antibiotics such as fluoroquinolones, clindamycin and third generation cephalosporin’s as well as prolonged hospitalization, antineoplastic chemotherapy, and severe underlying diseases (7, 8). Treatment of C. difficile infection (CDI) is challenging and demand new approaches because most of antibiotics used for the treatment of every kind of infections can potentially encourage CDI (9, 10). The currently available antibiotics that remain the first-line therapy for CDI are metronidazole and vancomycin (11, 12).
Antibiotic-associated diarrhea is mostly linked to strains of C. difficile that produce a range of virulence factors including toxins and adherence factors (13). Toxin A (TcdA) and toxin B (TcdB) are two homologous exotoxin and main virulence factors of toxigenic C. difficile that encoded by the tcdA and tcdB genes, respectively. Expression of these toxins causing proinflammatory and cytotoxic effects including disruption of the actin cytoskeleton and impairment of tight junctions in human intestinal epithelial cells that are responsible for the clinical symptoms of CDI (13).
The incidence of CDIs is an important quality indicator to reflect the effectiveness of the basic hospital policies include infection control and antimicrobial stewardship. The objectives of the present study were to estimate the burden of CDI and to acquire information on the overall rates of community- and hospital-acquired CDI in western Asia. Results from this survey indicate progress across Persian Gulf countries towards comprehensive monitoring and reporting of CDI.
Methods
Search strategies
A systematic literature search was performed to identify papers published from the eight Persian Gulf countries in western Asia including Iran, Iraq, Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, and the United Arab Emirates in the Web of Science, PubMed, Scopus, and Google Scholar electronic databases within Jan of 2000 to Dec of 2017. The keywords and terms were searched using Medical Subject Headings (MeSH) such as “Clostridium difficile” or “Clostridioides difficile” or “C. difficile” or “Clostridium difficile infection (CDI)” or “Pseudomembranous colitis” in combination with “Names of countries” in the title, abstract and keywords fields.
Selection criteria
Two reviewers independently screened the search results at the databases with the related keywords and analysis the titles, abstracts, and full texts to applied eligibility for inclusion according to inclusion criteria, and the disagreement between reviewers was resolved by consensus. English and Persian or Arabian language articles with English abstract indexed in PubMed, Scopus or Web of Science with the following criteria were considered in our study: 1) Clearly mentioned to method used for C. difficile and toxins detection; 2) cross-sectional or retrospective studies investigating the prevalence of toxigenic C. difficile collected from diarrhea samples. Meanwhile, exclusion criteria were: 1) studies that did not report a standardized method for detection of C. difficile and toxins; 2) studies with sample size was less than 10 isolates; 3) studies that origin of samples was unclear or isolates obtained from formed stool or environment sources, and 4) studies which focused on non-toxigenic C. difficile or the prevalence of toxigenic strains was unclear. Furthermore, reviews and systematic review articles, case reports, and articles which were only available in abstract form were ignored.
Definition
According to the European Center for Disease Prevention and Control (ECDC), an episode of CDI was defined as a patient with diarrhea whose stool takes the shape of the container, and it is positive for C. difficile toxin A and/ or B without other etiology (14).
Quality assessment
The quality of eligible studies was judged independently by two authors using the STROBE checklist (Strengthening the Reporting of Observational Studies in Epidemiology). Items related title and abstract, introduction, methods, results, discussion, and other information were determined and a score was assigned to each item. One score was assigned to each question and studies achieved at least eight quality scores were considered eligible and included in the study (15).
Data extraction
For all selected studies, the following details were extracted: the first author’s name, the study performing time, publication date, research location, sample type, patients age range, nature of patients (hospitalized or outpatient), toxins detection methods, primary sample size, the frequency of toxigenic C. difficile, source of infection, origin of infection, and proportion of toxigenic strains in each gender.
Statistical analysis
Analysis of data was performed using Comprehensive Meta-Analysis Software Ver. 2.2 (BioStat Company). Meta-analysis was performed using random-effects model to estimate the pooled prevalence and corresponding 95% confidence interval (CI). Statistical heterogeneity between and within groups was determined using Cochran’s Q statistic and the I2 index. The funnel plot, Begg’s rank correlation test, and Egger’s weighted regression tests were used to evaluate possible publication bias (P<0.05 is indicative of publication bias).
