Abstract
Objective:
Quadrivalent human papillomavirus (QHPV) vaccine has been advised for routine vaccination of pre-adolescent girls globally, and a two-dose QHPV vaccination schedule has been introduced in Indonesia to vaccinate 5th and 6th grade elementary school female students. This post-marketing surveillance study evaluated the possible adverse events following immunization with the two-dose QHPV vaccine in Indonesia.
Methods:
Girls studying in grade 6 of five designated elementary schools in Jakarta, receiving their 2nd dose of QHPV vaccine and provided informed consent (represented by their parents), were included in the study. Students who had received other immunizations either simultaneously or <1 month ago were excluded. Local and systemic reactions noted at 30 min, and 72 h to 28th day, after the immunization were recorded using a Children Symptom Dairy Card/Kartu Harian Anak Sekolah (KHAS/Student Daily Card).
Results:
A total of 500 students from 20 schools were included. No serious adverse events were reported during the study period. Fever (systemic reaction) of mild intensity was noted in 1.6 % (n=8) of participants, which subsided after day 6. Local reactions such as pain, redness and swelling were noted in 59.6% (n=295), 23.6% (n=118), and 17.2% (n=86) of participants, respectively. These resolved without any intervention in majority of the cases after day 5.
Conclusion:
These results along with the safety data from the pre-licensure clinical trials confirm the favorable safety profile of QHPV vaccine in pre-adolescent girls. The school-based two-dose QHPV immunization program in Indonesia is a safe and effective strategy for optimizing HPV vaccine coverage among pre-adolescent girls.
Keywords: Human papillomavirus vaccine, immunization, elementary school, post marketing, Indonesia
Introduction
Cervical cancer is listed as the 2nd most common cause of cancer as well as cancer related deaths among women in Indonesia. It has been estimated that every day, 26 Indonesian women die because of cervical cancer (Bruni et al., 2017). The association between oncogenic human papillomavirus (HPV) strains and cervical cancer is well known. Among these, HPV types 16 and 18 have been commonly associated with cervical cancer (Burd, 2003).
Sexual activity and age significantly influences the transmission of HPV. The HPV infection is often asymptomatic and barrier methods cannot prevent the risk of transmission completely (Kaarthigeyan, 2012). Prophylactic vaccination during younger ages has been noted to be 96%-100% effective in preventing HPV related cervical cancers (Barr and Tamms, 2007). As per the recent World Health Organization (WHO) position paper (WHO, 2017), HPV vaccines are recommended as part of a comprehensive and coordinated strategy for preventing cervical cancer as well as other HPV-related diseases. The WHO recommends that girls aged 9-14 years should be the primary target for HPV vaccination program. Accordingly, the HPV vaccination program has been implemented globally in many countries with 71 countries (37%) including the HPV vaccine in their national immunization program (NIP) for girls (WHO, 2017).
In Indonesia, the Indonesian Ministry of Health has implemented a school-based immunization program called Bulan Imunisasi Anak Sekolah (BIAS) in 1998. The BIAS program was carried out along with a school health program called Usaha Kesehatan Sekolah (UKS). As a part of this, measles, diphtheria and tetanus vaccines are administered to children studying in the first grade of elementary school, while tetanus and diphtheria (Td) vaccine is administered to those in the second and fifth grade of elementary school (School Immunization in Indonesia, 2014).
In 2016, the Indonesian Ministry of Health initiated a trial project for assessing the inclusion of HPV vaccination in the BIAS program, in the DKI Jakarta province using the quadrivalent (QHPV) vaccine. This demonstration project is a mandatory step before implementation of the NIP and this would be expanded to other provinces on an annual basis. Accordingly, the QHPV immunization program was implemented in Surabaya and Yogyakarta in 2017 (Indonesian Ministry of Health, 2017). The DKI QHPV vaccination coverage (first dose) in 2016 among girls in fifth grade of elementary school was 92.0% (n=66,094), and among the girls in sixth grade for the second dose (in 2017) was 99.98% (n=48,044). In 2017, the overall coverage in a new cohort of girls in fifth grade of elementary school in Jakarta was 93.2% (n=50,894), while Yogyakarta and Surabaya provinces reported a coverage of 99.8% (n=7,649) and 95.1% (n=22,010), respectively (Internal Data, 2018).
