Abstract
Objectives:
There is no consensus regarding the surgical or immunosuppressive treatment of idiopathic granulomatous mastitis (IGM). This study aimed to introduce a clinical classification system for IGM that might facilitate its treatment and predict recurrence.
Methods:
We analyzed the management of 68 patients with IGM at (Princess Basma Teaching Hospital and King Abdulla University Hospital (1994-2016) to find out if distinct patterns of presentation exist according to the following parameters: presence of a painful or painless breast mass, local inflammation, abscess formation, communication to the skin, and extra-mammary manifestation.
Results:
We identified four distinct patterns of IGM: A: (13.23 %) A hard, painless breast mass. B: (52.94 %) A hard, painful breast mass with gross inflammation. C: (26.47 %) A breast abscess-like presentation. D: (7. 35 %) A subacute presentation with ulceration, sinus, or fistula formation. Erythema nodosum might complicate any of these patterns. Wide local excision in pattern A was curative with zero recurrence rate. The recurrence rates in patterns B and C were 22.20 % and 50.00 %. Patterns B, C, and D were treated by a combination of surgery and prednisolone. In keeping with this, recent literature is in favor of a wider use of immunosuppression especially in the presence of pus and extra-mammary findings.
Conclusion:
IGM could be classified into 4 distinct patterns according to the presenting signs and symptoms. These patterns correlated with treatment, recurrence rate, and the gross operative findings. This is the first step toward a classification for IGM. Multicenter and Meta-analysis studies are essential for a comprehensive prognostic classification. Treatment of IGM in any institution should be the responsibility of a multidisciplinary team.
Keywords: Idiopathic granulomatous mastitis, immunosuppressive therapy, wide local excision, recurrence
Introduction
Kessler and Wolloch (1972) were first to identify idiopathic granulomatous mastitis (IGM) as a separate entity from other types of mastitis. Histologically, IGM is characterized by the presence of chronic granulomatous lobulitis in the absence of an obvious etiology. Clinically, patients present with a hard breast lump that imitates carcinoma (Kessler and Wolloch, 1972; Yaghan, 2004; Bani- Hani et al., 2004) Breast abscesses, fistulas, and sinuses are occasional findings (Prasad et al., 2017; Gudimani et al., 2015). Ultra-sonographic and mammographic findings are nonspecific and are occasionally interpreted as malignant (Yaghan, 2004, Prasad et al., 2017; Fazzio et al., 2016).
The etiology of IGM is still not clear. The most widely adopted theory considers IGM to be a local autoimmune disease that involves both humoral and cell-mediated immunity and results in non-caseating granulomas (Deng et al., 2017). No specific triggering antigens or infectious agents have been identified. IGM has been linked to parity, lactation, and pregnancy (Mahmodlou et al., 2017; Poniecka et al., 2001). Associations with alpha1-antitrypsin deficiency and hyperprolactinemia have also been reported (Bani- Hani et al., 2004; Mahmodlou et al., 2017; Altintoprak et al., 2014).
A wide local excision with steroid use is the most common treatment (Prasad et al., 2017; Deng et al., 2017; Mahmodlou et al., 2017). There is growing evidence that early introduction of immunosuppressive therapy might be useful in reducing the extent of surgery (Oran et al., 2013) Steroids are the most widely used agents in this context (Pandeyet et al., 2014). However, several reports have shown that other agents, such as methotrexate (Akbulut et al., 2011) or a combination of steroids and azathioprine (Konan et al., 2012), are alternative options.