The present study designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
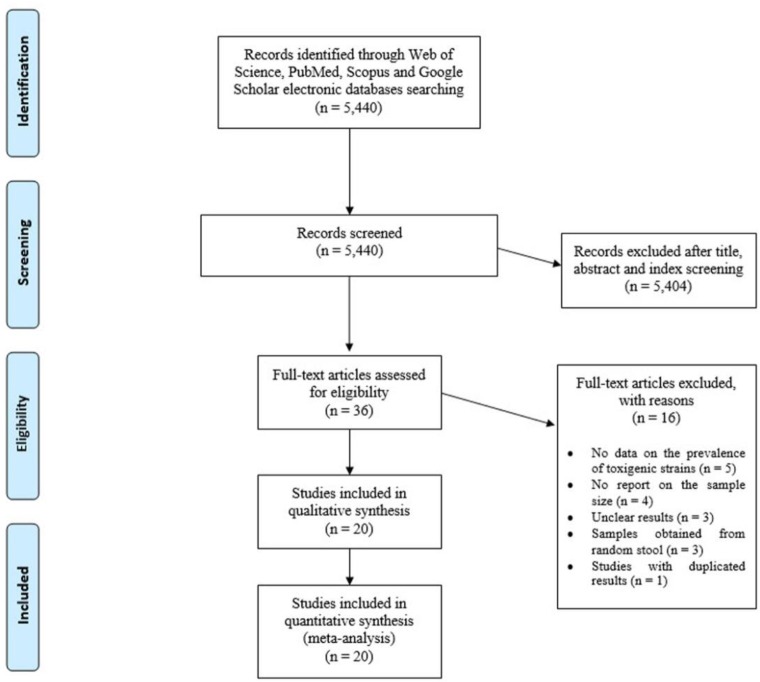
Initially, 5440 citations were yielded from the database search. Among them, 5404 were excluded on the initial screening of the index, title and abstract and 36 were reviewed in full text. Of 36 full text reviewed articles, five studies did not report the prevalence of toxigenic strains of C. difficile, four studies did not report primary samples size, three studies had unclear results, three studies performed on random stools, and results of one study duplicated in their recent study. Finally, 20 studies were eligible for inclusion and were subjected to meta-analysis. A flowchart of the literature search, the selection procedures and reasons for exclusion are presented in Fig. 1. The characteristics of the included studies in the meta-analysis are available in Table 1.
Fig. 1:
Flow chart of the literature search strategy and study selection
Table 1:
Characteristics of studies included in the meta-analysis
| First author | Publication year | Preformed time | Country | Sample type | Age range | Hospitalized (H) or outpatient (OP) | Detection method | Sample size | Toxigenic C. difficile No. | HA/CA No. | M/F No. | Ref |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sadeghifard | 2010 | 2002–2006 | Iran | Diarrheal stool | No limit | H | Cytotoxicity assay | 942 | 57 | - | 30/27 | (16) |
| Nazemalhosseini-Mojarad | 2011 | UN | Iran | Diarrheal stool | No limit | H and OP | ELISA | 356 | 19 | - | 13/6 | (17) |
| Nasri | 2012 | 2009–2010 | Iran | Diarrheal stool | No limit | H | ELISA | 162 | 36 | - | 24/12 | (18) |
| Jalali | 2012 | 2010–2011 | Iran | Diarrheal stool | No limit | H | Molecular | 86 | 17 | 13/4 | 9/8 | (19) |
| Goudarzi | 2013 | 2010–2011 | Iran | Diarrheal stool | No limit | H | Molecular | 350 | 75 | - | 39/36 | (20) |
| Farshad | 2013 | 2012 | Iran | Nosocomial diarrhea | No limit | H | Cytotoxicity assay and ELISA | 122 | 9 | 9/- | 5/4 | (21) |
| Azizi | 2013 | 2010 | Iran | Nosocomial diarrhea | No limit | H | Molecular | 98 | 15 | - | - | (22) |