The acceptance towards HPV immunization program is significantly influenced by vaccine confidence. In some countries, HPV immunization program has not been successful owing to adverse opinion among the public and media, reduced confidence in the safety profile of the vaccine, and also the inability of the government to quickly handle vaccine related issues (State of Vaccine Confidence, 2014; HPV Vaccination in Japan, 2014; Case Study C, 2018).
As with all vaccines, the safety of HPV vaccine has been evaluated in large pre-licensure clinical trials and is being monitored through post-marketing surveillance systems worldwide. The Global Advisory Committee on Vaccine Safety (GACVS) considers HPV vaccines to be extremely safe (Weekly Epidemiological Record, 2017).
Nevertheless, as per the policy requirements, Indonesian government needs local safety data for every vaccine planned to be included in the NIP. Thus, the objective of this study was to evaluate the potential systemic and local adverse events following immunization (AEFI) with the QHPV vaccine, in a school-based program. The study also intends to provide safety data to support the inclusion of QHPV vaccine in the NIP in Indonesia.
Materials and Methods
Subjects and Study Design
This was a prospective cohort study, which assessed the systemic and local reactions noted within 28 days following administration of the 2nd dose of quadrivalent vaccine (Gardasil™ - Human Papillomavirus Quadrivalent - Types 6, 11, 16, and 18– Recombinant Vaccine, Merck and Co., Inc.). Girls studying in sixth grade of elementary school in 5 districts of the Special Province of Jakarta (North Jakarta, West Jakarta, Central Jakarta, East Jakarta and South Jakarta), were assessed between July 2017 and February 2018. The QHPV vaccine immunization program in Indonesia was first implemented in Jakarta, and hence this province was chosen for the study. Ethical approval was obtained from the Ethics Committee Medical Research Faculty of Medicine Universitas Indonesia, Cipto Mangunkusumo Hospital, Jakarta (Protocol Number: 17-03-0243).
Girls studying in the sixth grade of elementary schools in Jakarta province area who had received at least 1 dose of the QHPV vaccine previously (in 5th grade) during the government pilot project in 2016, and agreed to provide informed consent (from parents/guardians) indicating that they would accept and follow all terms and conditions during the observational period, were included (Figure 1). The second dose of the QHPV vaccine was administered to all the study participants in October 2017 as per the protocol under standard vaccination precautions. Subjects who were administered any other vaccine/s either simultaneously or within one month of HPV immunization were excluded. Subjects were observed for a maximum period of 28 days after receiving the second dose. The observation period covered the entire period from initial visit to the last follow-up visit at 28 days. Loss to follow up was considered if the included subjects withdrew consent, or did not complete the observation period.
Figure 1.
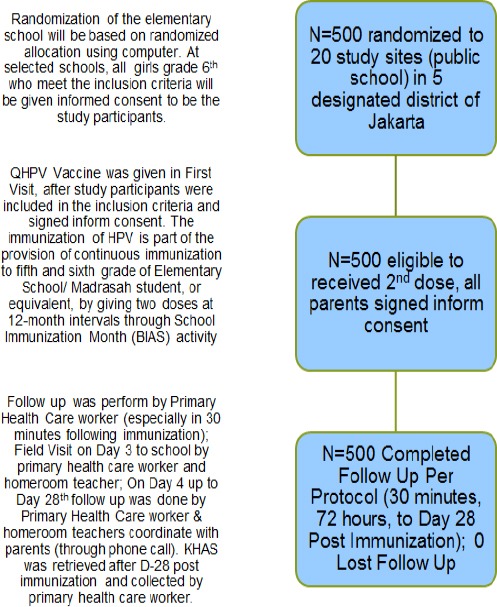
Flowchart Describing the Study Protocol
Data Collection
Local and systemic reactions were recorded using a children symptom card known as Kartu Harian Anak Sekolah (KHAS/Student Daily Card) after 30 minutes, 24, 36, 48, and 72 hours, and up to 28 days, after immunization. All reactions (if any) from the first 30 minutes up to 72 hours after immunization were recorded by a primary health care worker, while rest of the reactions (if any), from 72 hours up to 28 days after immunization, were recorded by the students, parents, or home room teachers. These records were later validated by a primary health care worker (Figure 2). The homeroom teacher and primary health care worker were trained for filling the KHAS questionnaire. They were also trained to observe, record, and handle potential adverse reactions. The five study sites were chosen from a stratified randomized list of government elementary schools in Jakarta.