In summary, the management of IGM faces some inherent challenges leading to its classical description as a mysterious entity that is difficult to diagnose and treat. Such challenges include the rarity of IGM, its clinical resemblance to breast carcinoma both clinically and radiologically, and its unknown etiology (Yaghan, 2004; Prasad et al., 2017; Fazzio et al., 2016). The similarity of the clinical presentation of IGM to breast carcinoma creates temporary fear and anxiety. The extent of surgery and indication and timing of immunosuppressive treatment are still determined by the preference of the treating surgeon rather than by evidence-based recommendations. Another challenging aspect of IGM is a high rate of local recurrence (Bani- Hani et al., 2004). With the exception of one recent report (Uysal et al., 2018), statistically based data on the predictors of recurrence of IGM are still lacking. In a recent publication (Yılmaz et al., 2018) retrospectively analyzed data about 53 patients with IGM to establish a severity score to predict the possibility of recurrence of IGM. The number of births, duration of lactation, BMI, presence of fistulas, abscess formation, and luminal inflammation were found to be significantly different between recurring and non-recurring patients. However, they concluded that the relatively low number of patients with recurrences was a major limitation in their study, which also rendered their data unsuitable for regression analysis. In view of the rarity of IGM, multi-center studies are needed to overcome this limitation.
There has been no previous classification system (s) for IGM. An ideal classification system should include elements related to the clinical picture, radiological findings, pathological variants, etiology, and prospects of treatment and prognostication. Unfortunately, the above-mentioned challenges during the management of IGM currently constitute an obstacle toward a comprehensive classification for IGM. The medical community will still need time until mature multicenter and meta-analysis studies are designed to provide statistically sound data. Bearing all these limitations in mind, this study aimed to explore whether our group of patients with IGM tend to have distinct patterns on initial presentation. If so, the presenting symptoms and signs may constitute practical parameters in proposing a clinically-based classification for IGM. In order to be useful, the proposed classification should give a practical clue to the treatment of IGM, and predicts the likelihood of its recurrence. This is a very early step in reaching a comprehensive classification for IGM in the future.
The absence of specific mammographic, ultrasonic, or MRI findings of IGM (Yaghan, 2004, Prasad et al., 2017; Fazzio et al., 2016) adds to a current need for a clinically-based classification.
Materials and Methods
Data collection
Pertinent clinical data regarding patients who were treated for IGM at (Princess Basma Teaching Hospital and King Abdulla University Hospital during the period from January 1994 to December 2016 were analyzed. These are the major tertiary centers in the North of Jordan and are affiliated to the Faculty of Medicine - Jordan University of Science and Technology. Data were collected by reviewing the computerized database and traditional patient files. The institutional review board (IRB) at King Abdulla University Hospital and its affiliated hospitals approved this study (575/2017).
Inclusion criteria
For the sake of this study, diagnosis of IGM depended on the availability of a histopathological report consistent with the diagnosis of IGM according to the original histological description by (Kessler and Wolloch, 1972). The characteristic histopathologic features of a non-caseating granulomatous inflammation centered on breast lobules, with the presence of epithelioid histiocytes, lymphocytes, plasma cells, polymorphonuclear leukocytes, and multinucleated Langhans-type giant cells constituted the main diagnostic requirement (Kessler and Wolloch, 1972).
Exclusion criteria
Secondary granulomatous processes associated with tuberculous mastitis, ductectasi, fat necrosis, post-surgical granulomatous reactions against foreign material, fungal infection, and sarcoidosis were excluded. Periodic acid-Schiff (PAS) and Ziehl-Neelsen (ZN) acid-fast stains should be negative.
Clinical patterns of IGM
The presenting symptoms and signs were the bases of the clinical classification reflecting real clinical practice. These included: Absence or presence of a mass, presence or absence of pain, degree of local inflammation, the presence of an associated abscess, communication to the skin via a fistula sinus or ulcer, and the presence or absence of extra-mammary systemic manifestation. Patients who shared a similar clinical picture were grouped in one pattern. The next step was to find out if such clinical patterns correlate with the received surgical and immunosuppressive treatment, rate of local recurrence, duration at initial presentation, size of the mass, the gross intra operative findings, and extra-mammary manifestations. The rarity of IGM and the retrospective nature of the study (like other available current reports) limit the applicability of advanced statistical analysis. Simple descriptive analysis and frequency counts were the only applicable option. Although this is a drawback, the clinical significance of such approach remains valid.