| Alinejad | 2015 | 2013–2014 | Iran | Nosocomial diarrhea | 6–60 months | H | ELISA | 38 | 8 | 8/- | 6/2 | (23) |
| Rezazadeh Zarandi | 2017 | 2014–2015 | Iran | Diarrheal stool | No limit | H | Molecular and ELISA | 233 | 11 | - | - | (24) |
| Azimirad | 2017 | 2011–2012 | Iran | Diarrheal stool | No limit | H | Molecular | 105 | 18 | - | 9/10 | (25) |
| Sandokji | 2009 | 2007–2008 | Saudi Arabia | Diarrheal stool | >2 yr | H | ELISA | 258 | 56 | 56/- | - | (26) |
| Al-Tawfiq | 2010 | 2007–2008 | Saudi Arabia | Diarrheal stool | No limit | H and OP | ELISA | 913 | 42 | 26/16 | 23/19 | (27) |
| AL-Eidan | 2013 | 2011 | Saudi Arabia | Diarrheal stool | Adult | H | ELISA | 2927 | 171 | 98/73 | - | (28) |
| Senok | 2017 | 2014–2015 | Saudi Arabia | Diarrheal stool | No limit | H and OP | Molecular | 210 | 31 | - | - | (29) |
| Jamal | 2010 | 2003–2005 | Kuwait | Diarrheal stool | No limit | H and OP | ELISA | 697 | 73 | 56/17 | - | (30) |
| Jamal | 2014 | 2012 | Kuwait | Diarrheal stool | >2 yr | OP | Molecular | 409 | 13 | -/13 | - | (31) |
| Jamal | 2015 | 2011–2013 | Kuwait | Diarrheal stool | >2 yr | OP | ELISA | 2548 | 16 | -/16 | - | (32) |
| Albert | 2016 | 2014–2015 | Kuwait | Diarrheal stool | No limit | H | Molecular | 109 | 3 | - | - | (33) |
| Alrifai | 2009 | 2004–2005 | Iraq | Nosocomial diarrhea | 1–60 months | H | ELISA | 81 | 17 | - | (34) | |
| Al-Thani | 2014 | 2011–2012 | Qatar | Diarrheal stool | >1 yr | H and OP | EIA and Molecular | 1,532 | 122 | 98/14 | 72/50 | (35) |
Abbreviations: ELISA: enzyme-linked immunosorbent assay; EIA: Enzyme Immunoassay; HA: hospital-acquired; CA: community-acquired; M: male; F: female.
Of the total number of included studies, 10 of which were from Iran (16–25), four from Saudi Arabia (26–29), four from Kuwait (30–33), and one for each of Iraq (34), and Qatar countries (35). Totally, 13 studies conducted only among hospitalized patients, five studies contained both hospitalized and outpatients and two studies enrolled only outpatients.