Figure 2.
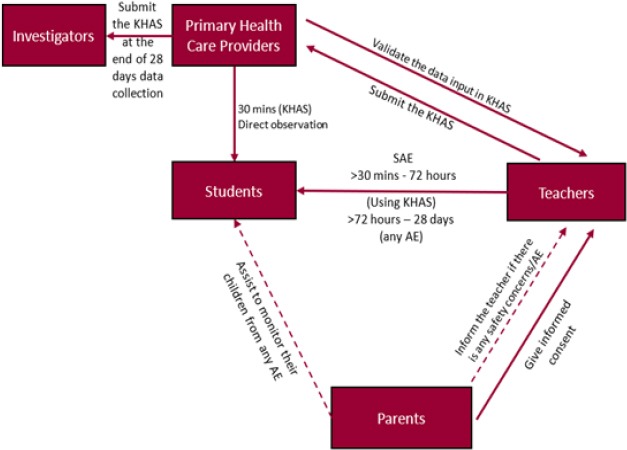
Protocol for Recording Symptoms
Definition of signs and symptoms recorded
Systemic reactions were defined as the occurrence of one or more symptoms (estimated or not expected) including fever and other systemic reactions, within 28 days of immunization. Body temperature > 38°C was considered representative of fever (Heininger, 2013). In this study, fever was categorized into mild (38.0-38.5°C), moderate (38.6 – 39.0°C), and severe (>39°C), based on the temperature noted.
Local reaction was defined as any changes over the skin at and around the injection site along with other associated symptoms. Local reactions that were recorded included pain, redness, and swelling around the injection site, and any other local reaction (if any). Pain was categorized based on its severity as follows: mild: subjects react if the injection site is touched; moderate: subject feels pain if the injection site is touched; and severe: pain when the arm is moved.
Redness, indurations, swelling, and other local reactions at the injection site were measured using plastic bracelets (plastic bangle) of 3 different diameters and were categorized as follows: mild: if the lesion was restricted to a diameter <2.5 cm; moderate: if the diameter of the lesion was between 2.5-5 cm; and severe: if the diameter of the lesion was >5cm (Heininger, 2013; Indonesian NRA, 2011; Gidudu et al., 2012).
Other systemic reactions were grouped based on their severity as: light/mild: tolerable reactions; moderate: reactions that cause discomfort/ somewhat affect daily activities; severe: reactions that are bothersome, affect daily activities, and need medication.
Statistical Analysis
A descriptive analysis of the data was carried out to calculate the frequency of adverse events (AEs) and serious adverse events (SAEs) in the study population at different timelines. The number and percentage of subjects with the frequency of local/systemic reaction, whether or not related to vaccine, were calculated for each of the timeline defined earlier. The data was analyzed using the Statistical Package for Social Sciences 24 (SPSS24) software.
Results
A total of 500 girls studying in sixth grade across 5 elementary schools in the Jakarta province of Indonesia were included in the study. Demographic data along with clinical history of the study participants has been enumerated in Table 1.
Table 1.
Demographic Data of Participants
| Parameter | n (%) |
|---|---|
| Age (years) | |
| 10 | 1 (0.2) |
| 11 | 194 (38.8) |
| 12 | 281 (56.2) |
| 13 | 23 (4.6) |
| 14 | 1 (0.2) |
| Regionwise distribution | |
| North Jakarta | 100 (20) |
| South Jakarta | 100 (20) |
| Central Jakarta | 100 (20) |
| West Jakarta | 100 (20) |
| East Jakarta | 100 (20) |
| Medical/Clinical History before HPV Vaccination | |
| Asthma | 1 (0.2) |
| Flu and Cough | 2 (0.4) |
| Lump on back neck | 1 (0.2) |
| Lump on right neck | 1 (0.2) |
| Fever | 1 (0.2) |
| Dyspepsia | 9 (1.8) |
| Bronchitis | 1 (0.2) |
| Nasal Congestion | 1 (0.2) |
| No Symptom | 483 (96.6) |
| Total participants | 500 (100) |
Serious adverse events
No SAEs were noted following immunization with the QHPV vaccine in any of the participants during the study duration. Additionally, none of the subjects with a history of other conditions prior to vaccination experienced any SAEs.
