Abstract
Hypertension prevalence is on the rise in low and middle income countries like South Africa, and migration and concomitant urbanization are often considered to be associated with this rise. However, relatively little is known about the relationship between blood pressure (BP) and internal migration - a highly prevalent population process in LMICs. This study employs data for a group of 194 adult men and women from an original pilot dataset drawn from the Agincourt Health and Demographic Surveillance System in northeast South Africa. Migrants in the sample are identified, tracked, and interviewed. The relationship between BP and migration distance and the number of months an individual spends away from his/her home village is estimated using robust OLS regression, controlling for a series of socioeconomic, health, and behavioral characteristics. This study finds migrants who move further distances and for longer durations to have significantly higher systolic and diastolic BP compared with shorter-term migrants and those who remain nearby or in their home village. These associations remain robust and statistically significant when adjusting for measures of socioeconomic conditions, as well as body mass index (BMI), and the number of meals consumed per day. Migration, both in terms of distance and time away, explains significant variation in BP among migrants in a typical South African context. This finding suggests the need for further studies of nutritional and psychosocial factors associated with geographic mobility that may be important factors for understanding rising hypertension in LMICs.
Keywords: Migration and Social Mobility, Population Health, Demography
Introduction
The association between the rise in prevalence of cardiometabolic risk factors such as hypertension and internal migration, particularly from rural to urban areas, in low and middle income countries (LMICs) is a component of the health and demographic transitions linked to economic development processes (Omran 1971; Frenck et al. 1991; Caldwell 1993; Collinson et al. 2014). Available data indicate that hypertension in LMICs has increased, but with significant national and subnational variation in blood pressure (BP) levels and hypertension prevalence (Addo et al. 2007; Tollman et al. 2008; Day et al. 2014; Gómez-Olivé 2017). Sub-Saharan Africa (SSA) is one region that has moderately high levels of elevated BP and has experienced no reduction in such levels from 1975 to 2015 (NCD Risk Factor Collaboration 2017). South Africa has comparatively high hypertension and adult age-specific BP levels. Despite key historical and political economic differences between South Africa, the SSA region and other LMICs, they all share substantial underestimation of, and recent temporal increases in, hypertension (Addo et al. 2007; Kayima 2013). Hypertension poses an increasingly important public health challenge in South Africa and other LMICs.
The World Health Organization (WHO) Study on Global Ageing and Adult Health (SAGE) finds hypertension prevalence among older adults (50+) to range from 32.3 percent in India to a staggering 77.9 percent in South Africa (Lloyd-Sherlock et al. 2014; Gómez-Olivé et al. 2017). Hypertension prevalence in other nationally representative samples are lower but still striking: hypertension prevalence in South Africa’s National Income Dynamics Study (NIDS) in 2008 was 33.9 percent among individuals aged 15+, 43.6 percent among individuals aged 25+, and 69.8 percent among individuals aged 45+ (Day et al. 2014). Thorogood et al. (2007) reported hypertension (systolic BP ≥ 140 mmHg, or diastolic BP ≤ 90 mmHg or use of antihypertensive medication) prevalence in 2002–03 was 43% in a random stratified sample of residents (non-migrants) aged 35 or older in Agincourt, South Africa, with no difference between men and women. Only 37% of those with hypertension regularly used medication. Clark et al. (2015) using data collected in Agincourt between 2010–2011 from individuals 18 years and older found that age-adjusted hypertension (systolic BP ≥ 140 mmHg, or diastolic BP ≥ 90 mmHg or use of antihypertensive medication) prevalence was 37% and 39% percent in men and women, respectively.
In South Africa, circular labour migration was a key consequence of the Apartheid system of residential social re-engineering that has left a legacy of continued temporary migration from rural and peri-urban parts of the country (Murray 1981; Poset and Casale 2003; Hosegood et al. 2005). Historically, for political and economic motivations, a ‘Bantustan’ system was developed by the national government, and sanitized by the term ‘homelands’, to restructure settlement patterns and livelihood strategies of the Black African population. This system provided necessary labour to urban areas while forcing unemployed family members to remain in densely settled, rural, and peri-urban areas or ‘homelands’ (Hosegood et al. 2005; Collinson et al. 2007). Temporary migration played a key role in the success of the national economy, but also placed a heavy burden on rural households (Reed 2013). Nowadays, temporary labour migration remains a primary strategy used by rural households to access employment and fight against poverty (Hosegood et al. 2005; Collinson 2010) in a context of extreme spatial inequality.
The influence of migration and associated political economic forces on HIV in South Africa have been studied (Lurie et al. 2003; Lurie and Williams 2014; Dobra et al. 2017), but there is limited data and little understanding about how population processes like migration and urbanization affect BP and non-communicable cardiometabolic conditions, despite the awareness that they are pervasive and important upstream risk factors. Previous findings on the relationship between migration and health in general, and migration and BP specifically, are mixed. While studies in LMICs often show higher hypertension prevalence in urban populations compared with rural residents (Ibrahim and Damasceno 2012), this finding is not universal across contexts. For instance, Ebrahim et al. (2010), using data from the Indian Migration Study established that the odds of hypertension in urban and migrant men were almost twice that of rural men. Increased odds of hypertension were also evident for rural women, but migration-associated differences in BP, lipids, glucose and insulin were most prominent among men. Unwin et al. (2010) in Tanzania, on the other hand, reported that some cardiovascular risk factors—such as high cholesterol and high triglycerides—increase with migration while BP decreased. In this same study, migrants reported lower physical activity, increased weight, more meat consumption, but also more fruit and vegetable consumption. HDL cholesterol and total cholesterol increased, and systolic BP fell by 5.4mmHg for men and 8.6mmHg for women. Examining diet and caloric intake in the Indian Migration Study, Bowen et al. (2011) also found migration and urban life to be associated with both positive (more fruit) and negative (more fat and saturated fat) dietary changes.
Hendricks et al. (2012) compared hypertension among four study populations in Nigeria (rural), Kenya (rural), Tanzania (urban), and Namibia (urban) and found somewhat higher levels of hypertension in urban than in rural areas (age-adjusted prevalence was 19.3 percent in Nigeria, 21.4 percent in Kenya, 23.7 percent in Tanzania, 38 percent in Namibia) but a major limitation of that study is that it does not make the rural/urban comparison within each country. In Namibia, Ekezie et al. (2011) found that, when stratified by sex, the urban Igbo population had significantly higher BP among both men and women. Similar urban and rural differences in hypertension have been reported for Burkina Faso and Cameroon (Fezeu et al. 2010; Soubeiga et al. 2017).
Across LMICs, increased hypertension prevalence has been associated with urbanization and migration (Ibrahim and Damasceno 2012). But, much of the existing literature on migration and non-communicable disease risk compares risk factors such as hypertension and its determinants between rural and urban populations to make conclusions about the relationship between migration and health. Study designs that do not identify migrants and their migratory behaviour over time cannot shed light on the dynamic social and bio-behavioural processes that may lead to BP elevations in this population.
