Abstract
Purpose:
Exosomal proteases are important in regulation of molecular signaling from growth factor receptors and adhesion molecules and also the regulation of cell motility and protein folding. The aim of this study was to evaluate the level of ADAM10, ADAM17 and 20S proteasomes in exosomes isolated from colorectal cancer patients (CRCPs) in relation with clinical and histopathological parameters.
Methods:
Blood plasma exosomes of 60 CRCPs at stage T2-4N0-2M0-1 and 10 control subjects (CSs) with colorectal polyps were isolated using ultrafiltration in combination with ultracentrifugation. The level of tetraspanin-associated (ADAM20 and ADAM17) and tetraspanin-non-associated (20S proteasome) proteases were evaluated by flow cytometry and western blot analysis.
Results:
The ADAM10-/ADAM17- population predominated in plasma exosomes of CRCPs and the level of ADAM10+ exosomes was significantly higher in exosomes of CSs compared with CRCPs. No difference was found between subpopulations of ADAM10/ADAM17 exosomes and level of exosomal 20S proteasomes in terms of sex, age and tumor grade. Simultaneous decrease of ADAM10+/ADAM17-subpopulation of exosomes and level of exosomal 20S proteasomes in patients with metastatic CRC was observed compared with patients with non-metastatic CRC. The level of ADAM17+ exosomes significantly reduced in exosomes of CRCPs with metabolic syndrome compared to CRCPs without metabolic syndrome (3.97±0.71 (%) vs. 13.04±1.34 (%), respectively (p<0.05). A decrease in the 20S proteasomes level in plasma exosomes was revealed in CRCPs with metabolic syndrome compared with CRCPs without metabolic disorders (1.90±0.25 (r.u.) vs. 2.92±0.42 (r.u.) respectively ((p<0.05).
Conclusion:
According to findings of this study, it seems that exosomal proteases can be promising molecular predictors of hematogenous metastasis in patients with non-metastatic CRC. Further studies on subpopulation composition of exosomes CRCPs are need for elucidating the role of tetraspanin-associated and tetraspanin-non-associated exosomal proteases in CRC development and progression.
Keywords: Exosomes, ADAM10, ADAM17, 20S proteasome, colorectal cancer
Introduction
Colorectal cancer (CRC) remains one of the most common tumors of the gastrointestinal tract, and ranks 2 or 3 in the structure of cancer morbidity for both sexes in most of the countries(Yunusova et al., 2016). CRC initially manifests as metastatic in almost 20% of cases. About a third of patients with non-metastatic CRC experience tumor relapse or hematogenous liver metastases within three years (Loree and Kopetz, 2017). Therefore, it seems necessary to search for molecular predictors of hematogenous metastasis in patients with non-metastatic CRC with the aim of personalizing adjuvant treatment.
At present, extracellular vesicles (EVs), including exosomes (30-100 nm) and microvesicles (100-1,000 nm) species, are of great importance in the processes of tumor invasion and metastasis. They are detected in many biological fluids, such as plasma, blood serum, urine, saliva, breast milk, spinal fluid, as well as in pathological effusions (e.g. ascites) (Li et al., 2017). Tetraspanins CD9, CD63, and CD81 are common exosomal biomarkers (Yunusova et al., 2016; Tamkovich et al., 2018). Enzymes constitute up to 32% of the protein composition of exosomes (Mathivanan et al., 2010). Proteases , enzymes of the hydrolase class, have an important role during functional activity of EVs. Exosomes carry a complex of proteases and their activators. ADAM10 and ADAM17 are transmembrane “molecular scissors”, and are involved in shedding of limited proteolysis of premembrane proteins, leading to the cleavage of the extracellular domain of transmembrane proteins (Matthews et al., 2017). Substrates of sheddases are growth factor receptors (EGFR1, HER2, TGFβ-IIIR), adhesion receptors (CD44 and L1CAM), and Fas-L receptor. As a result of ADAM10 and ADAM17 shedding, signaling from growth factor receptors, Fas-L receptor, and adhesion molecules is modified, and soluble forms of receptors such as sCD44, sEGFR1, sHER2, sTGFbeta-IIIR, sFasL are documented in biological fluids (Lee et al, 2010; Levine, 2008). The primed proteolysis of CD23, L1CAM, and CD44 mediated by ADAM10, can occur in multivesicular bodies within the cells. This process also occurs in exosomes released from tumor cells, as shown for RPMI 8866 in the B-cell line of chronic myeloid leukemia, OVMz, and SKOV3 of the ovarian carcinoma cell lines (Stoeck et al., 2006). The tetraspanin-non-associated exosomal proteases mainly include 20S proteasomes and metalloproteinase PAPP-A (Yunusova et al., 2013; Kondakova et al., 2014). The 20S proteasome is a cylindrical multicatalitic structure consisting of two inner β rings flanked by two outer α rings. Proteomic studies revealed seven alpha and seven beta chains of 20S proteasomes in exosomes originating from mesenchymal stem cells (Lai et al., 2013). In cells and in exosomes 20S proteasomes are unable to recognize and bind polyubiqutinilated proteins; however, these proteasomes degrade some damaged and foreign proteins in an ATP and ubiquitin independent manner (Sharova and Zakharova, 2008). The important roles of these exosomal proteases are the regulation of molecular signaling from growth factor receptors and adhesion molecules and the regulation of cell motility and protein folding. The aim of this study was to evaluate the level of ADAM10, ADAM17 and 20S proteasomes in exosomes in colorectal cancer patients in association with clinical and histopathological parameters for search for promising exosomal markers associated with invasion and metastasis.