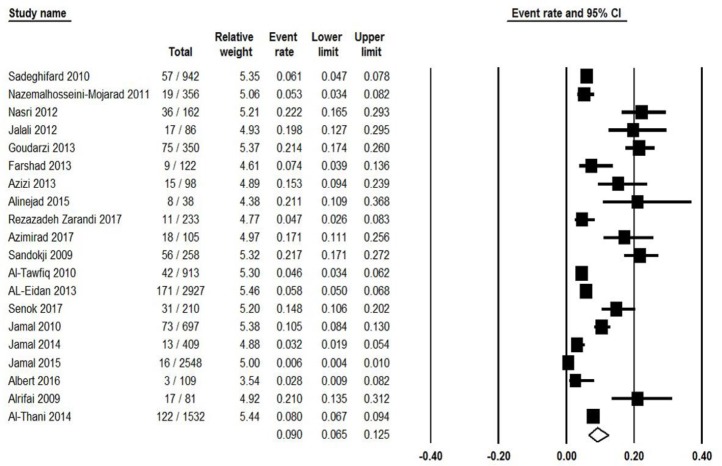
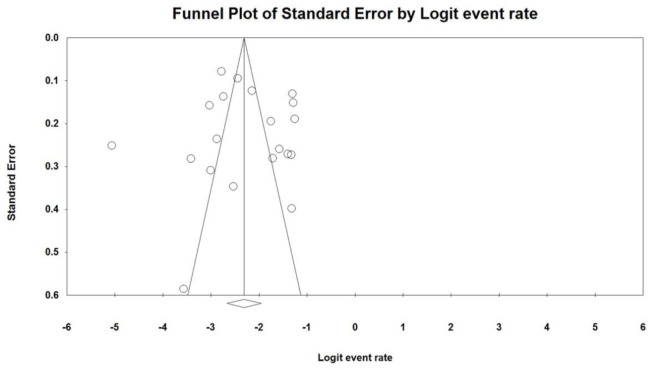
Twenty studies reported the prevalence of toxigenic strains of C. difficile among patients from Persian Gulf countries, of these the pooled prevalence of CDI was 9% (95% CI: 6.5%–12.5%) ranging from 0.6% to 22.2% (Fig. 2). There was a significant heterogeneity among the included studies (χ2 = 410.893; P<0.001; I2 =95.4%). Moreover, a symmetric funnel plot of the included studies showed no evidence of publication bias (Fig. 3). Additionally, Begg’s and Egger’s tests were performed to quantitatively evaluate the probable publication bias among studies. According to the results of Begg’s test (z=0.097, P=0.92) and Egger’s test (t=0.34, P=0.74), no evidence of publication bias was observed.
Fig. 2:
Forest plot of the pooled prevalence of CDI in western Asia
Fig. 3:
Funnel plot of meta-analysis on CDI in western Asia
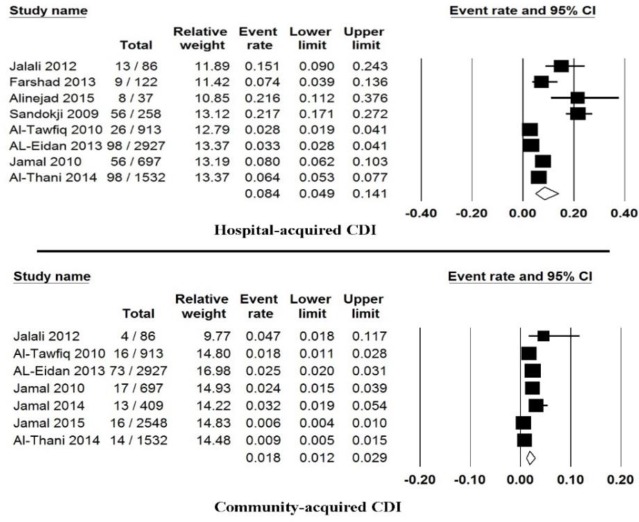
Totally, eight studies showed the prevalence of hospital-acquired CDI, from those studies the prevalence of CDI was estimated 8.4% (95% CI: 4.9%–14.1%) ranging from 2.8% to 21.7% (Fig. 4).
Fig. 4:
Forest plot of the pooled prevalence of community- or hospital-acquired
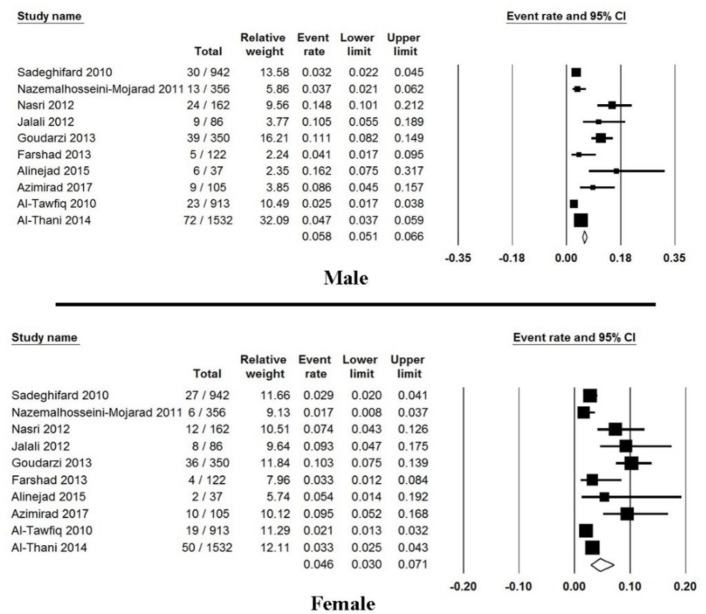
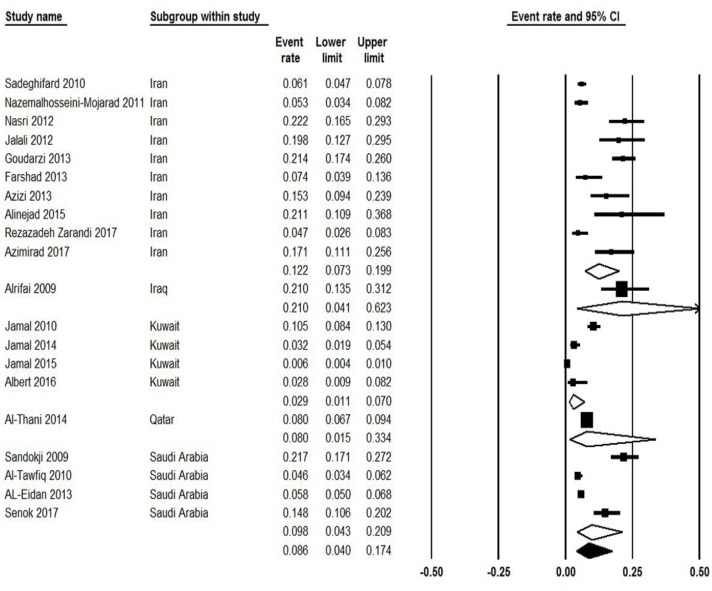
Moreover, seven studies reported the prevalence of community-acquired CDI, from those studies the prevalence of CDI was estimated 1.8% (95% CI: 1.2%–2.9%) ranging from 0.6% to 4.7% (Fig. 4). Ten studies showed the incidence of CDI regarding gender, from those studies the occurrence of CDI among male and female patients was estimated 6% (95% CI: 4%–9%) and 4.6% (95% CI: 3.0%–7.1%), respectively (Fig. 5). Finally, subgroups analysis between countries were done and results were presented in Fig. 6.
Fig. 5:
Forest plot of the pooled prevalence of male and female patients with CDI
Fig. 6:
Forest plot of subgroups analysis of the pooled prevalence of CDI between countries in western Asia
Discussion