Results
Initially we identified 152 patients with the diagnosis of granulomatous mastitis. After applying the above inclusion and exclusion criteria, 84 patients were having secondary granulomatous mastitis. The remaining 68 patients were having IGM and they were the subject of this study.
Demographic and clinical characteristics of the patients
The pertinent clinical features are summarized in Table 1. The mean age at diagnosis was 37.75 years (SD ± 6.4, Range: 11-55). Diagnosis of IGM was confirmed by a true-cut biopsy in 48 patients (70.58 %), frozen section in 17 patients (25 %), and excisional biopsy in 2 patients. The permanent pathology reports for the cases diagnosed by frozen section were consistent with the per-operative diagnosis of IGM.
Table 1.
General Characteristics of Patients Diagnosed with Idiopathic Granulomatous Mastitis between 1994 and 2016 (n=68)
| C | Number | Percentage (%) | Notes |
|---|---|---|---|
| Presence of mass | 68 | 100 | Mean size: 5.8 cm (Range: 1-15) Mean duration: 3.01 months (Range 0.25-18) |
| Presence of pain | 58 | 85.30 | (Variable from mild to severe) |
| Abscess-like presentation | 18 | 26.47 | |
| Axillary lymphadenopathy | 15 | 22.05 | |
| Nipple retraction | 5 | 7.35 | |
| Ulcer, sinus, or fistula formation | 5 | 7.35 | |
| Surgery | |||
| -Wide local excision | 65 | 95.59 | |
| -Mastectomy | 2 | 2.94 | |
| -None | 1 | 1.47 | (Refused treatment) |
| Prednisolone | 29 | 42.64 | (Recurrent and severe cases) |
| Parity | |||
| -Parous | 65 | 95.58 | |
| -Nulliparous | 3 | 4.42 | |
| Pre-menopausal | 68 | 100 | |
| Pregnancy at presentation | 9 | 13.23 | |
| Lactation at presentation | 9 | 13.23 |
Ultrasonography was performed for 45 patients (66.17 %) and mammography for 36 patients (52.94 %). These techniques were mainly useful for ruling out neoplastic breast diseases rather than confirming the diagnosis of IGM. Surgical treatment consisted of a wide local excision in 65 patients and mastectomy in two patients. Prednisolone was given to 29 (42.64%) patients.
Nineteen patients (27.94 %) developed local recurrence. The mean time to local recurrence was 10.64 months (Range 1-72). Ipsilateral recurrence occurred in 15 patients out of the 19 patients (78.95 %) while four patients developed contralateral recurrence (21.05%). All patients with recurrence were treated by re-excision and prednisolone treatment. The rate of local recurrence in each pattern is summarized in Table 2.
Table 2.
Classification of Idiopathic Granulomatous Mastitis Based on the Clinical Presentation (n= 68)
| Pattern A | Pattern B | Pattern C | Pattern D | |
|---|---|---|---|---|
| Main classifying features | Mass: no pain, no inflammation | Mass: +pain +Inflammation | Abscess-like | Mass: + ulcer, sinus, or fistula |
| Number of patients | 9 (13.23 %) | 36 (52.94%) | 18 (26.47%) | 5 (7.35%) |
| Associated pain | No | Mild to moderate | Severe | Moderate |
| Local inflammation | No | Mild to moderate | Severe | Severe |
| Erythema nodosum | - | 1 patient | 2 patients | - |
| Recurrence | 0 (00 %) | 8 (22.20 %) | 9 (50.00%) | 2(40.0%) |
| Steroid treatment | 0 (00 %) | 10 (27.78%) | 16 (88.90%) | 3 (60.0 %) |
| Operative finding | Hard mass | Hard mass | -Hard mass + pus -Difficult margin identification | Ulcerative mass ± pus |
Patterns of clinical presentation
Four distinct patterns of presentation were identified: A, B, C, D. So, in this study group, IGM was classified into four groups accordingly Table 2. The classification was primarily based on the initial clinical presentation and presence or absence of extra-mammary findings. The following local signs and symptoms tended to follow distinct patterns: the presence or absence of variant degrees of mastalgia, the presence or absence of varying degrees of local inflammatory signs, and the presence or absence of pus. Among our study group of patients, the size of the mass at initial presentation, and the duration of symptoms did not serve as a practical categorizing element.