The purpose of this study is to examine the association of migration with BP among migrants in South Africa by taking advantage of a pilot study that tracked migrants as they moved. Migration is operationalized directly in both time and space as a measure of how many months individuals spend away from their home village and how far they move away. The first part of the analysis considers initial cross-sectional associations with BP and the second examines the consistency of the results across two dimensions of migration: distance and time away. While this study does not resolve particular mechanisms, it does suggest informed hypotheses about mechanisms through which migration is plausibly affecting BP among movers.
Methods
This analysis uses pilot study data for the Migrant Health Follow Up Study (MHFUS). Undertaken in 2012, the pilot is based on a sample of 390 individuals drawn from the Agincourt Health and Socio-Demographic Surveillance System (HDSS) in South Africa. The Agincourt HDSS is a rural surveillance site in northeast South Africa located near the Mozambican border. The site covers an area of 420km2 and comprises 27 villages (Kahn et al. 2012). The HDSS method, based on annual visits to every household in the surveillance area, registers and updates all births, deaths, and in- and out-migrations taking place within the surveillance area. Permanent migration, where an individual relocates to an alternative place of residence with a permanent intention, is distinguished from temporary or circular migration, where an individual is absent for a period of more than six months but retains links with the origin household within the surveillance area. The pilot study sample included individuals who had migrated out of the HDSS site after 2011 (312 temporary migrants and 31 permanent migrants) and individuals who were lost to follow up in the Agincourt-based Ha Nakakela study – an HlV/non-communicable disease study undertaken in 2010 (58 temporary migrants, and 88 non-migrants) (Gómez-Olivé et al. 2013). The MHFUS pilot sample was randomly split in halves: one half was assigned to an in-person survey while the other half was surveyed over the phone. Fieldworkers collected anthropometric and biometric measures including BP, height, and weight from the 201 individuals who were surveyed in-person. Of the 201 individuals, the final analytical sample comprises 194 individuals – 113 women and 81 men – with anthropometric and biometric measures and non-missing values on covariates.
The outcome variables of interest are systolic and diastolic BP, which were measured following the WHO SAGE protocol (WHO 2006; Lloyd-Sherlock 2014). Blood pressure was measured with a Boso blood pressure instrument (BOSCH + SOHN, Jungingen, Germany), taken on the left wrist with participants in sitting position, at rest and with the wrist resting against their chest at the level of their heart and the left elbow held by the free hand. BP measurements were taken three time with intervals of 3 minutes. BP values are based on the mean of the 2nd and 3rd measurements. This method is consistent with prior Agincourt HDSS studies (Clark et al. 2015). Excluding the first measurement in estimates of BP minimizes the “white coat effect,” albeit at the expense of disregarding some information about the individual’s initial physiological response to having his/her BP taken. Hypertension was defined using the WHO cutoff of systolic BP≥140 mmHg, or diastolic BP≥90 mmHg, or on anti-hypertensive medication (WHO 2013).
Migration is operationalized in two ways. First in terms of the distance in relation to the individual’s home village (classification stratum: near, middle, far). Participants who lived within or near the closest major town to the field site (but not within the HDSS site itself) and those who lived in a nearby game farm, are classified as “near” migrants. Those who lived 1–2 hours drive from the field site are classified as “mid”, and those beyond a 2-hour drive (many in the major metropolitan destination) are classified as “far” migrants. Given the small cell sizes, individuals are dichotomized based on whether they are classified as living in or near their home village (0) or as living at an intermediate or far distance away from the home village (1).
Second, migration is measured as the number of months spent away from the home village in the past 12 months (continuous variable from 0–12). For missing cases (n=23), a value is imputed for the number of months away by assigning individuals the mean number of months away for the distance stratum into which they were originally classified by the HDSS. All missing cases were in the first (near) stratum and were assigned a value of 2.735 months away. A dummy variable indicates whether months away has been imputed (1), or not (0). The imputed dummy is included in all regressions where the number of months away is the key independent variable of interest.
Age (centered on its mean) and age-squared are included in the regression models since increasing cross-sectional age is associated with increased systolic BP. BP levels were slightly lower in older individuals >60 years, due to cohort effects, selective mortality, or both. Other indicators include sex, the standard measure of body mass index (BMI, kg/m2), employment status, high school completion, and a 3-category variable that captures whether an individual typically consumes three meals per day (0= reference category), fewer than three meals per day (1), or more than three meals per day (2). These covariates are included as measures or proxies for risk factors for high BP since these mechanisms have been posited in the literature as determinants of increased BP through biology, social position, work-stress, or diet. Finally, an interaction between sex and the dummy variable set for meal consumption is included because meals consumed is the only covariate that operates in a different direction for men compared with women in an analysis stratified by sex (not shown); consuming more than three meals per day is associated with higher BP for women, but lower BP for men.
The relationship between BP and migration is estimated with robust OLS regression using STATA 14.0 and the rreg command. The analysis begins with a reduced model containing only migration (distance or time away), age, age squared, and sex and progresses with sequential additions of controls for socioeconomic status (employment status, education), adiposity (body mass index [BMI])), and food consumption behaviour (dummies for meals consumed per day). With the introduction of each new set of covariates, the improvement in model fit as well as the sensitivity of the migration variable coefficients to the addition of controls is presented. The variance explained increases noticeably (and usually statistically significantly) as the models become progressively more inclusive. Although Akaike information criterion (AIC) values, which penalize model complexity could be seen as favoring models with fewer parameters, we still opt for the more inclusive models, since they represent the range of the most substantive meaningful covariates.
Both unstandardized coefficients for direct interpretation with respect to original units, as well as standardized coefficients for continuous variables for ease of coefficient-size comparison, are presented. Graphs furthermore show predicted values of BP from estimates of robust regressions with age, age squared, employment status, high school completion status, BMI, number of meals consumed per day, and an interaction between meals per day and sex; we hold all covariates at their means unless otherwise specified. A robust regression approach is preferred to deal with the potential influence of outliers in the data rather than applying probability weights, because weighting has the consequence of significantly up-weighting very few individuals given the sampling strategy and small sample size. The results are therefore not generalizable to the rural population of South Africa, but these findings do suggest additional hypotheses for further inquiry as data become available from the scaled-up study.
Results
The mean age of the sample is 36.5 years, the sample is 58.3 percent female, slightly overweight with mean BMI of 25.7 kg/m2, and most individuals consume three meals per day. In the sample 71.7 percent of individuals are not employed and 28.4 percent have completed high school. Mean systolic BP is 139.5 mmHg and diastolic BP is 96.6 mmHg with a hypertension prevalence of 67.5 percent (Table 1). 78.9 percent of the sample live near or at home, while 21.1 percent migrated to a location at an intermediate or far distance away from the home village. 44.3 percent of the sample are non-migrants (spent 0 months away from their home village). The rest of the sample spent at least one month away from home in the past 12 months for an overall mean of four months absent. 50.52 percent are temporary migrants (spent between 1 and 11 months away) and 5.15 percent are permanent migrants1 (spent all 12 months away) (not shown). Not all individuals who reported having migrated in our study moved to an urban place, but both historically and in these data the pursuit of employment has driven many migrants—particularly those who travel longer distances—toward urban destinations. In these data, 72.7 percent of far-migrants for whom we have rural-urban locational information moved to urban places while only 42.9 percent of near-migrants migrated to urban locations (not shown). Further away moves thus generally correspond with urbanization, while closer moves often do not.