Materials and Methods
Patients
Blood samples of control subjects (CSs) (n = 10, 44.3±3.1 years) and patients with stage T2-4N0-2M0-1 colorectal cancer (n=60, 58.6±1.6 years) were obtained from the Cancer Research Institute of Tomsk National Research Medical Center. Control subjects underwent videocolonoscopy. Neither colorectal cancer nor other malignant neoplasms was detected in these patients, but they had colorectal polyps. Exclusion criteria for patients with colorectal cancer (CRCPs) were multiple primary CRC, CRC of Ia stage (T1N0M0), mid-rectal, and low-rectal cancer.
All patients with CRC, depending on the presence of the metabolic syndrome, were divided into two subgroups: patients with metabolic syndrome (33 pts.) and patients without metabolic syndrome (27 pts.). Based on International Diabetes Federation (2005), patients were included in metabolic syndrome group if they had abdominal type of obesity (waist circumference greater than 94 cm for men and 80 cm for women - at Caucasians), had high blood serum triglyceride level (more than 1.7 mmol/l), previously underwent dyslipidemia treatment, experienced reduction in high density lipoproteins (less than 1.03 mmol/L for men and 1.29 mmol/L for women), had high blood pressure (systolic over 135 mm. Hg, or diastolic more than 85 mm. Hg) or underwent hypertension therapy, had increased fasting blood glucose (more than 5.6 mmol/L), or suffered from diabetes mellitus type II. Clinical and histopathological parameters of patients are presented in the Table 1. This study was approved by Local Medical Research Ethics Committee , and all patients provided written informed consent.
Table 1.
Clinical and Histopathological Parameters of Colorectal Cancer Patients
| Parameters | N (%) |
|---|---|
| Sex | |
| Male | 29 (48.3) |
| Female | 31 (51.7) |
| Age | |
| ≤59 | 18 (30) |
| >59 | 42 (70) |
| Stage | |
| Non-metastatic | |
| Stage II | 27 (45) |
| Stage III | 25 (41.6) |
| Metastatic | 8 (13.4) |
| Tumor grade | |
| G1-G2 | 53 (88.3) |
| G3 | 7 (11.7) |
| Presence of metabolic syndrome | |
| Yes | 33 (55) |
| No | 27 (45) |
N, number of patients
Exosome isolation
Blood plasma exosomes were isolated using a combination of ultrafiltration and ultracentrifugation. Blood samples (18 ml) were collected in K3EDTA spray-coated vacutainers. The blood cells were pelleted by centrifugation at 1,200 g (bucket rotor, Labofuge 400R, Thermo Fisher) for 20 min at 4°C. To remove the cell debris, plasma samples were centrifuged at 17,000 g (angular rotor, centrifuge 5415R, Eppendorf) for 20 min at 4°C. To remove vesicles larger than 100 nm, the supernatant was diluted 5-fold with PBS (10 mM phosphate buffer, 0.15 M NaCl, pH 7.5) and filtered through 100 nm pore-size filter (Minisart high flow, 16553-K, Sartorius). For the exosome precipitation, the filtrate was centrifuged at 100,000 g (bucket rotor, Optima XPN 80, Beckman Coulter, USA) for 90 min at 4°C, the pellet was resuspended in 10 ml PBS, and re-centrifuged under the same conditions. The isolated exosomes were resuspended in 200 μl of PBS, aliquoted, frozen in liquid nitrogen, and stored at -80°C.