Adverse events
A total of 514 adverse events (systemic and local reactions) were reported during the study period. The details regarding the same have been enumerated below. All these events resolved within 3 days.
Systemic reactions
Fever
There were no incidences of moderate or severe fever during the study period. Mild fever was reported in 1.6% participants (n=8), with the highest peak noted on the 5th day after immunization (Figure 3). Mean fever duration was 1.75 days (Figure 4).The fever subsided after the 6th day.
Figure 3.
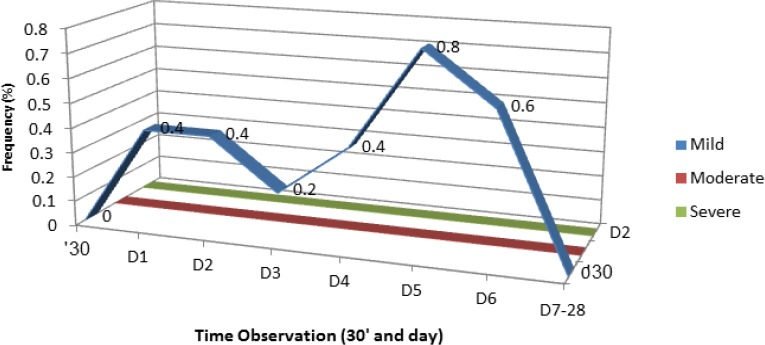
Subjects Distribution by Fever Level and Time of Observation
Figure 4.
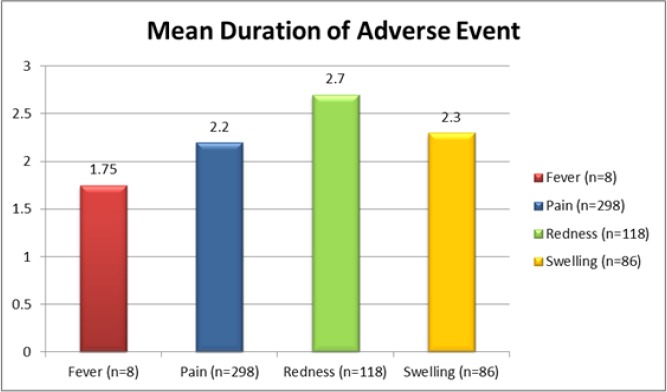
Mean Duration of Adverse Event in Day for Systemic and Local Reaction Regardless of Severity
Other systemic reactions
Other systemic reactions reported included malaise (0.2%; n=1), myalgia (0.2%; n=2), and arthralgia (0.2%; n=1). All these symptoms were transient and resolved subsequently without any intervention.
Local reactions
Pain
Majority of the participants (59.6%; n=298) experienced some degree of pain during the study period. Severe pain was reported within the first 30 minutes by 12% (n=62) of the participants and it resolved by the 3rd day (Table 2). Mild and moderate pain subsided after day 12 and 14, respectively. The mean duration of pain was 2.2 days (Figure 4).
Table 2.