Table 1:
Migrant Health Follow-Up Pilot Study Descriptive Statistics of Dependent, Key Independent, and Covariate Variables employed in the Analysis of BP in rural South Africa
| N = 194 | ||
|---|---|---|
| Mean/Percent | SD | |
| Covariates | ||
| Age (yrs) | 36.5 | 17.3 |
| Sex (%) | ||
| Male (reference) | 41.8 | |
| Female | 58.3 | |
| Body Mass Index (kg/m2 ) | 25.7 | 6.3 |
| Meals in the last Day (%) | ||
| 1–2 meals | 27.3 | - |
| 3 meals (reference) | 57.2 | - |
| 4–7 meals | 15.5 | - |
| Employment (%) | ||
| Unemployed | 71.7 | - |
| Employed | 28.4 | - |
| Completed High School (%) | 32.0 | - |
| Key Independent Variables | - | |
| Months Away | 4.0 | 4.5 |
| Distance | ||
| Near or in Home Village (reference) | 78.9 | - |
| Mid/Far | 21.1 | - |
| Dependent Variables | ||
| Systolic BP (mmHg) | 139.5 | 20.2 |
| Diastolic BP (mmHg) | 96.6 | 16.0 |
| Hypertension (%) | 67.5 | - |
Source: Migrant Health Follow Up Pilot Study
The number of months an individual has spent away from the home village is significantly and positively associated with both adjusted higher systolic (Table 2, models 1 – 5) and diastolic BPs (Table 2, models 6 – 10). In the reduced systolic BP model (model 1), each standard deviation increase in time spent away from the home village—4.5 months—is associated with a 0.175 standard deviation difference—equivalent to a 3.50 mmHg higher systolic BP (p<.05). This association remains robust and statistically significant with adjustments for measures of socioeconomic condition, as well as other health and behaviour covariates (Table 2, models 2–4). In Model 5, which includes the gender-meals/day interaction, the same 4.5-month duration of absence predicts 3.35 mmHg elevation in BP, slightly smaller than in models 1–4, but still statistically significant at the .05 percent level. The model fit improves with each additional set of covariates, with BMI (model 3) and the consumption-gender interactions (model 5), especially enhancing the models; the final full model (model 5) explains 18.8 percent of the variation in systolic BP, more than twice that of the reduced model (model 1).
Table 2:
Determinants of Systolic and Diastolic BP from robust OLS regressions of months away and covariates
| Model 1 | Model 2 | Model 3 Systolic BP | Model 4 | Model 5 | Model 6 | Model 7 | Model 8 Diastolic BP | Model 9 | Model 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Months Away | 0.792** | 0.796** | 0.878*** | 0.850*** | 0.753** | 0.639** | 0.724*** | 0.762*** | 0.749*** | 0.669** |
| (0.316) | (0.324) | (0.320) | (0.320) | (0.307) | (0.263) | (0.270) | (0.271) | (0.273) | (0.270) | |
| [0.175] | [0.176] | [0.194] | [0.188] | [0.166] | [0.178] | [0.202] | [0.213] | [0.209] | [0.187] | |
| Age | 0.409*** | 0.473*** | 0.408*** | 0.424*** | 0.355*** | 0.409*** | 0.456*** | 0.431*** | 0.439*** | 0.390*** |
| (0.113) | (0.116) | (0.117) | (0.117) | (0.114) | (0.0940) | (0.0968) | (0.0992) | (0.1000) | (0.100) | |
| [0.350] | [0.405] | [0.349] | [0.363] | [0.304] | [0.443] | [0.493] | [0.466] | [0.475] | [0.422] | |
| Age Squared | −0.00808* | −0.0109** | −0.00829* | 0.00896* | −0.00616 | −0.0162*** | −0.0188*** | −0.0178*** | −0.0183*** | −0.0163*** |
| (0.00430) | (0.00451) | (0.00452) | (0.00456) | (0.00443) | (0.00358) | (0.00375) | (0.00383) | (0.00389) | (0.00389) | |
| [−0.177] | [−0.238] | [−0.181] | [−0.196] | [−0.135] | [−0.447] | [−0.519] | [−0.492] | [−0.506] | [−0.449] | |
| Female | −7.255*** | −7.982*** | −10.34*** | −11.10*** | −12.31*** | −3.283 | −4.256* | −5.036** | −5.588** | −6.730** |
| (2.724) | (2.783) | (2.848) | (2.885) | (3.607) | (2.267) | (2.315) | (2.415) | (2.462) | (3.173) | |
| Employed | −5.721* | −7.317** | −7.996** | −8.721*** | −5.407** | −5.875** | −6.420** | −6.864** | ||
| (3.205) | (3.185) | (3.245) | (3.127) | (2.667) | (2.701) | (2.770) | (2.751) | |||
| High School Graduate | 2.705 | 1.806 | 2.565 | 3.019 | 0.366 | 0.0322 | 0.434 | 0.631 | ||
| (3.085) | (3.045) | (3.078) | (2.990) | (2.567) | (2.582) | (2.627) | (2.630) | |||
| Body Mass Index | 0.649*** | 0.664*** | 0.765*** | 0.239 | 0.243 | 0.327* | ||||
| (0.230) | (0.231) | (0.223) | (0.195) | (0.197) | (0.196) | |||||
| [0.202] | [0.207] | [0.238] | [0.0940] | [0.0957] | [0.129] | |||||
| 3 meals/day (ref) | ||||||||||
| Less than 3 meals/day | −3.638 | −0.641 | −1.797 | 0.218 | ||||||
| (3.120) | (4.257) | (2.663) | (3.745) | |||||||
| More than 3 meals/day | 1.698 | −12.48* | 2.947 | −7.358 | ||||||
| (3.875) | (6.630) | (3.308) | (5.832) | |||||||
| 3 meals/day*female (ref) | ||||||||||
| Less than 3 meals/day*Female | −7.031 | −4.512 | ||||||||
| (6.013) | (5.290) | |||||||||
| More than 3 | ||||||||||
| meals/day*Female | 21.07*** | 15.07** | ||||||||
| (8.038) | (7.071) | |||||||||
| Months Away Imputed | 0.332 | −0.112 | −0.245 | −0.662 | −1.193 | −0.820 | −1.349 | −1.362 | −1.554 | −1.779 |
| (4.257) | (4.271) | (4.193) | (4.200) | (4.020) | (3.542) | (3.553) | (3.555) | (3.585) | (3.537) | |
| Constant | 141.8*** | 144.1*** | 128.3*** | 129.4*** | 127 1*** | 100.3*** | 103.0*** | 97.07*** | 97.56*** | 95.79*** |
| (2.848) | (3.308) | (6.510) | (6.542) | (6.369) | (2.370) | (2.752) | (5.520) | (5.584) | (5.602) | |
| Observations | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 |
| R-squared | 0.0921 | 0.109 | 0.140 | 0.148 | 0.188 | 0.123 | 0.141 | 0.147 | 0.153 | 0.178 |
| AIC | 202.809 | 198.835 | 202.864 | 202.946 | 212.791 | 175.055 | 173.126 | 177.077 | 184.208 | 199.746 |
Standard errors in parentheses
Normalized beta coefficients for continuous variables in brackets
p<0.01
p<0.05
p<0.1
age variable is centered on the mean
The relationship between time away from home and diastolic BP is similar to that of systolic BP: an additional 4.5 months away is associated with a 0.178 standard deviation change, or 2.84 mmHg higher, in diastolic BP in the reduced model (model 6) and a 0.187 standard deviation change—equivalent to a 2.99 mmHg increase—in the full model (model 10) Older age (at time of survey) is significantly associated both with higher diastolic and systolic BPs, but the further association with age-squared indicates a decreasing increment to BP with each additional year among older individuals (Table 2). Women and employed individuals have appreciably lower systolic and diastolic BPs compared with men and those unemployed, respectively (Table 2). BMI is positively and significantly associated with systolic BP. A one standard deviation increase in BMI, i.e. 6.29 kg/m2, is associated with 4.8 mmHg higher systolic BP (model 5). While the relationship between BMI and diastolic BP is positive, it is not statistically significant in models 8 and 9, and only marginally significant in the full model. Finally, consuming more than three meals per day, compared with three meals per day, is marginally associated with lower systolic BP (model 5), but the interactions in the full models suggest that this relationship is in fact positive for women (model 6).