Electron microscopy of exosomes
For negatively stained samples, the isolated exosomes were adsorbed on copper grids with a carbon stabilized form-substrate for 1 min and 10 seconds and contrasted with a 2% phosphoric-tungstic acid. Grids were studied using Jem 1400 transmission electron microscope (Jeol, Japan), and the images were obtained with a digital camera Veleta (Olympus Corporation, Japan).
Protein quantification
To evaluate the protein concentration of exosomes, a NanoOrange Protein Quantitation kit (Molecular Probes, USA) was used in accordance with the manufacturer’s recommendations.
Flow cytometry analysis
The 4 μm-diameter aldehyde/sulphate latex beads (Thermo Fisher Scientific, USA) were incubated with anti-CD9 (ab134375, Abcam) or anti-CD24 (bsm-50424M, Bioss) antibodies at room temperature for 14 hours at gentle agitation. The aliquots of exosomes (about 30 μg exosomal protein) were incubated with antibody-coated latex beads in 100 μl of PBS at 4°C for 14 hours at gentle agitation. The reaction was blocked with 0.2 M glycine for 30 min at 4°C. The exosomes-antibody-bead complexes were washed twice with washing buffer (2% exosome depleted bovine serum in PBS) and incubated with a blocking immunoglobulin G (BD Biosciences, USA) at room temperature for 10 min with washing. Then, there was incubation with FITC-conjugated anti-tetraspanins (CD63, CD81, CD9, and CD24) antibodies (BD Biosciences, USA) for 50 min at 4°C. The complexes were washed twice with washing buffer and acquired per sample in a FACS Canto II (BD Biosciences). Data were analyzed using FACSDiva version 6.1 Software. The median fluorescence intensity (MFI) of the exosomes was compared with the isotypic control (BD bioscience, USA).
Analysis of ADAM10/ADAM17 subpopulations of exosomes
Aliquots of exosomes (about 30 μg exosomal protein) were incubated with 3×105 latex beads labeled anti-CD9 antibody in 150 μl of PBS at 4°C overnight at gentle agitation, blocked in 0.2 M glycine for 30 min, and stained with anti-ADAM10 (CD156c)-PE (5µl on test, Biolegend, USA) and anti-ADAM17/TACE antibody (dilution 1:10, LifeSpan BioSciences, USA) for 20 min at room temperature. Then, the complexes were stained with anti-Rabbit IgG secondary antibody, Alexa Fluor 488 (dilution 1:3,000, Thermo Fisher Scientific, USA). Single beads were gated and acquired in a Cytoflex (Becman Coulter, USA). Data were analyzed using CytExpert 2.0 Software.
Western blot analysis
Aliquots of exosomes were incubated for 90 min on ice with 7 μl lysis buffer (125 mM Tris- HCl, pH 7-8, 750 mM NaCl, 0.5% SDS, 5% Triton X-100) and 3 μl of protease inhibitors cocktail (1.3 mM Aprotinin (Sigma, USA), 0.33 mM Pepstatin A (ICN, USA), 1 μg/ml Leupeptin (ICN, USA)). Further, the samples of lysed exosomes were incubated with sample buffer for 7 min at 95° C and centrifuged at 13,000 g for 5 min. After that, centrifuged samples were applied to a 13% PAA gel for SDS-PAGE electrophoresis according to Lemmli. Following electrophoresis, proteins were transferred to the PVDF membrane (Immobylon, Millipore, USA). The membrane was blocked with 1X iBind Solution (Invitrogen, USA). Primary antibody to 20S proteasomes (Anti-Proteasome 20S alpha+beta antibody ab22673, Abcam, UK, dilution 1:2,000) binding, washing, secondary antibody (goat anti-rabbit IgG-HRP, Santa Cruz Biotechnology, 1:5000 dilution) binding (1/5,000 dilution) and washing were performed using an automated Western blotting device iBind Western Device (Thermo Scientific, USA) for 3 hours. Further, the membrane was incubated with the Amersham ECL western blotting detection analysis system (Amersham, USA). The visualization was performed on the system ChemiDoc Touch (Bio-Rad, USA). The density of the bands was estimated using “ImageLab” computer program. The results were standardized taking into account the level of CD63 in exosomes, and expressed as a related units of the level of the target protein in the exosomes in the control patients.
Statistical analysis
Statistical analysis was performed using Statistica (10.0). Each data set was first tested for a Gaussian distribution by using a Shapiro-Wilk normality test. All data were expressed as medians using interquartile ranges or means with standard errors. To evaluate the difference, Mann-Whitney U-test and Kruskal-Wallis test were used. P-values <0.05 were considered to be statistically significant.