Distribution of Local Pain and Redness According to the Time of Observation and Severity Level
| No. | Adverse Event Following Immunization* | Post Marketing Surveillance, Jakarta, 2017 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 30’ | D1 | D2 | D3 | D4 | D5 | D6 | D7-28 | ||
| Local Reaction | |||||||||
| 1 | Pain (n= 298; 59.6%) | ||||||||
| Severity Time | 30’ | D1 | D2 | D3 | D4 | D5 | D6 | D7-28 | |
| Mild | 13.80% (n=69) | 15.20% (n=76) | 12.60% (n=63) | 14.20% (n=71) | 3% (n=15) | 1% (n=5) | 0.60% (n=3) | 2.80% (n=14) | |
| Moderate | 18.40% (n=92) | 15.80% (n=79) | 14.40%(n=72) | 7.60% (n=38) | 1% (n=5) | 0 | 0 | 0.80% (n=4) | |
| Severe | 12.40% (n=62) | 5.80% (n=29) | 4.40% (n=22) | 1% (n=5) | 0 | 0 | 0 | 0 | |
| 2 | Redness (n= 118; 23.6%) | ||||||||
| Severity Time | 30’ | D1 | D2 | D3 | D4 | D5 | D6 | D7-28 | |
| Mild | 18.80% (n=94) | 11.60% (n=58) | 9.60% (n=48) | 9.20% (n=46) | 6.40% (n=32) | 4.00% (n=20) | 2.00% (n=10) | 8.20% (n=41) | |
| Moderate | 2.60% (n=13) | 2.40% (n=12) | 0.40% (n=2) | 0 | 0.20% (n=1) | 0 | 0 | 0 | |
| Severe | 0.40% (n=2) | 0.40% (n=2) | 0 | 0 | 0.40% (n=2) | 0 | 0 | 0 | |
Adverse events were evaluated after 30 minutes (30’) and on days 1 to 28 (D1, D2, D3, D4, D5, D6, D7-28)
Redness
Redness at the site of injection (regardless of severity) was experienced by 23.6% (n=118) of the participants with the redness being of mild severity in majority of these cases (Table 2). The redness was noted within 30 minutes after the injection, gradually decreased, and resolved after 12 days following immunization. The mean duration of redness at the site of injection was 2.7 days (Figure 4).
Swelling
Swelling at the site of injection (regardless of severity level) was noted in 17.2% (n=86) of the participants and was of mild severity in majority of the cases. The swelling occurred within 30 minutes after the injection, gradually decreased, and completely subsided after 10 days following immunization (Figure 5). The mean duration of swelling was 2.3 days (Figure 4).
Figure 5.
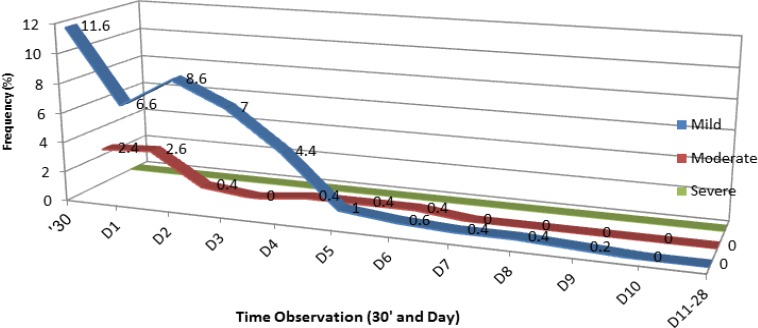
Distribution of Local Swelling According to the Time of Observation and Level of Severity
Discussion
There were no SAEs reported in the current study following immunization with the QHPV vaccine. Among systemic reactions, mild fever was noted in 1.6% of the participants. This incidence was much lower compared to earlier phase III QHPV clinical trials (9.9% in QHPV vaccine group, age 9-26 years old) (Kohl et al., 2007; MSD Data on File). Data from other school based post licensure/registry study (2009-2013 in Slovenia) also reported a higher incidence of fever (10%) following immunization with QHPV vaccine (Subelj et al., 2016).
The overall pain prevalence in the current study was found to be 59.6%. This was slightly higher (53%) compared to another post licensure study (Slade et al., 2009), but lower (81.3%) compared with a phase III HPV clinical trial (MSD Data on File). Pain associated with immunization can influence the compliance rate among adolescents. In a study which evaluated the prevalence of non-compliance to immunizations reported that about 40% of the adolescents (aged between 13 and 17 years) avoided immunization due to fear of needles (Taddio et al., 2012). Nevertheless, the mean duration of pain in the current study was about 2 days, which seems to be an acceptable duration. Steps taken to create awareness about the importance of these vaccinations can be useful in improving the compliance to such immunization programs.