Table 3 reports results using migration as a distance measure in association with BP. These are largely similar to those shown above for time away from home. Individuals who migrate away from the home village to either intermediate or far-away places have significantly higher systolic and diastolic BP compared with individuals who do not migrate or do so only locally (Table 3). Moving away is associated with higher systolic BP (that ranges from 8.6 mmHg to 10.38 mmHg) compared with those who stay close by or in their home village (p<.05). Similarly, far-migrants have diastolic BP 8.27 mmHg higher than near and non-migrants, controlling for the full set of covariates (p<.001). In these alternative models using a distance measure for migration, the covariates age, sex, and BMI are positive and statistically significant, as is the sex-conditioned meal consumption interaction for women who consume more than three meals per day. As with prior models 6–10, BMI is positively but not statistically significantly associated with diastolic BP. Being employed is inversely and highly significantly associated with both systolic and diastolic BP. One notable difference between the months-away and distance migration models is that sex is not significantly associated with diastolic BP in the distance models, while it is in the months-away models.
Table 3:
Determinants of Systolic and Diastolic BP from robust OLS regressions of distance away and covariates
| Model 11 | Model 12 | Model 13 Systolic BP | Model 14 | Model 15 | Model 16 | Model 17 | Model 18 Diastolic BP | Model 19 | Model 20 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stayed home or near village (ref) | ||||||||||
| Migrated away | 8.597** | 9.387*** | 9.671*** | 10.38*** | 8.747** | 7.505*** | 8.925*** | 8.992*** | 9.185*** | 8.274*** |
| (3.413) | (3.607) | (3.542) | (3.573) | (3.468) | (2.833) | (2.999) | (3.015) | (3.083) | (3.030) | |
| Age^ | 0.420*** | 0.489*** | 0.423*** | 0.461*** | 0.355*** | 0.407*** | 0.450*** | 0.427*** | 0.443*** | 0.394*** |
| (0.111) | (0.115) | (0.116) | (0.116) | (0.114) | (0.0925) | (0.0957) | (0.0987) | (0.0997) | (0.0992) | |
| [0.359] | [0.419] | [0.363] | [0.395] | [0.304] | [0.44] | [0.487] | [0.462] | [0.479] | [0.426] | |
| Age Squared | −0.00846** | −0.0118*** | −0.00930** | −0.0104** | −0.00626 | −0.0161*** | −0.0188*** | −0.0180*** | −0.0186*** | −0.0165*** |
| (0.00425) | (0.00448) | (0.00450) | (0.00450) | (0.00442) | (0.00353) | (0.00373) | (0.00383) | (0.00389) | (0.00386) | |
| [−0.185] | [−0.258] | [−0.203] | [−0.227] | [−0.137] | [−0.444] | [−0.521] | [−0.497] | [−0.513] | [−0.457] | |
| Female | −6.408** | −7.210*** | −9.335*** | −10.35*** | −11.60*** | −2.514 | −3.421 | −4.032* | −4.685* | −6.131* |
| (2.715) | (2.757) | (2.814) | (2.835) | (3.607) | (2.253) | (2.292) | (2.396) | (2.447) | (3.151) | |
| Employed | −6.557** | −7.932** | −8.822*** | −9.108*** | −5.546** | −5.838** | −6.393** | −6.842** | ||
| (3.209) | (3.186) | (3.215) | (3.124) | (2.669) | (2.712) | (2.774) | (2.730) | |||