Results
Characterization of plasma exosomes
The morphology of exosomes was confirmed by transmission electron microscopy method. Clearly, structured particles of a cup-shaped low electron density with a preserved membrane were found during the preparations of exosomes isolated from the blood plasma of CSs and CRCPs (Figure 1A, B). It was revealed that their morphology was not differ from that of exosomes in patients with other malignant neoplasms (Tamkovich et al., 2017; Tamkovich et al., 2018). During the preparations, particles called “non-vesicles” were found, which were very low or low density lipoproteins. Transmission electron microscopy showed that ultrafiltration in combination with ultracentrifugation allowed high-quality exosome preparations to be isolated with sufficient quantity for microscopic and other types of research.
Figure 1.
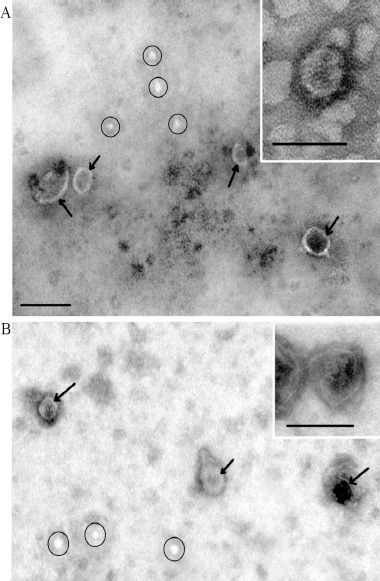
Total View of Exosome Preparation Obtained from Blood of: A – control subject, B – patient with colorectal cancer. Inserts shows exosomes. Arrows indicates exosomes, ellipses – «non-vesicles». Scale bars correspond to 100 nm. Electron microscopy, negative staining by phosphotungstate acid.
The isolated exosomes were also characterized by flow cytometry to examine the presence of exosomal markers (CD24, CD9, CD81, and CD63). The combination of conjugated and unconjugated antibodies made it possible to identify different subpopulations of exosomes. The decrease in the MFI of the exosome subpopulations was distributed as follows: CD24/CD9>CD9/CD81>CD24/CD63>CD9/CD63 from the plasma of CSs and
CD9/CD81>CD9/CD63>CD24/CD9>CD24/CD63 from the plasma of CRCPs. The compositions of exosomes subpopulation isolated from the blood plasma of both groups were different (Table 2). The CD9/CD81 subpopulation in plasma exosomes increased, while CD24/CD9 subpopulation of exosomes decreased in CRCPs compared to CSs. A NanoOrange Protein kit was used to determine the protein concentration. There was no statistically significant difference between both groups regarding the level of exosomal protein.
Table 2.
Expression of CD 24, CD 63 and CD 81 on the Surface of Exosomes from Plasma of CSs and CRCPs.
| Groups | CD 9+ exosomes | CD 24+ exosomes | ||
|---|---|---|---|---|
| CD 63 expression | CD 81 expression | CD 9 expression | CD 63 expression | |
| CSs | 513±76 | 645±97 | 1048±120 | 523±75 |
| CRCPs | 720±84 | 1020±105* | 680±85* | 500±71 |
- differences were significant compared to the control subjects (CSs). Data represent median fluorescence intensity (MFI) ± SEM.
Analysis of ADAM10/ADAM17 subpopulations of exosomes
Phenotyping of exosomes by flow cytometry implies analyzing of exosomes using antibody-coated beads. According to the results obtained in the previous stage of our work, we used anti-CD9 coated latex beads for the detection of ADAM10/ADAM17 subpopulations of exosomes in CSs and CRCPs plasma (Figure 2A). Distributions of subpopulations of ADAM10/ADAM17 of CSs and of CRCPs were different (Figure 2B, C, D). The ADAM10-/ADAM17- population predominated in plasma exosomes of CRCPs and the level of ADAM10+ exosomes were significantly higher in exosomes of CSs than CRCPs (Figure 2D). No difference was identified between subpopulations of ADAM10/ADAM17 exosomes in terms of sex, age, and tumor grade. However, a statistically significant decrease in the level of ADAM10+/ADAM17- exosomes was found in patients with metastatic CRC compared to CRCPs at stage III (Figure 3).
Figure 2.