Among other local reactions, the prevalence of swelling was lower (17.2% vs.22%) compared to the post licensure study by Slade et al., (2009), and with the phase III HPV clinical trial (17% vs. 24.2%) (MSD Data on File). The prevalence of redness was also lower (24% vs.28%) compared to the post licensure study (Slade et al., 2009), and almost similar (23%) to that noted in the phase III HPV clinical trial (MSD Data on File).
These variations may be the result of a difference in the sample size used in each study. Additionally, the current study used the services of homeroom teachers and primary health care workers to record the AEs, while phase III clinical trial/other post licensure studies commonly employ clinical pediatricians/specialist health care professionals. The level of education of the parents/students/teachers may also have had an impact on interpretation of the instructions in the KHAS card.
Vaccines are approved for use by the regulatory authorities only after they are proven to be safe and effective. However, vaccines may often be associated with minor AEs in majority of the cases and serious reactions in rare cases. Therefore, it is essential to monitor the incidence of AEFI to ensure continued acceptance towards mass immunization programs (Vaccine Safety Basics, 2014; Stillo et al., 2015).
A meta-analysis which carried out a head-to-head comparison of bivalent, quadrivalent and 9-valent HPV vaccines, reported a lower risk of pain and redness with the QHPV vaccine compared to the other 2 types. Additionally, the risk of swelling as well as systemic symptoms was also lower with the QHPV vaccine. The study concluded that although the HPV vaccines were associated with an overall higher risk of injection site symptom compared to placebo or other (hepatitis A and B) vaccines, most of the adverse events were transient (Ogawa et al., 2017).
The administration of HPV vaccines has been suggested to provide health as well as social benefits, especially in those living in low-resource settings (Nicol et al., 2016). Public HPV vaccination program has been accordingly implemented in numerous developing countries. However, the success and feasibility of mass immunization program for adolescents depends on the efficient integration of public health programs with schooling systems (Fregnani et al., 2013). Further, age appropriate education about the complication of HPV infections and the role of immunization in preventing these, can significantly increase the compliance among young adolescents (Naleway et al., 2012).
It has been proven that all HPV vaccines are safe and well tolerated in general. Therefore, efforts to increase the vaccination coverage should be made, as it is an important tool to decrease the HPV disease burden (Stillo et al., 2015). This is especially true in developing countries such as Indonesia. In a recent study, it was reported that the QHPV vaccination substantially reduced the incidence of genital warts, cervical intraepithelial neoplasia and cervical cancer among Indonesian women. Further, it also improved the quality of life and was considered to be a cost-effective method as it could reduce the total disease cost by 31.8% (Kosen et al., 2017). The safety of QHPV vaccine among adolescents has also been proved in a long term study where it was reported to be associated with clinically effective protection and sustained antibody titers over a period of 10years (Ferris et al., 2017).
The current study has a few limitations. An active surveillance was not conducted following the administration of the first dose of QHPV vaccine. Further, no safety signals were recorded following the administration of the first dose of QHPV vaccine. Although the history of local/systemic reactions experienced following the first dose was recorded before the administration of the second dose, this data may not be considered valid for passive surveillance reporting system (as the memory recall ability of the subjects cannot be validated). Further, adequate education of the health care providers and the society would be required for successful implementation of passive surveillance reporting system and active reporting of any AEs following administration of HPV vaccines. This combination of both active and passive safety surveillance systems can provide a comprehensive means of monitoring QHPV vaccine safety, and represent one of the most extensive safety evaluations in Indonesia. Therefore, the information gathered in the current study may be insufficient to deduce a systematic causality relationship. Nevertheless, temporal relationship of an AE to a vaccine does not mean causality (Siegrist et al., 2007; Clothier et al., 2013).
In conclusion, the implementation of 2nd dose of HPV4 vaccination among girls studying in sixth grade of elementary school was associated with a good and tolerable safety profile. The QHPV immunization program is a safe and effective strategy for optimizing HPV vaccine coverage in public programs and hence, its inclusion in the Indonesia NIP seems beneficial. The outcome of the current study as well as the extensive post-approval safety surveillance data should be considered to reinforce and implement the national recommendation for HPV immunization among preadolescents and adolescents in Indonesia.