| High School Graduate | 2.036 | 1.270 | 2.060 | 2.513 | −0.479 | −0.717 | −0.194 | −0.0223 | ||
| (3.099) | (3.055) | (3.063) | (3.003) | (2.576) | (2.600) | (2.643) | (2.623) | |||
| Body Mass Index | 0.606*** | 0.637*** | 0.747*** | 0.187 | 0.202 | 0.294 | ||||
| (0.227) | (0.226) | (0.221) | (0.193) | (0.195) | (0.193) | |||||
| [0.189] | [0.199] | [0.233] | [0.0738] | [0.0795] | [0.116] | |||||
| 3 meals/day (ref) | ||||||||||
| Less than 3 meals/day | −5.438* | −2.391 | −2.958 | −0.988 | ||||||
| (3.096) | (4.233) | (2.672) | (3.698) | |||||||
| More than 3 meals/day | 0.00740 | −13.78** | 1.820 | −8.638 | ||||||
| (3.855) | (6.577) | (3.327) | (5.746) | |||||||
| 3 meals/day*female (ref) | ||||||||||
| Less than 3 meals/day*Female | −6.334 | −4.101 | ||||||||
| (5.975) | (5.220) | |||||||||
| More than 3 | ||||||||||
| meals/day*Female | 21.03*** | 15.17** | ||||||||
| (7.992) | (6.982) | |||||||||
| Constant | 142.9*** | 145.6*** | 131.0*** | 132.6*** | 129.0*** | 100.6*** | 103.6*** | 99.04*** | 99.69*** | 97.73*** |
| (2.640) | (3.113) | (6.267) | (6.249) | (6.147) | (2.191) | (2.588) | (5.335) | (5.393) | (5.370) | |
| Observations | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 | 194 |
| R-squared | 0.095 | 0.114 | 0.142 | 0.159 | 0.188 | .125 | .145 | .147 | .153 | .181 |
| AIC | 190.396 | 188.411 | 188.682 | 177.894 | 233.158 | 177.097 | 177.276 | 185.167 | 195.373 | 199.255 |
Standard errors in parentheses
Normalized beta coefficients for continuous variables in brackets
p<0.01
p<0.05
p<0.1
age variable is centered on the mean
We examined the sensitivity of our results to the inclusion of permanent migrants in the sample. We repeated the analyses without the 5 percent of migrants who were away from home all 12 months in the past year and found our results to be consistent and robust.2
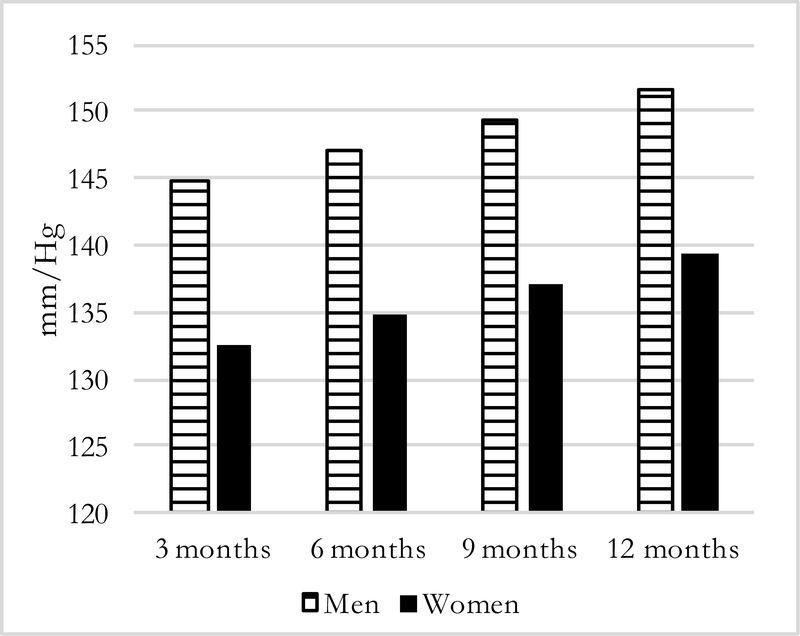
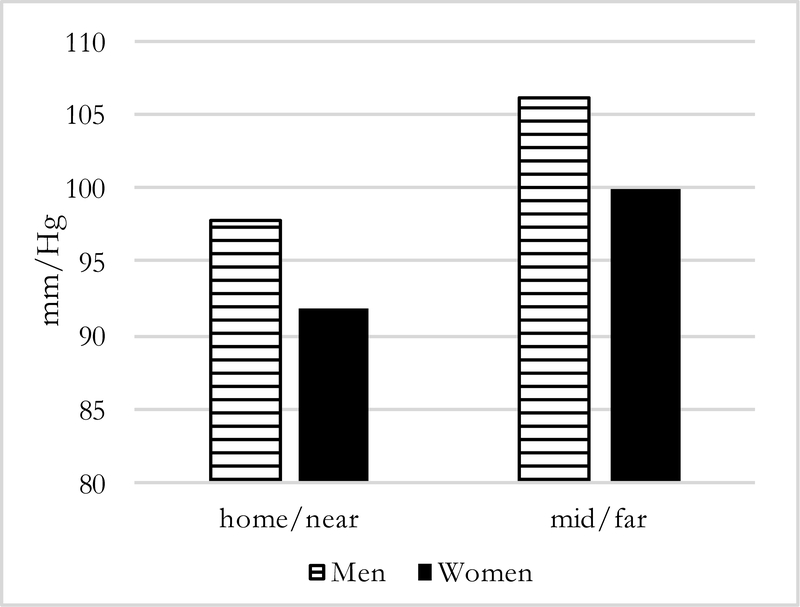
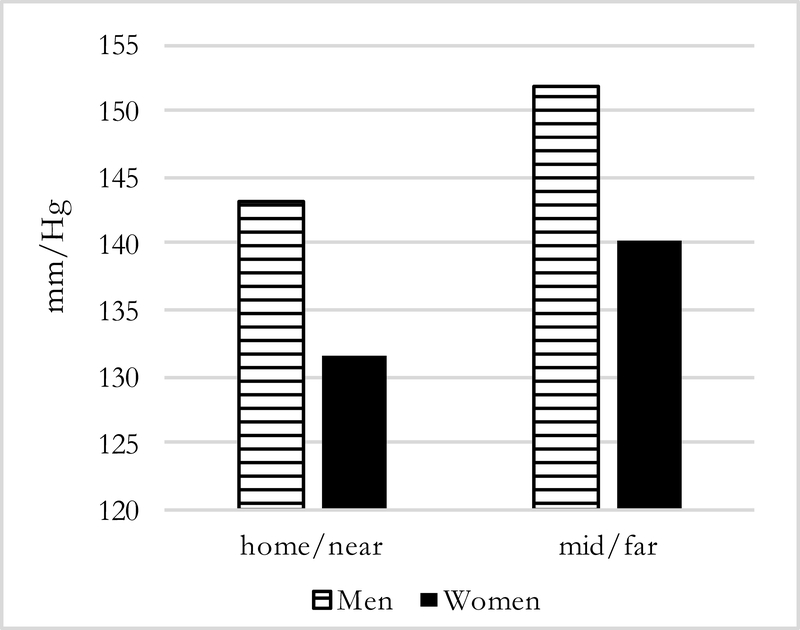
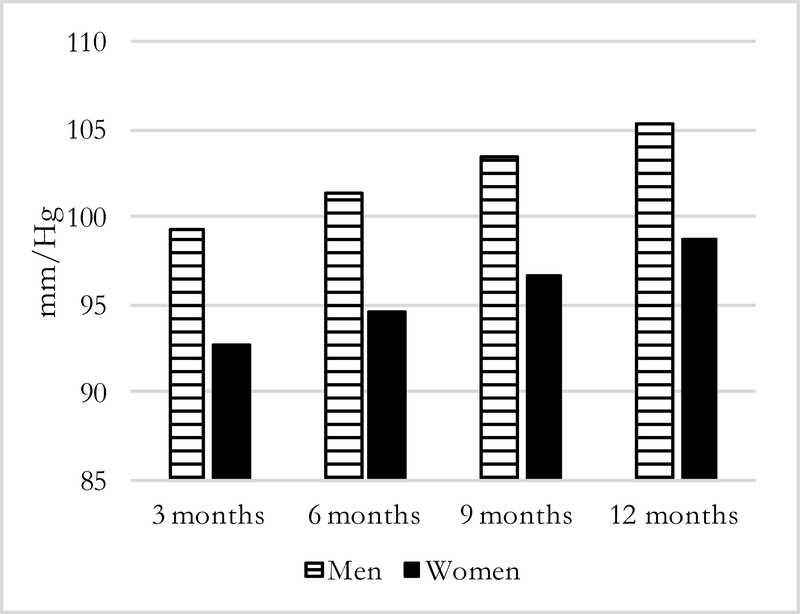
Figures 1–4 present predicted values generated from the most inclusive regression models. They illustrate a prediction of higher systolic and diastolic BP with more months away, as well as further distance, for both men and women—although BP levels are higher for men in all instances. In this simulation, women who spend three months away from home have systolic/diastolic BPs of 132.4/92.6 mmHg compared with 139.3/98.6 mmHg if they spend twelve months away from the home village in the past year. For men, the corresponding values are 144.8/99.3 mmHg if a migrant is away for three months compared with 151.6/105.4 mmHg if he is away for twelve months. For distance moves (Figures 3–4) the simulated differences in systolic and diastolic BPs are similarly striking: a man who migrated further away who has average characteristics on covariates is expected to have systolic and diastolic BPs of 151.9/106.2 mmHg compared with 143.2/97.8 mmHg for a man who stayed nearby. A female far-migrant has predicted BP 140.3/100.0 mmHg compared with 131.5/91.8 mmHg if she stayed in the home village or migrated only locally.