The emergence of the hypervirulent C. difficile strains increase the prevalence and severity of CDI; however, epidemic strain of NAP1/BI/027 is not common in our region and is a public health problem for other countries, mainly western countries (36). Thus, it is essential to gain a close estimation of the burden of CDI for the development of effective healthcare practice. To the best of our knowledge, the present study is the largest comprehensive survey to date estimated the pooled prevalence of CDI 9% from the Persian Gulf region. Moreover, the prevalence of CDI differed greatly between studied countries and even hospitals in the same country ranging from 0.6% to 22.2%. Despite the discrepancy in literature, our findings are consistent with the median values reported in previous studies. In this regard, the prevalence of CDI in South and East Asia were reported 10.9% in India (37), 14% in China (38), and 14.3% in South-Korea (39). These reports, accompanied by results from a meta-analysis study that indicated the prevalence of CDI in Eastern Asia is higher than other parts (40). In American countries, this rate was reported 8% in Brazil (41), and 13.7% in the USA (42). While a hospital-based survey within 34 European countries showed much more discrepancy in the prevalence of CDI ranging from 0% in Luxembourg to 39% in Poland (43). These variations might be due to differences in predominant epidemic strains, geographical distribution, studied population or the sensitivity of detection methods.
In the present study, from those studies that reported the origin of infection, the prevalence of hospital-acquired CDI was estimated at 8.4%, while the prevalence of community-acquired CDI was 1.8%. International estimation of the burden of community- or hospital-acquired CDI is challenging since in most of the studies the origin of infection was not investigated. Comparison with available reports indicate that the prevalence of community-acquired CDI in our study (1.8%) is closest to reports from Brazil (1%) (41), South-Korea (1.6%) (39), and Europe (∼2%) (43), whereas it is lower than report from China (8%) (44).