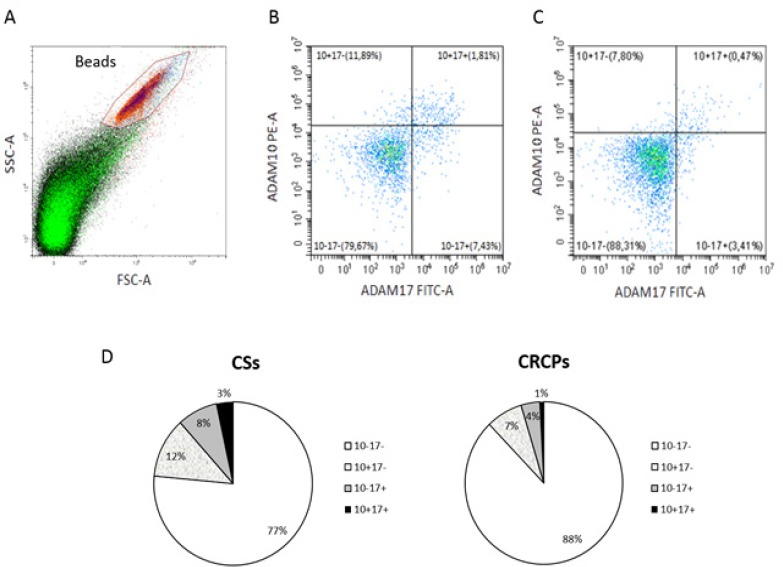
Differences between ADAM10/ADAM17 Exosomes Are Present in Plasma of CSs and CRCPs. A - Forward scatter area (FSC-A) and side scatter area (SSC-A) dot plot representing exosomal samples with aldehyde/sulphate latex beads. B, C – Double labeling ADAM10 versus ADAM17 of plasma exosomes of CSs (B) and CRCPs (C). D - Data show percentage ADAM10/ADAM17 subpopulations of plasma exosomes. Flow cytometry analysis of plasma exosomes
Figure 3.
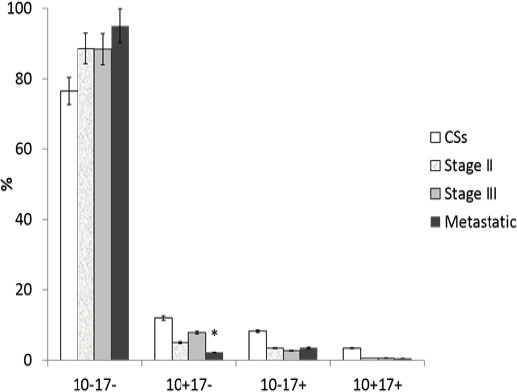
ADAM10/ADAM17 Subpopulations (in Percentage) of Plasma Exosomes of CRCPs, Depending on the Stage of the Colorectal Cancer. *- difference was significant (p<0.05) compared to CRCPs with stage III
20S proteasomes level of exosomes
Western blot analysis showed that the 20S proteasomes level in plasma exosomes of CRCPs was 1.8 times higher than that in the exosomes of CSs (Figure 4A). Significant difference in sex, age, and tumor grade in the 20S proteasomes level was not found. Maximal 20S proteasomes level in plasma exosomes was observed in CRCPs at stage II CRC. Significant difference was detected in the level of exosomal 20S proteasomes between metastatic and Stage II CRCPs. (Figure 4B).
Figure 4.
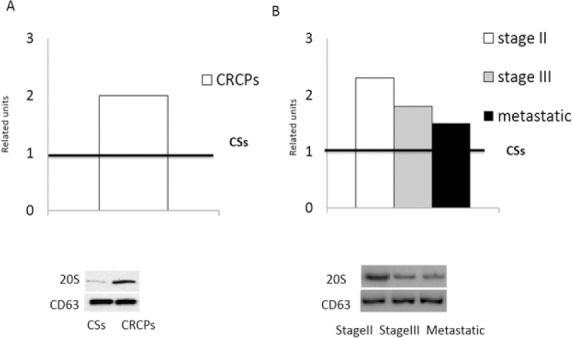
. 20S Proteasomes Level in Plasma Exosomes of CRCPs Compared to CSs (A). 20S proteasomes level in exosomes of CRCPs, depending on the stage of the colorectal cancer (B). The results were standardized taking into account the level of CD63 in exosomes and were expressed as a related units of the level of the target protein in the exosomes in the CSs. Vestern blot analysis of plasma exosomes
Relation between tetraspanin-associated and tetraspanin-non-associated exosomal proteases and metabolic syndrome in colorectal cancer patients.