Figure 1:
Predicted Systolic BP, by months away and sex =194)
Figure 4:
Predicted Diastolic BP, by distance and sex (N=194)
Figure 3:
Predicted Systolic BP, by distance and sex (N=194)
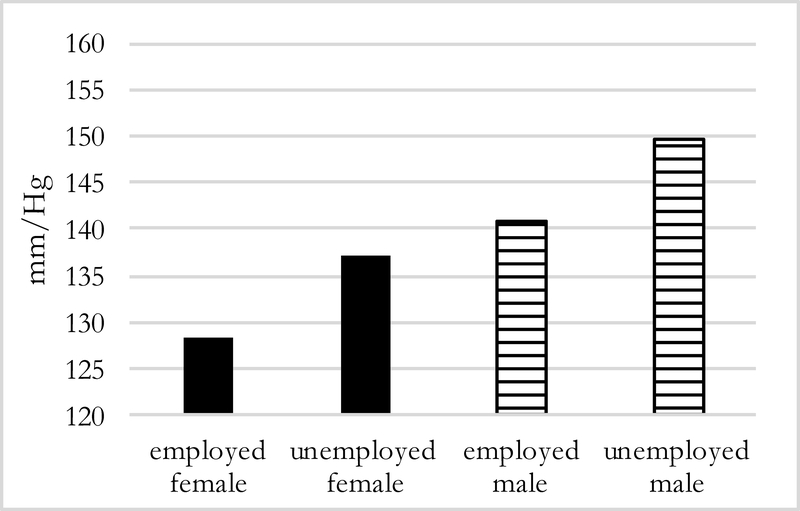
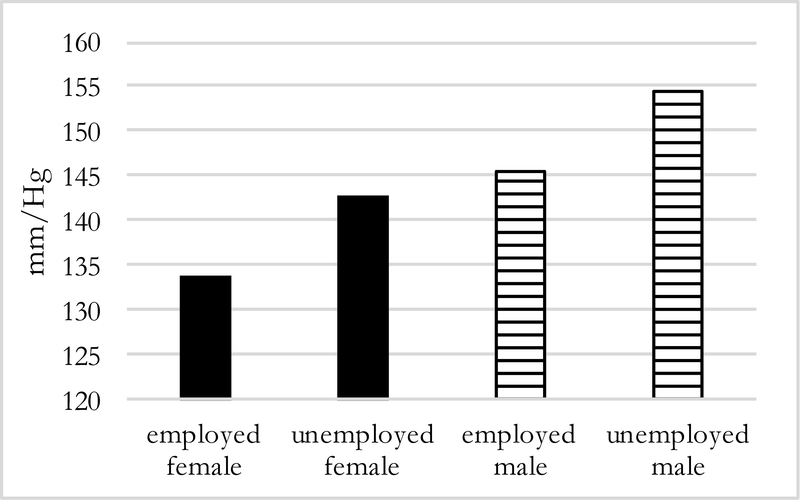
Predicted values of systolic BP by employment status but holding absence at 6 months (Figure 5), show that employed men and women have lower systolic BP than those who are unemployed. The same pattern is evident when keeping distance constant (Figure 6): among men and women who migrate further distances, those who are unemployed have higher systolic BP by about 9 mmHg.
Figure 5:
Predicted Systolic BP of migrants 6 months away, by sex and employment status (N=194)
Figure 6:
Predicted Systolic BP of mid/far migrants by sex, and employment status (N=194)
Discussion
The results indicate that circular migration in the past year is significantly and positively associated with higher BP levels among persons from the Agincourt HDSS of northeast South Africa. Individuals who move further away and for longer periods have significantly higher BPs than those who stay close to home or migrate for shorter periods. Other studies of the Agincourt HDSS reveal that migration is most commonly temporary, and involves moves to urban destinations that are driven by a search for employment that consequently exposes individuals to new and additional health risk factors (Collinson et al. 2007, 2010; Reed 2013). The findings raise several compelling questions: what is it about longer durations and greater distance from origin locations that affects BP among migrants for the worse? What is the role of destination places in BP? Does the relationship between migration and BP depend on the type of employment or employment status, social networks at destinations, living conditions at destinations, the health selectivity of those who undertake migration in the first place, or the ease or frequency of return to the home village? Because of the cross-sectional design and study sample size these questions remain largely unanswered here. A potential explanation, though, may be that social and psycho-social factors alongside, and in combination with, nutritional and other environmental changes, may affect BP (Strogat et al. 1997; Gorman and Sivaganesan 2007).
Migrants might experience more stress as they move further away for longer periods as a result of greater dislocation from exiting social networks and support, be less able or willing to engage in familiar social practices and behaviours, and may be more likely to experience physical and social environments more varied and dissimilar from those closer to home (Uchino 2006; Lu 2009). The interrupted social support due to migration may also disrupt prioritizing health care for hypertension as a recent study in Nigeria showed (Osamor 2015). The ability to secure employment, rely on friends and relatives for support, and return home periodically are potentially important mechanisms through which migration can influence BP negatively.
The lower BP among employed migrant men and women may reflect their higher incomes, purchasing power, and social integration, which could counteract anticipated psychosocial and material stressors for South African Black migrants to urban areas (Turok 2012). It may also reflect greater awareness of the health risks from elevated BP and /or opportunities for recognition and treatment of high BP in the context of more healthcare access or job-related health insurance. These scenarios affecting BP levels remain speculative but will be studied in full in the larger population-based MHFUS research on migration, urbanization and health. This study will follow a cohort of migrants and non-migrants recruited from the Agincourt HDSS study area for a period of 5 years from 2018 to 2022 (Ginsburg et al. 2016; Collinson et al. 2016). The fine-grained individual longitudinal approach should help further understanding of how the economic and social conditions experienced during migration from rural to urban areas (such as employment) may affect BP.
There is no consistent evidence of the association between the number of meals consumed per day and BP. Models (not shown) that included a measure of micronutrients (e.g. potassium-rich foods) showed no statistically significant association with BP. The measures of meal consumption and diet used are probably too crude to capture any true underlying association between diet and BP, which is difficult to detect in all but the largest studies that also collect dietary biomarkers (Farquhar et al. 2015).
This study has important limitations. The pilot sample is not representative of the rural South African population in general, or the Agincourt study site in particular. The pilot study was designed to locate migrants who had been lost to follow up in previous surveys (in part to demonstrate the feasibility of follow-up and hence the ability to maintain cohort integrity); migrants who could not be found in their rural houses of origin in 2011–2012 are, therefore, intentionally oversampled and overrepresented in the sample.