- Pattern A: nine patients (13.23 %) presented with a hard, painless breast mass mimicking carcinoma, with no signs of local inflammation Figure 1. Patients falling into this group were cured by a wide local excision with a zero recurrence rate.
Figure 1.
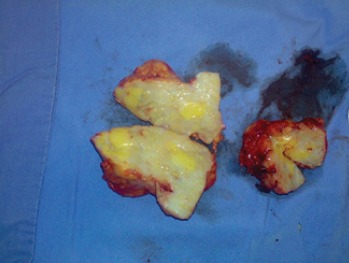
Pattern A of Idiopathic Granulomatous Mastitis. This patient presented with a large, painless breast mass in the absence of any signs of inflammation. At operation, a hard mass was found. The cut surface was whitish and glistening.
- Pattern B: 36 patients (52.94 %) presented with a hard, painful breast mass. A variable degree of local inflammatory signs was present but was not suggestive of the presence of an abscess Figure 2A, 2b. The rate of local recurrence in this group was 22.2 %.
Figure 2.
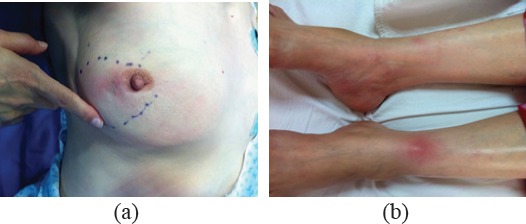
Pattern B of Idiopathic Granulomatous Mastitis. This pregnant woman presented with a painful breast mass. There was evidence of local inflammation (a). The patient also had erythema nodosum (b). Thus, the patient was classified as pattern Be.
- Pattern C: 18 patients (26.47 %) presented with a hard breast mass associated with an abscess. Severe pain, local inflammation, and pus were present. The recurrence rate in this group was 50 %.
- Pattern D: five patients (7.35 %) presented with a subacute to chronic presentation. Patients presented with a hard mass communicating to the skin and leading to the formation of an ulcer, sinus, or fistula Figure 3.
Figure 3.
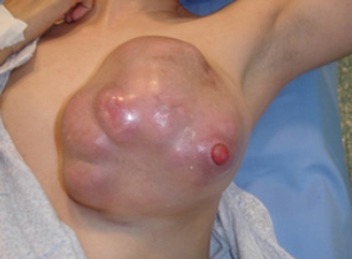
Pattern D of Idiopathic Granulomatous Mastitis. This patient presented with a huge mass destroying the breast and fungating through the remnants of the nipple. The lesion was stony hard.
Erythema nodosum (EN) affected one patient in pattern B and two patients in pattern C Figure 2B. In the proposed classification Table 2, the letter e in lowercase will be added to the main pattern of IGM when extra-mammary findings are present. For example, a patient who falls into pattern A and has EN will be classified as Ae. A summary for our proposed clinically based classification for IGM is provided in Figure 4.
Figure 4.
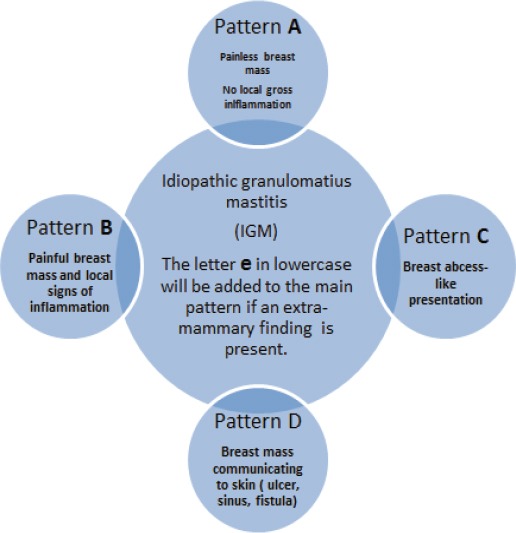
Clinically Based Classification for IGM. Four distinct patterns (A, B, C, D) were identified. The letter e in lowercase indicates extra-mammary findings. As an example, a patient presenting with an ulcerating mass and erythema nodosum will be classified as De.