In our study, the incidence of metabolic syndrome in CRCPs was 55%. Metabolic syndrome was more common in women (64%) than in men (36%). We investigated a possible association between the ADAM10/ADAM17 subpopulations of exosomes and the presence of metabolic syndrome in CRCPs. The level of ADAM17+/ADAM10- exosomes significantly reduced in CRCPs with metabolic syndrome (3.97±0.71%) compared to CRCPs without metabolic syndrome ((13.04±1.34 (%)) (p<0.05) (Figure 5A). The decrease in the 20S proteasomes level in plasma exosomes was found in CRCPs with metabolic syndrome compared to CRCPs without metabolic disorders, 1.90±0.25 (r.u.) and 2.92±0.42 (r.u.), respectively, (p<0.05) (Figure 5B).
Figure 5.
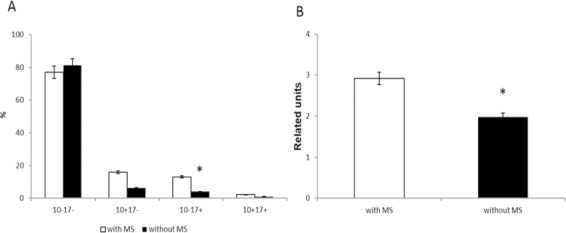
Expression of Tetraspanin-Assosiated and Tetraspanin-Non-Assosiated Proteases Depending on the Presence of Metabolic Syndrome: ADAM10/ADAM17 subpopulations (in percentage) in plasma exosomes of CRCPs with and without MS (A) and 20S proteasomes level (in related units) in plasma exosomes of CRCPs with and without MS (B). Note: MS – metabolic syndrome. *- differences were significant (p<0.05) compared to CRCPs with MS
Discussion
There are contradictory data on the number of circulating exosomes in patients with CRC. Apparently, this is due to various approaches in the isolation of exosomes and various approaches in the protein determination in isolated vesicles. Tian et al., (2018) reported that there was no significant difference in the mean concentration of EVs between cancer patients and healthy controls. At the same time, another group of researchers showed that the fraction of exosomes in CRCPs was statistically higher than healthy controls. High levels of exosomes in plasma of patients were correlated with high levels of carcino-embryonic antigen and with poorly differentiated tumors (Silva et al., 2012). A few data on the composition of subpopulation of exosomes of CRCPs have been found. CD147 and CD9 double-positive EVs were significantly higher in serum from CRCPs than healthy donors (Yoshioka et al., 2014). CD9/CD81 subpopulation of exosomes predominated in blood plasma of CRCPs and accounted for 31.8% (Tian et al., 2018), which corresponds to our data.
The role of proteases in the progression of cancer has been well studied, but the role of exosomal proteases in the formation and progression of malignant tumors has been poorly studied, especially in tumor cell lines. Data on the level of exosomal proteases in circulating exosomes in cancer patients are almost absent, not clarifying their role in the development of cancer. It is known that ADAM10 exhibits proteolytic activity in multivesicular bodies; hence, mature ADAM10 is already included in exosomes (Mathews et al., 2010). The inclusion of mature ADAM10 in exosomes and its proteolytic activity is regulated by tetraspanins CD9, CD81, and CD82 (Arduise et al., 2008). ADAM17 transfers to the endoplasmic reticulum in an inactive latent form, and interacts with the inactive iRHOM2 protein, promoting the translocation of pro-ADAM17 into the Golgi complex, which is activated by furin protein-convertase (Lopez-Verrilli et al., 2013). Following cell cultures (A549 epithelial cell line of alveolar pulmonary carcinoma, MDA-MB-231 cell line of epithelial breast cancer, human embryonic kidney line HEK293, and THP-1 human leukemia cell line), the release of ADAM17 in exosomes was observed (Groth et al., 2016). In our study, the loss of both proteases of ADAM10 and ADAM17 from surface of exosomes was associated with CRC formation.
A comprehensive proteomic analysis revealed a secretory path- and status-dependent signature of the exosomes released from tumor-associated macrophages associated with 20S proteasome subunits and ribosomal proteins (Zhu et al., 2015). Changes in chymotrypsin-like activity of proteasomes in CRC tissues, compared with correspond normal tissues, were observed in combination with an increased expression of immune subunits and/or proteasome activator PA28β associated with activity of 20S proteasome. The high level expression of 20S proteasomes in the exosomes of CRCPs was reported to cause increased expression of both 26S and 20S pools in the tumor tissue (Kondakova et al., 2014). High 20S level of proteasomes in circulating exosomes in CRCPs appears to be necessary for recipient cells to realize a multitude of proteasome functions in tumor cells and a tumor microenvironment (Sharova and Zakharova, 2008). The phenomenon of simultaneous decrease of ADAM10+/ADAM17- subpopulation of exosomes and exosomal 20S proteasomes in patients with metastatic CRC compared with patients with non-metastatic CRC requires further study. The loss of both tetraspanin-associated and tetraspanin-non-associated exosomal proteases in CRCPs was associated with the progression of cancer and hematogenous metastasis.