While the selective nature of the sample adds to understanding of the relationship between migration and health and is useful in generating hypotheses, the higher BP levels and hypertension prevalence in the MHFUS pilot sample compared to others in rural South Africa and Agincourt (Lloyd-Sherlock et al. 2014; Clark et al. 2015; Ntuli et al. 2015) may be partially attributable to differences in study timing, design, and adult age sampling. Thorogood et al.’s (2007) study of those 35 years and older occurred a full decade prior, 2002–03, to the MFHUS pilot study, and did not include migrants. Clark et al.’s (2015) sample from 2010–11 is younger (adults age 18 and older) and suffers significant non-response. These data with a mean age of 36.5 years are consistent with reports that hypertension prevalence rates have increased in the last decade throughout rural and urban Africa. Use of the wrist BP monitor can lead to falsely elevated BP values if arm and hand position is not performed as described above and in the WHO SAGE study (Thorogood et al. 2007; Lloyd-Sherlock et al. 2014). But proper arm position was consistently checked with participant’s wrist held at the level of the heart to avoid higher BPs due to greater hydraulic pressure when the wrist is lower than the heart (Topouchian et al 2006). This was the technique used in prior studies by the Agincourt research team (Clark et 2015). Thus, the high BP levels recorded appear not to be affected by measurement technique errors. Very high BP among these South African migrants are consistent with that from the SAGE and NIDS 2008 studies (Tollman et al. 2008). Furthermore, confidence in these data and statistical findings are bolstered by their broad consistency with the findings of others in the field: migration is associated with significantly higher systolic and diastolic BP (Ebrahim et al. 2010; Bowen et al. 2011).
Going beyond the prior studies, this research suggests the need for further inquiry by carefully examining and recording migration directly and in detail as a process that occurs both in space and time; an HDSS platform can facilitate such enquiry. Psychosocial stressors such as social dislocation and stress that are associated with migration distance and time away might be understudied but important dimensions of migrant health in LMICs like South Africa (WHO 2010). The present findings will guide future research to pay attention to these additional dimensions of the migration process in relation to the place of origin and its influence on BP and related risk factors. Since migration and urbanization are occurring rapidly throughout SSA, a goal of this study has been to stimulate other research on the multifactorial drivers of elevated BP and hypertension in LMICs.
Figure 2:
Predicted Diastolic BP, by months away and sex (N=194)
Acknowledgments
Funding
We acknowledge support from NIH, P30AI042853 to the Providence/Boston Center for AIDS Research (CFAR) and 1R01HD083374-01A1 Migration, Urbanization and Health in a Transition Setting to the Population Studies and Training Center, Brown University. The MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt) gratefully acknowledges funding from The Wellcome Trust, UK (grants 058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z), the Medical Research Council, South Africa. We further gratefully acknowledge the South African Medical Research Council for funding Carren Ginsburg’s Career Development Award.
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
Ethical Approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Our definition of permanent migrants is different from the “permanent migrant” classification in the Agincourt HDSS, in which permanent migrants are defined as individuals who have permanently moved out of the study site and are therefore not followed.
In the most inclusive SBP model, the coefficient on the “months away” variable without the permanent migrants is 0.772 vs. 0.753 with them included (coefficient in each specification significant at the 5 percent level), and is 9.44 vs. 8.75 on the “migrate away” variable with and without the permanent migrants (both significant at 5 percent). In the most inclusive DBP model, the coefficient on the “months away” variable without the permanent migrants is 0.811 vs. 0.669 with them included (both significant at 5 percent), and 9.12 vs. 8.274 on the “migrate away” variable with and without the permanent migrants (both significant at 5 percent).
Contributor Information
Chantel F. Pheiffer, Department of Sociology, Brown University, Providence, RI, USA
Stephen T. McGarvey, International Health Institute, Brown University, Providence, RI, USA
Carren Ginsburg, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Parktown, South Africa..
Mark Collinson, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Parktown, South Africa..
F. Xavier Gómez-Olivé, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Parktown, South Africa..
Stephen Tollman, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Parktown, South Africa..
Michael J. White, Brown University, Department of Sociology, Providence, RI, USA and University
References
- Addo J, Smeeth L and Leon DA (2007) Hypertension In Sub-Saharan Africa: A Systematic Review. Hypertension; 50(6), 1012–1018. [DOI] [PubMed] [Google Scholar]
- Bowen L, Ebrahim S, De Stavola B, Ness A, Kinra S, Bharathi AV, Prabhakaran D et al. (2011) Dietary intake and rural-urban migration in India: a cross-sectional study. PLoS One; 6(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JC. (1993) Health Transition: The Cultural, Social, and Behavioural Determinants of Health in the Third World. Social Science Medicine 36(2), 125–135. [DOI] [PubMed] [Google Scholar]
- Clark SJ, Gómez-Olivé FX, Houle B, Thorogood M, Klipstein-Grobusch K, Angotti N, Kabudula C et al. (2015) Cardiometabolic Disease Risk and HIV Status in Rural South Africa: Establishing a Baseline. BMC Public Health 15(1), 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson MA, Tollman SM, and Kahn K (2007) Migration, Settlement Change and Health in Post-Apartheid South Africa: Triangulating Health and Demographic Surveillance with National Census Data. Scandinavian Journal of Public Health 35(69_suppl), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson MA, White MJ, Ginsburg C, Gómez-Olivé FX, Kahn K, and Tollman SM (2016) Youth migration, livelihood prospects and demographic dividend: A comparison of the Census 2011 and Agincourt Health and Demographic Surveillance System in the rural northeast of South Africa. African Population Studies 30(2), 2629–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson MA, White MJ, Bocquier P, McGarvey S, Afolabi SA, Clark SJ, Kahn K, and Tollman SM (2014) Migration and the Epidemiological Transition: Insights from the Agincourt sub-district of northeast South Africa. Global Health Action 7, 23514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinson MA (2010) Striving against Adversity: The Dynamics of Migration, Health and Poverty in Rural South Africa. Global Health Action 3(1), 5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C, Groenewald P, Laubscher R, Chaudhry S, Van Schaik N, and Bradshaw D (2014) Monitoring of Non-Communicable Diseases Such as Hypertension in South Africa: Challenges for the Post-2015 Global Development Agenda. South African Medical Journal 104(10), 680. [DOI] [PubMed] [Google Scholar]