The strengths of this systematic review based study were the large number of patients from the major countries of Middle East with a study design adherence to international guidelines for the estimating burden of CDI. Based on the ECDC experiences, continuous or periodical surveillance supplemented with epidemiological and microbiological data is a practical strategy for monitoring CDI (45). Meanwhile, increasing the national coverage of hospital-based CDI surveillance will improve the national estimates of the CDI burden (43, 45).
As the main limitation of the present study, our report was not representative of the actual rate in the Persian Gulf region. Since our estimation was based on published data, so the burden of infection would be expected to be different if a network of data from more hospitals and laboratories in this region were available. Moreover, differences in the severity of illness of patients or antibiotics prescription might be an effect on reporting rates (43).
Conclusion
The results of the present study provide good epidemiological information about the distribution of CDI in the Persian Gulf region in western Asia. The prevalence of CDI in western Asia is lower than southern and eastern region. Moreover, the lower prevalence of community-acquired CDI compared to hospital-acquired CDI, indicate that the source of infection in western Asia is more likely in the hospitals. These findings highlighting the importance of active surveillance, and the demand for improving infection control policy in reducing the risk of CDI in hospitals.
Ethical consideration
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
None declared.
Footnotes
Funding support
Self-funding.
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Revelas A. (2012). Healthcare-associated infections: A public health problem. Niger Med J, 53(2):59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarani H, Balouchi A, Masinaeinezhad N, Ebrahimitabas E. (2015). Knowledge, Attitude and Practice of Nurses about Standard Precautions for Hospital-Acquired Infection in Teaching Hospitals Affiliated to Zabol University of Medical Sciences (2014). Glob J Health Sci, 8(3):193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dancer SJ. (2014). Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev, 27(4):665–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asadian M, Sadeghi J, Rastegar Lari A, et al. (2016). Antimicrobial resistance pattern and genetic correlation in Enterococcus faecium isolated from healthy volunteers. Microb Pathog, 92:54–9. [DOI] [PubMed] [Google Scholar]
- 5.Heinlen L, Ballard JD. (2010). Clostridium difficile infection. Am J Med Sci, 340(3):247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Depestel DD, Aronoff DM. (2013). Epidemiology of Clostridium difficile infection. J Pharm Pract, 26(5):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bignardi GE. (1998). Risk factors for Clostridium difficile infection. J Hosp Infect, 40(1):1–15. [DOI] [PubMed] [Google Scholar]
- 8.Surawicz CM. (2015). Clostridium difficile infection: risk factors, diagnosis and management. Curr Treat Options Gastroenterol, 13(1):121–9. [DOI] [PubMed] [Google Scholar]
- 9.Sedigh Ebrahim-Saraie H, Heidari H, Amanati A, et al. (2018). A multicenter-based study on epidemiology, antibiotic susceptibility and risk factors of toxigenic Clostridium difficile in hospitalized patients in southwestern Iran. Infez Med, 26(4):308–15. [PubMed] [Google Scholar]
- 10.Khan FY, Abu-Khattab M, Anand D, et al. (2012). Epidemiological features of Clostridium difficile infection among inpatients at Hamad General Hospital in the state of Qatar, 2006–2009. Travel Med Infect Dis, 10(4):179–85. [DOI] [PubMed] [Google Scholar]
- 11.Nasiri MJ, Goudarzi M, Hajikhani B, et al. (2018). Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: A systematic review and meta-analysis. Anaerobe, 50:32–7. [DOI] [PubMed] [Google Scholar]