Metabolic syndrome is one of the leading risk factors for the development of cardiovascular diseases, type II diabetes mellitus, pathology of reproductive system, and some prevalent cancers, including endometrial cancer, postmenopausal breast cancer, and colorectal cancer (Yunusova et al., 2018). In accordance with our previously published data and previous studies, metabolic syndrome occurred in approximately 60% of cases in CRCPs at stages of II and III (Yunusova et al., 2015; Yunusova et al., 2016). The presence of metabolic syndrome was associated with an increase in the blood quantity of procoagulant EVs expressing tissue factor (TF +), derived from platelets (annexin V/CD41 + and CD62P +), endothelial cells (CD31 + / CD41- and CD62E +), and leukocytes (CD45 +) when compared with normal-weight healthy subjects (Martinez and Andriantsitohaina, 2017). At present, the properties of exosomes of CRCPs with obesity or metabolic syndrome have not been clarified yet. It has been shown that both EVs and exosomes carry a complex of markers associated with obesity and insulin resistance, and also it has been reported that adipocyte-derived exosomes promote cancer metastases (Eitan et al., 2017; Wang et al., 2017). The loss of ADAM17 and 20S proteasomes in exosomes was associated with the presence of metabolic syndrome in CRCPs. Studying subpopulations of tetraspanin-associated and tetraspanin-non-associated proteases in adipocyte-derived exosomes can help clarify the role of obesity in the redistribution of exosomal proteases.
In conclusion, significant differences in the level of tetraspanin-associated and tetraspanin-non-associated proteases of circulating exosomes in CRCPs were found in comparison with patients with colorectal polyps. Simultaneous decrease of ADAM10+/ADAM17-subpopulation of exosomes and exosomal 20S proteasomes in patients with metastatic CRC, compared with patients with non-metastatic CRC, was also observed. The loss of ADAM17 and 20S proteasomes in exosomes was associated with the presence of a metabolic syndrome in CRCPs.
Acknowledgments
This research was funded by Russian Foundation for Basic Research and the government of the Tomsk region of the Russian Federation(grant No: 18-415-703006).
References
- 1.Arduise C, Abache T, Li L, et al. Tetraspanins regulate ADAM10-mediated cleavage of TNF-alpha and epidermal growth factor. J Immunol. 2008;181:7002–13. doi: 10.4049/jimmunol.181.10.7002. [DOI] [PubMed] [Google Scholar]
- 2.Eitan E, Tosti V, Suire CN, et al. In a randomized trial in prostate cancer patients, dietary protein restriction modifies markers of leptin and insulin signaling in plasma extracellular vesicles. Aging Cell. 2017;16:1430–3. doi: 10.1111/acel.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groth E, Pruessmeyer J, Babendreyer A, et al. Stimulated release and functional activity of surface expressed metalloproteinase ADAM17 in exosomes. Biochim Biophys Acta. 2016;1863:2795–2808. doi: 10.1016/j.bbamcr.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Kondakova IV, Spirina LV, Koval VD, et al. Chymotrypsin-like activity and subunit composition of proteasomes in human cancers. Mol Biol. 2014;48:384–9. [PubMed] [Google Scholar]
- 5.Kondakova IV, Yunusova NV, Spirina LV, et al. Association between intracellular proteinase activities and the content of locomotor proteins in tissues of primary tumors and metastases of ovarian cancer. Russian J Bioorganic Chem. 2014;40:681–7. doi: 10.1134/s1068162014060089. [DOI] [PubMed] [Google Scholar]
- 6.Lai RC, Tan SS, Teh BJ, et al. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. J Proteomics. 2012;12:971907. doi: 10.1155/2012/971907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SB, Schramme A, Doberstein K, et al. ADAM10 is upregulated in melanoma metastasis compared with primary melanoma. J Invest Dermatol. 2010;130:763–73. doi: 10.1038/jid.2009.335. [DOI] [PubMed] [Google Scholar]
- 8.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. 2008;283:14177–81. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W, Li C, Zhou T, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16:145. doi: 10.1186/s12943-017-0706-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61:1795–806. doi: 10.1002/glia.22558. [DOI] [PubMed] [Google Scholar]