- Dobra A, Barnighausen T, Vandormael A, and Tanser F (2017) Space-Time Migration Patterns and Risk of HIV Acquisition in Rural South Africa. AIDS 31(1), 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim S, Kinra S, Bowen L, Andersen E, Ben-Shlomo Y, Lyngdoh T, et al. (2010) The Effect of Rural-to-Urban Migration on Obesity and Diabetes in India: A Cross-Sectional Study. PLoS Med 7(4), e1000268 10.1371/journal.pmed.1000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekezie J, Anyanwu EG, Danborno B, and Anthony U (2011) Impact of urbanization on obesity, anthropometric profile and BP in the Igbos of Nigeria. North American Journal of Medical Sciences 3(3), 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar WB, Edwards DG, Jurkovitz CT, and Weintraub WS (2015) Dietary Sodium and Health. Journal of the American College of Cardiology 65(10), 1042–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fezeu L, Kengne AP, Balkau B, Awah PK, and Mbanya JC (2010) Ten-Year Change in Blood Pressure Levels and Prevalence of Hypertension in Urban and Rural Cameroon. Journal of Epidemiology & Community Health 64, 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk J, Bobadilla JL, Stern C, Frejka T, and Lozano R (1991). Elements for a Theory of the Health Transition. Health Transition Review 1, 21–38. [PubMed] [Google Scholar]
- Ginsburg C, Collinson MA, Iturralde D, van Tonder L, Gómez-Olivé FX, Kahn K, and Tollman S (2016) Migration and settlement change in South Africa: Triangulating Census 2011 with longitudinal data from the Agincourt Health and Demographic Surveillance System in the rural north-east. Southern African Journal of Demography 17(1), 133–198. [Google Scholar]
- Gómez-Olivé FX, Ali SA, Made F, Kyobutungi C, Nonterah E, Micklesfield L et al. (2017) Regional and Sex Differences in the Prevalence and Awareness of Hypertension: An H3Africa AWI-Gen Study Across 6 Sites in Sub-Saharan Africa. Journal of Global Health 12(2), 81–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Olivé FX, Angotti N, Houle B, Klipstein-Grobusch K, Kabudula C, Menken J, Williams J, Tollman S, and Clark SJ (2013) Prevalence of HIV among Those 15 and Older in Rural South Africa. AIDS Care 25, 1122–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman BK and Sivaganesan A (2007) The Role of Social Support and Integration for Understanding Socioeconomic Disparities in Self-Rated Health and Hypertension. Social Science & Medicine 65 (5), 958–975. [DOI] [PubMed] [Google Scholar]
- Hendriks ME, Wit FW, Roos MT, Brewster LM, Akande TM, de Beer IH, Mfinanga SG et al. (2012) Hypertension in sub-Saharan Africa: cross-sectional surveys in four rural and urban communities. PloS One 7(3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosegood V, Benzler J, and Solarsh GC (2005) Population Mobility and Household Dynamics in Rural South Africa: Implications for Demographic and Health Research. Southern African Journal of Demography 10:43–68. [Google Scholar]
- Ibrahim MM and Damasceno A (2012) Hypertension in Developing Countries. The Lancet 380(9841), 611–619. [DOI] [PubMed] [Google Scholar]
- Kahn K, Collinson MA, Gomez-Olive FX, Mokoena O, Twine R, Mee P, Afolabi SA, et al. (2012) Profile: Agincourt Health and Socio-Demographic Surveillance System. International Journal of Epidemiology 41, 988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayima J, Wanyenze RK, Katamba A, Leontsini E, and Nuwaha F (2013) Hypertension Awareness, Treatment and Control in Africa: A Systematic Review. BMC Cardiovascular Disorders 13(1): 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, and Chatterji S (2014) Hypertension among Older Adults in Low- and Middle-Income Countries: Prevalence, Awareness and Control. International Journal of Epidemiology 43(1), 116–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y (2009) Rural-urban migration and health: Evidence from longitudinal data in Indonesia. Social Science & Medicine 70, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie MN, Williams BG, Zuma K, Mkaya-Mwamburi D, Garnett GP, Sturm AW, Sweat MD, Gittelsohn J, and Abdool Karim SS (2003) The Impact of Migration on HIV-1 Transmission in South Africa: A Study of Migrant and Nonmigrant Men and Their Partners. Sexually Transmitted Diseases 30(2), 149–156. [DOI] [PubMed] [Google Scholar]
- Lurie MN and Williams BG (2014) Migration and Health in Southern Africa: 100 Years and Still Circulating. Health Psychology and Behavioral Medicine 2(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C (1981) Families divided: the impact of migrant labour in Lesotho. Johannesburg: Ravan. [Google Scholar]
- NCD Risk Factor Collaboration (2017) Worldwide Trends in Blood Pressure from 1975 to 2015: A Pooled Analysis of 1479 Population-Based Measurement Studies with 19.1 Million Participants. Lancet 389(10064), 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntuli ST, Maimela E, Alberts M, Choma S, and Dikotope S (2015) Prevalence and Associated Risk Factors of Hypertension amongst Adults in a Rural Community of Limpopo Province, South Africa. African Journal of Primary Health Care & Family Medicine 7(1), 847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omran AR (1971) The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. The Milbank Memorial Fund Quarterly 83, 509–538. [PubMed] [Google Scholar]
- Osamor PE (2015) Social Support and Management of Hypertension in South-West Nigeria: Cardiovascular Topic. Cardiovascular Journal Of Africa 26(1), 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posel D and Casale D (2003) What Has Been Happening to Internal Labour Migration in South Africa, 1993—1999? South African Journal of Economics 71(3), 455–479. [Google Scholar]
- Reed HE (2013) Moving Across Boundaries: Migration in South Africa, 1950—2000. Demography 50(1), 71–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubeiga JK, Millogo T, Bicaba BW, Doulougou B and Kouanda S (2017) Prevalence and Factors Associated with Hypertension in Burkina Faso: A Countrywide Cross-Sectional Study. BMC Public Health 17(1), 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogat DS, Croft JB, James SA, Keenan NL, Browning SR, Garret JM, and Curtis AB (1997) Social Support, Stress, and Blood Pressure in Black Adults. Epidemiology 8(5), 482–487. [DOI] [PubMed] [Google Scholar]
- Thorogood M, Connor M, Tollman S, Hundt GL, Fowkes G and Marsh JA (2007) Cross-Sectional Study of Vascular Risk Factors in a Rural South African Population: Data from the Southern African Stroke Prevention Initiative. BMC Public Health 7(1), 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollman SM, Kahn K, Sartorius B, Collinson MA, Clark SJ and Garenne ML (2008). Implications of Mortality Transition for Primary Health Care in Rural South Africa: A Population-Based Surveillance Study. The Lancet 372(9642), 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topouchian Jirar A.; El Assaad Mohamed A.; Orobinskaia Ludmila V.; El Feghali Ramzi N.; Asmar Roland G. Validation of two automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension: the Omron M6 (HEM-7001-E) and the Omron R7 (HEM 637-IT). Blood Pressure Monitoring, 2006; 11: 165–171. [DOI] [PubMed] [Google Scholar]
- Turok I (2012) Urbanisation and Development in South Africa: Economic Imperatives, Spatial Distortions and Strategic Responses Urbanization And Emerging Population Issues Working Paper 8, International Institute For Environment And Development. United Nations Population Fund: London [Google Scholar]
- Uchino BN (2006) Social Support and Health: A Review of Physiological Processes Potentially Underlying Links to Disease Outcomes. Journal of Behavioral Medicine 29(4), 377–387. [DOI] [PubMed] [Google Scholar]
- Unwin N, James P, McLarty D, Machybia H, Nkulila P, Tamin B, Nguluma M et al. (2010) Rural to urban migration and changes in cardiovascular risk factors in Tanzania: a prospective cohort study. BMC Public Health 10(1), 272–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2006) WHO SAGE Survey Manual: The WHO Study on Global Aging and Adult Health, Geneva, Switzerland. [Google Scholar]
- World Health Organization (2010) Hidden Cities: unmasking and overcoming health inequities in urban settings, Geneva, Switzerland. [Google Scholar]
- World Health Organization (2013) Global action plan for the prevention and control of noncommunicable diseases 2013–2020, Geneva, Switzerland. [Google Scholar]