The proposed 4 patterns (A, B, C, D) among our study group correlate with the treatment modality our patients received and the recurrence rate Table 2. None of the patients in pattern A received steroid treatment while 82.61 % of patients in patterns C and D received prednisolone. The proposed patterns also reflected the encountered gross intraoperative findings. In patterns A and B the mass was hard and adherent to the surrounding normal breast tissue Figure 1. The outer surface was reddish, with strands of yellowish fatty tissue. Upon sectioning, the fresh specimen typically has a whitish glistening surface with small-scattered cyst-like areas. Globules of fat tissue are present. In pattern C extensive tissue reaction surrounded the hard IGM mass. The mass was located deep in the associated pus. Occasionally, intervening pus gives a false impression of multiple masses. In pattern D IGM mass was stony hard. There was extensive fibrous tissue formation and significant parenchymal damage of the affected breast. These patients were in need of extensive surgery. In two of our patients, the lesion was so destructive that we had to perform a mastectomy, which was followed by reconstruction after disease quiescence Figure 3.
Discussion
Since its first classical description 45 years ago as a painless breast lump that mimics carcinoma (Kessler and Wolloch, 1972), the clinical spectrum of IGM has become wider. Additional local inflammatory signs, with occasional extra-mammary associations, were described (Prasad et al., 2017; Atak et al., 2016; Akin et al., 2017). These variable presentations, in the absence of unified recommendations for surgical and immunosuppressive treatment, and the high rate of local recurrence of IGM added extra challenges for the managing surgeon. There has been no previous classification system(s) for IGM. Rarity of IGM, absence of specific radiological findings, unknown etiology, and lack of sufficient multicenter-based data prompted us to propose a classification for IGM based on the symptomatology and the physical findings. With the presence of such inherent problems in the management of IGM a clinically- based classification might be a good starting point for the time being.
This study introduces a simple classification for IGM that provides therapeutic clues and helps to predict recurrence. The main classifying features in our proposed classification were the degree of inflammation (reflected by the degree of pain and cardinal signs of inflammation), and the presence or absence of extra-mammary findings. Four distinct patterns of IGM were identified among our study group (A, B, C, D), Table 2, and Figure 1-4.
In pattern A (a painless breast mass with no signs of local inflammation), the initial clinical picture exactly mimics carcinoma and is responsible for the traditional description of IGM as a mysterious surprising diagnosis after a histopathological study. Grossly, the mass is hard and adherent to the surrounding normal breast tissue Figure 1. Patients falling into this group are usually cured by wide local excision with no tendency toward recurrence; thus, additional systemic immunosuppression does not seem to be justified Table 2.
In pattern B (a painful breast mass with mild to moderate signs of local inflammation) Figure 2A, the rate of local recurrence increased to 22.2 %. The gross operative description of the mass is similar to that of pattern A. Patients with prominent inflammatory signs will possibly benefit from a short course of steroid treatment (Uysal et al., 2018). The presentation might mimic a locally advanced breast carcinoma. A high index of suspicion is the major contributing factor for early clinical diagnosis. However, the relatively short duration of symptoms compared to the size of the mass and the associated pain are the triggering clues. This was the most common pattern in the current study.
In pattern C (a breast abscess-like picture), a hard mass with varying amounts of pus is present. In our experience, complete excision in this pattern is the most difficult and time-consuming procedure compared to other patterns. The presence of an extensive tissue reaction, hardening of the associated mass, and difficulty in identifying adequate gross resection margins contribute to this difficulty. The recurrence rate in this group was 50.0 %. We suggest that the early introduction of systemic immunotherapy in this group reduces the recurrence rate and shortens the duration of illness. Our opinion is supported by the recent findings of Uysal (2018).