- 12.Di X, Bai N, Zhang X, et al. (2015). A meta-analysis of metronidazole and vancomycin for the treatment of Clostridium difficile infection, stratified by disease severity. Braz J Infect Dis, 19(4):339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Bella S, Ascenzi P, Siarakas S, et al. (2016). Clostridium difficile Toxins A and B: Insights into Pathogenic Properties and Extraintestinal Effects. Toxins (Basel), 8(5):E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamal WY, Rotimi VO. (2016). Surveillance of Antibiotic Resistance among Hospital- and Community-Acquired Toxigenic Clostridium difficile Isolates over 5-Year Period in Kuwait. PLoS One, 11(8):e0161411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bialvaei AZ, Kouhsari E, Salehi-Abargouei A, et al. (2017). Epidemiology of multidrug-resistant Acinetobacter baumannii strains in Iran: a systematic review and meta-analysis. J Chemother, 29(6):327–37. [DOI] [PubMed] [Google Scholar]
- 16.Sadeghifard N, Salari MH, Ghassemi MR, et al. (2010). The incidence of nosocomial toxigenic clostridium difficile associated diarrhea in Tehran tertiary medical centers. Acta Med Iran, 48(5):320–5. [PubMed] [Google Scholar]
- 17.Nazemalhosseini-Mojarad E, Azimirad M, Razaghi M, et al. (2011). Frequency of Clostridium difficile among patients with gastrointestinal complaints. Gastroenterol Hepatol Bed Bench, 4(4):210–3. [PMC free article] [PubMed] [Google Scholar]
- 18.Nasri MR, Khorvash F, Zolfaghari MR, Mobasherizadeh S. (2012). The relative frequency of clostridium difficile in fecal samples of hospitalized patients with diarrhea by elisa method. J Isfahan Med School, 29(167):2376–82. [Google Scholar]
- 19.Jalali M, Khorvash F, Warriner K, Weese JS. (2012). Clostridium difficile infection in an Iranian hospital. BMC Res Notes, 5:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goudarzi M, Goudarzi H, Alebouyeh M, et al. (2013). Antimicrobial susceptibility of clostridium difficile clinical isolates in iran. Iran Red Crescent Med J, 15(8):704–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farshad S, Azami M, Pouladfar G, et al. (2013). Prevalence and risk factors of Clostridium difficile-Associated diarrhea in Iranian hospitalized patients. Ann Trop Med Public Health 6(5):554–8. [Google Scholar]
- 22.Azizi O, Aslani MM, Azimi Rad M, et al. (2013). The frequency of toxigenic strains of Clostridium difficile in hospitalized patients with diarrhea in Tehran/Iran by PCR method, 2010. J Kerman Univ Med Sci, 20(2):129–37. [Google Scholar]
- 23.Alinejad F, Barati M, Satarzadeh Tabrisi M, Saberi M. (2015). Hospital acquired diarrhea in a burn center of Tehran. Iran J Microbiol, 7(6):310–4. [PMC free article] [PubMed] [Google Scholar]
- 24.Rezazadeh Zarandi E, Mansouri S, Nakhaee N, et al. (2017). Frequency of antibiotic associated diarrhea caused by Clostridium difficile among hospitalized patients in intensive care unit, Kerman, Iran. Gastroenterol Hepatol Bed Bench, 10(3):229–34. [PMC free article] [PubMed] [Google Scholar]
- 25.Azimirad M, Krutova M, Nyc O, et al. (2017). Molecular typing of Clostridium difficile isolates cultured from patient stool samples and gastroenterological medical devices in a single Iranian hospital. Anaerobe, 47:125–8. [DOI] [PubMed] [Google Scholar]
- 26.Sandokji AM, Murshid KR, El-Badry AA, et al. (2009). Infectious nosocomial diarrhea in the surgical wards: Role of parasites and microbes imply stool analysis. J Taibah Univ Med Sci, 4(1):73–81. [Google Scholar]
- 27.Al-Tawfiq JA, Abed MS. (2010). Clostridium difficile-associated disease among patients in Dhahran, Saudi Arabia. Travel Med Infect Dis, 8(6):373–6. [DOI] [PubMed] [Google Scholar]
- 28.Al-Eidan FA. (2013). Proton pump inhibitors and the increased risk of Clostridium difficile infections: A case-control study. Int J Pharma Bio Sci, 4(2):B735–41. [Google Scholar]