- 11.Loree J, Kopetz Recent developments in the treatment of metastatic colorectal cancer. Ther Adv Med Oncol. 2017;9:551–64. doi: 10.1177/1758834017714997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez MC, Andriantsitohaina R. Extracellular vesicles in metabolic syndrome. Circ Res. 2017;120:1674–86. doi: 10.1161/CIRCRESAHA.117.309419. [DOI] [PubMed] [Google Scholar]
- 13.Mathews JA, Gibb DR, Chen BH, Scherle P, Conrad DH. CD23 Sheddase A disintegrin and metalloproteinase 10 (ADAM10) is also required for CD23 sorting into B cell-derived exosomes. J Biol Chem. 2010;285:37531–41. doi: 10.1074/jbc.M110.141556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Matthews AL, Noy PJ, Reyat JS, Tomlinson MG. Regulation of A disintegrin and metalloproteinase (ADAM) family sheddases ADAM10 and ADAM17: The emerging role of tetraspanins and rhomboids. Platelets. 2017;28:333–41. doi: 10.1080/09537104.2016.1184751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharova N, Zakharova L. Multiple forms of proteasomes and their role in tumor fate. Recent patients on endocrine, metabolic and immune drug discovery. 2008;2:152–61. [Google Scholar]
- 17.Silva J, Garcia V, Rodriguez M, et al. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes Chromosomes Cancer. 2012;51:409–18. doi: 10.1002/gcc.21926. [DOI] [PubMed] [Google Scholar]
- 18.Stoeck A, Keller S, Riedle S, et al. A role for exosomes in the constitutive and stimulus-induced ectodomain cleavage of L1 and CD44. Biochem J. 2006;393:609–18. doi: 10.1042/BJ20051013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamkovich SN, Yunusova NV, Somov AK, et al. Comparative subpopulation analysis of plasma exosomes from cancer patients. Biochemistry (Moscow) Supplement Series B. Biomed Chem. 2018;12:151–5. [Google Scholar]
- 20.Tamkovich SN, Yunusova NV, Stakheeva MN, et al. Isolation and characterization of exosomes from blood plasma of breast cancer and colorectal cancer patients. Biochemistry (Moscow) Supplement Series B. Biomed Chem. 2017;11:291–5. doi: 10.18097/PBMC20176302165. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y, Ma L, Gong M, et al. Protein profiling and sizing of extracellular vesicles from colorectal cancer patients via flow cytometry. ACS Nano. 2018;12:671–80. doi: 10.1021/acsnano.7b07782. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Wu Y, Guo J, et al. Adipocyte-derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget. 2017;8:81880–91. doi: 10.18632/oncotarget.18737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshioka Y, Kosaka N, Konishi Y, et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yunusova NV, Kondakova IV, Kolomiets LA, et al. Serum adipokines and their receptors in endometrial and colon cancer patients: relationship with tumor invasion and metastasis. Voprosy Onkologii. 2015;61:619–23. [PubMed] [Google Scholar]
- 25.Yunusova NV, Kondakova IV, Kolomiets LA, et al. Molecular targets for the therapy of cancer associated with metabolic syndrome (transcription and growth factors) Asia Pac J Clin Oncol. 2018;14:134–40. doi: 10.1111/ajco.12780. [DOI] [PubMed] [Google Scholar]
- 26.Yunusova NV, Spirina LV, Frolova AE, et al. Association of IGFBP-6 expression with metabolic syndrome and adiponectin and IGF-IR receptor levels in colorectal cancer. Asian Pac J Cancer Prev. 2016;17:3963–9. [PubMed] [Google Scholar]
- 27.Yunusova NV, Spirina LV, Kondakova IV, et al. Relationship between the expression levels of PAPP-A metalloproteinase and growth and transcriptional factors in endometrial cancer. Biol Bull. 2013;40:253–9. [PubMed] [Google Scholar]
- 28.Yunusova NV, Tamkovich SN, Stakheeva MN, et al. The characterization of exosome from blood plasma of patients with colorectal cancer. AIP Conference Proceedings. 2016;1760:8470020070. [Google Scholar]
- 29.Zhu Y, Chen X, Pan QJ, et al. A comprehensive proteomics analysis reveals a secretory path- and status-dependent signature of exosomes released from tumor-associated macrophages. Proteome Res. 2015;14:4319–31. doi: 10.1021/acs.jproteome.5b00770. [DOI] [PubMed] [Google Scholar]