In pattern D (a hard breast mass communicating to the outside through ulceration, fistulae, and/or sinuses), the presentation mimics an ulcerating breast carcinoma or tuberculous mastitis Figure 3. Marked ipsilateral axillary lymphadenopathy is usually present. At operation, the mass is stony hard. There is extensive fibrous tissue formation. Despite the ugly appearance of the lesion, patients tend to have less pain compared to those with pattern C. Surprisingly, achieving a gross adequate resection margin is less difficult compared to pattern C. These patients are in need of extensive surgery and prolonged use of systemic treatment.
A growing evidence suggesting that the degree of inflammation, the presence of pus, and the presence of extra-mammary findings are indicative of the severity of IGM (Akin et al., 2017; Yılmaz et al., 2018) is in harmony with the potential clinical usefulness of our proposed classification. The observation that such patients will benefit from the early introduction of systemic steroids was supported by a recent study (Uysal et al., 2018). In this multicenter retrospective study involving 720 patients with IGM, a statistically significant association was found between IGM recurrence and the presence of breast infection. Such severe inflammatory signs were prominent features of patterns C and D in our proposed classification.
EN is a rare but recognized extra-mammary finding in IGM Figure 2B. Among the patients in this study group, EN affected patients with patterns B and C Table 2. However, in other reports, EN has been described in patients presenting with a clinical picture similar to the other patterns described in this study (Akin et al., 2017; Bani-Hani et al., 2004). We propose adding a lowercase e to the main pattern of IGM when extra-mammary findings are present Figure 4. Many investigators would recommend using an immunosuppressive treatment when EN is present (Atak et al., 2016; Akin et al., 2017).
Among our study group, the mean time to local recurrence was 10.64 months. We therefore recommend a minimum follow up period of one year for IGM, taking into consideration that the recurrence might occur in the contralateral side in about one fifth of the cases.
The natural history of IGM is not known. Weather the 4 patterns identified among our study group represent a progressive continuum, or various immunological host response remains unanswered. We are in favor of the second assumption because extra -mammary systemic manifestation, which seemingly indicate severity, can affect any of the four patterns, and the duration of symptomatology was not harmonizing with the recognized patterns.
This is the first proposal for a classification system for IGM. The classification was based on clinical grounds. The rarity of IGM and the retrospective nature of our study rendered our data unsuitable for deep statistical analysis. These drawbacks are shared by other available studies about IGM. Multicenter and meta- analysis studies will be mandatory to obtain statistically sound data regarding treatment modalities , treatment outcome, and a future comprehensive classification system . Only two reports of this type are available but with limited variables (Uysal et al., 2018; Lei et al., 2017). We presented our own approach in IGM. The treatment of IGM in any institution should be the responsibility of a multidisciplinary team until universal guidelines become available.
In conclusion, among our study group, IGM could be classified into 4 distinct patterns according to the presenting signs and symptoms. These patterns correlated with treatment, recurrence rate, and the gross operative findings. Further correspondences will explore the usefulness of our proposed clinical classification for IGM.
Funding Statement
None.
Acknowledgements
None.