- 29.Senok AC, Aldosari KM, Alowaisheq RA, et al. (2017). Detection of clostridium difficile antigen and toxin in stool specimens: Comparison of the C. difficile quik chek complete enzyme immunoassay and GeneXpert C. difficile polymerase chain reaction assay. Saudi J Gastroenterol, 23(4):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jamal W, Rotimi VO, Brazier J, Duerden BI. (2010). Analysis of prevalence, risk factors and molecular epidemiology of Clostridium difficile infection in Kuwait over a 3-year period. Anaerobe, 16(6):560–5. [DOI] [PubMed] [Google Scholar]
- 31.Jamal W, Pauline EM, Rotimi VO. (2014). Comparative performance of the GeneXpert C. difficile PCR assay and C. diff Quik Chek Complete kit assay for detection of Clostridium difficile antigen and toxins in symptomatic community-onset infections. Int J Infect Dis, 29:244–8. [DOI] [PubMed] [Google Scholar]
- 32.Jamal W, Pauline E, Rotimi V. (2015). A prospective study of community-associated Clostridium difficile infection in Kuwait: Epidemiology and ribotypes. Anaerobe, 35(Pt B):28–32. [DOI] [PubMed] [Google Scholar]
- 33.Albert MJ, Rotimi VO, Iqbal J, Chehadeh W. (2016). Evaluation of the xTAG Gastrointestinal Pathogen Panel Assay for the Detection of Enteric Pathogens in Kuwait. Med Princ Pract, 25(5):472–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alrifai SB, Alsaadi A, Mahmood YA, et al. (2009). Prevalence and etiology of nosocomial diarrhoea in children < 5 years in Tikrit teaching hospital. East Mediterr Health J, 15(5):1111–8. [PubMed] [Google Scholar]
- 35.Al-Thani AA, Hamdi WS, Al-Ansari NA, et al. (2014). Polymerase chain reaction ribotyping of Clostridium difficile isolates in Qatar: a hospital-based study. BMC Infect Dis, 14:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quesada-Gomez C, Lopez-Urena D, Acuna-Amador L, et al. (2015). Emergence of an outbreak-associated Clostridium difficile variant with increased virulence. J Clin Microbiol, 53(4):1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaishnavi C, Singh M, Mahmood S, Kochhar R. (2015). Prevalence and molecular types of Clostridium difficile isolates from faecal specimens of patients in a tertiary care centre. J Med Microbiol, 64(11):1297–304. [DOI] [PubMed] [Google Scholar]
- 38.Tang C, Cui L, Xu Y, et al. (2016). The incidence and drug resistance of Clostridium difficile infection in Mainland China: a systematic review and meta-analysis. Sci Rep, 6:37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon SS, Gim JL, Kim MS, et al. (2017). Clinical and molecular characteristics of community-acquired Clostridium difficile infections in comparison with those of hospital-acquired C. difficile. Anaerobe, 48:42–6. [DOI] [PubMed] [Google Scholar]
- 40.Borren NZ, Ghadermarzi S, Hutfless S, Ananthakrishnan AN. (2017). The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PLoS One, 12(5):e0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pires RN, Monteiro AA, Carneiro LC, et al. (2014). Clostridium difficile infection in Brazil: a neglected problem? Am J Infect Control, 42(4):459–60. [DOI] [PubMed] [Google Scholar]
- 42.Kilic A, Alam MJ, Tisdel NL, et al. (2015). Multiplex Real-Time PCR Method for Simultaneous Identification and Toxigenic Type Characterization of Clostridium difficile From Stool Samples. Ann Lab Med, 35(3):306–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bauer MP, Notermans DW, van Benthem BH, et al. (2011). Clostridium difficile infection in Europe: a hospital-based survey. Lancet, 377(9759):63–73. [DOI] [PubMed] [Google Scholar]
- 44.Zhang D, Chen J, Zhan H, et al. (2016). Clostridium difficile-associated clinical burden from lack of diagnostic testing in a Chinese tertiary hospital. J Hosp Infect:S0195-6701(16)30435-2. [DOI] [PubMed] [Google Scholar]
- 45.van Dorp SM, Kinross P, Gastmeier P, et al. (2016). Standardised surveillance of Clostridium difficile infection in European acute care hospitals: a pilot study, 2013. Euro Surveill, 21(29): 10.2807/1560-7917.ES.2016.21.29.30293. [DOI] [PubMed] [Google Scholar]