References
- 1.Akbulut S, Arikanoglu Z, Senol A, et al. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284:1189–5. doi: 10.1007/s00404-010-1825-2. [DOI] [PubMed] [Google Scholar]
- 2.Akin M, Karabacak H, Esendağlı G, et al. Coexistence of idiopathic granulomatous mastitis and erythemanodosum: successful treatment with corticosteroids. Turk J Med Sci. 2017;47:1590–2. doi: 10.3906/sag-1611-100. [DOI] [PubMed] [Google Scholar]
- 3.Altintoprak F, Kivilcim T, Ozkan OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. 2014;2:852–8. doi: 10.12998/wjcc.v2.i12.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atak T, Sagiroglu J, Eren T, Özemir İA, Alimoglu O. Strategies to treat idiopathic granulomatous mastitis: retrospective analysis of 40 patients. Breast Dis. 2015;35:19–4. doi: 10.3233/BD-140373. [DOI] [PubMed] [Google Scholar]
- 5.Bani-Hani KE, Yaghan RJ, Matalka II, Shatnawi NJ. Idiopathic granulomatous mastitis: time to avoid unnecessary mastectomies. Breast J. 2004;10:318–2. doi: 10.1111/j.1075-122X.2004.21336.x. [DOI] [PubMed] [Google Scholar]
- 6.Deng JQ, Yu L, Yang Y, et al. Steroids administered after vacuum-assisted biopsy in the management of idiopathic granulomatous mastitis. J Clin Pathol. 2017;70:827–1. doi: 10.1136/jclinpath-2016-204287. [DOI] [PubMed] [Google Scholar]
- 7.Fazzio RT, Shah SS, Sandhu NP, Glazebrook KN. Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging. 2016;7:531–9. doi: 10.1007/s13244-016-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gudimani SC, Rohit KC, Mithun VV, Gowda CKN, Deepa AB. Idiopathic granulomatous mastitis: diagnostic and therapeutic challenges to general surgeon. Breast Dis. 2015;35:67–2. doi: 10.3233/BD-140375. [DOI] [PubMed] [Google Scholar]
- 9.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. Am J Clin Pathol. 1972;58:642–6. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 10.Konan A, Kalyoncu U, Dogan I, et al. Combined long-term steroid and immunosuppressive treatment regimen in granulomatous mastitis. Breast Care (Basel) 2012;7:297–1. doi: 10.1159/000341388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei X, Chen K, Zhu L, et al. Treatments for idiopathic granulomatous mastitis: Systematic review and meta-analysis. Breastfeed Med. 2017;12:415–21. doi: 10.1089/bfm.2017.0030. [DOI] [PubMed] [Google Scholar]
- 12.Mahmodlou R, Dadkhah N, Abbasi F, Nasiri J, Valizadeh R. Idiopathic granulomatous mastitis: dilemmas in diagnosis and treatment. Electron Physician. 2017;9:5375–9. doi: 10.19082/5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oran EŞ, Gürdal SÖ, Yankol Y, et al. Management of idiopathic granulomatous mastitis diagnosed by core biopsy: a retrospective multicenter study. Breast J. 2013;19:411–8. doi: 10.1111/tbj.12123. [DOI] [PubMed] [Google Scholar]
- 14.Pandey TS, Mackinnon JC, Bressler L, et al. Idiopathic granulomatous mastitis-a prospective study of 49 women and treatment outcomes with steroid therapy. Breast J. 2014;20:258–6. doi: 10.1111/tbj.12263. [DOI] [PubMed] [Google Scholar]
- 15.Poniecka AW, Krasuski P, Gal E, et al. Granulomatous inflammation of the breast in a pregnant woman. Acta Cytol. 2001;45:797–1. doi: 10.1159/000328309. [DOI] [PubMed] [Google Scholar]
- 16.Prasad S, Jaiprakash P, Dave A, Pai D. Idiopathic granulomatous mastitis: an institutional experience. Turk J Surg. 2017;33:100–3. doi: 10.5152/turkjsurg.2017.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uysal E, Soran A, Sezgin E Granulomatous mastitis study group. Factors related to recurrence of idiopathic granulomatous mastitis: what do we learn from a multicentre study? ANZ J Surg. 2018;88:635–9. doi: 10.1111/ans.14115. [DOI] [PubMed] [Google Scholar]
- 18.Yaghan RJ. The magnetic resonance image findings of idiopathic granulomatous mastitis. Saudi Med J. 2004;25:1715–9. [PubMed] [Google Scholar]
- 19.Yılmaz TU, Gürel B, Güler SA, et al. Scoring idiopathic granulomatous mastitis: An effective system for predicting recurrence? Eur J Breast Health. 2018;14:112–6. doi: 10.5152/ejbh.2018.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]