Acknowledgements
We would like to acknowledge the efforts of Arum Handayani, SE1; Santi Ikrari, SKM1; Poppy Brilia Safitri, SE1; Ade Putra, SKM1; Eka Desti Purwanti, SKM2; Widyastuti, MD, MKM3; drg.Nina, MKM3; Anuj Walia, MD, DNB4; Kim Jin Oh, MD, PhD5; Suria Nataatmadja, MD6; Mellisa H.Wiyono, MD6; and Marcillia Rizka Aryadi, MD6 in data analysis and data collection.
We would also like to acknowledge the funding by Merck Sharp Dohme Indonesia for this study. The views expressed in this article are those of the authors and not of the funding organization.
1) National Committee of Adverse Event Following Immunization, Jakarta
2) Immunization Sub-Directorate at Directorate of Surveillance, Immunization and Health Quarantine of Indonesia Ministry of Health
3) Special Province of Jakarta Health Office
4) Merck and Co, Global Medical
5) Merck Sharp and Dohme, Regional Medical
6) Merck Sharp and Dohme Indonesia, Medical Department
References
- 1.Barr E, Tamms G. Quadrivalent human papillomavirus vaccine. Clin Infect Dis. 2007;45:609–7. doi: 10.1086/520654. [DOI] [PubMed] [Google Scholar]
- 2.Bruni L, Barrionuevo-Rosas L, Albero G, et al. ICO/IARC information Centre on HPV and Cancer (HPV Information Centre) Human Papillomavirus and Related Diseases in the World. Summary Report 27 July 2017. [Summary Report] 2017. Retrieved from http://www.hpvcentre.net/statistics/reports/XWX.pdf .
- 3.Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi: 10.1128/CMR.16.1.1-17.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clothier HJ, Lee KJ, Sundararajan V, et al. Human papillomavirus vaccine in boys: background rates of potential adverse events. Med J Aust. 2013;198:554–8. doi: 10.5694/mja12.11751. [DOI] [PubMed] [Google Scholar]
- 5.CSIS report on The HPV Vaccination in Japan; Issues and Options (2014) Retrieved from http:// www.rho.org/files/CSIS_HPV_vac_issues_options_Japan_2014.pdf .
- 6.Ferris D, Samakoses R, Block SL, et al. 4-Valent human papillomavirus (4vHPV) vaccine in preadolescents and adolescents after 10 years. Pediatrics. 2017;140:e20163947. doi: 10.1542/peds.2016-3947. [DOI] [PubMed] [Google Scholar]
- 7.Fregnani JH, Carvalho AL, Eluf-Neto J, et al. A school-based human papillomavirus vaccination program in Barretos, Brazil: Finalresults of a demonstrative study. PLoS One. 2013;8:e62647. doi: 10.1371/journal.pone.0062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gidudu JF, Walco GA, Taddio A, et al. Immunization site pain: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2012;30:4558–7. doi: 10.1016/j.vaccine.2012.03.085. [DOI] [PubMed] [Google Scholar]
- 9.Heininger U. Standardized case definitions for adverse events following immunization. Lancet. 2013;381:2250–1. doi: 10.1016/S0140-6736(13)61477-4. [DOI] [PubMed] [Google Scholar]
- 10.Indonesia Ministry of Health News for 3 New Vaccine Implementation. 2017. [News Release] Retrieved from http://www.depkes.go.id/article/view/17020100001/ini-rencana-pelaksanaan-3-vaksinasi-baru-untuk-lengkapi-imunisasi-dasar-.html .
- 11.Indonesian NRA. Dissemination of Technical Guidelines for Implementing Pharmacovigilance for the Pharmaceutical Industry. [Webpage] 2011. Retrieved from http://www.pom.go.id/mobile/index.php/view/berita/1257/Sosialisasi-Pedoman-Teknis-Penerapan-Farmakovigilans-bagi-Industri-Farmasi.html .
- 12.Internal Data from Sub-Directorate at Directorate of Surveillance, Immunization and Health Quarantine of Indonesia Ministry of Health. 2018 [Google Scholar]
- 13.Kaarthigeyan K. Cervical cancer in India and HPV vaccination. Indian J Med Paediatr Oncol. 2012;33:7–12. doi: 10.4103/0971-5851.96961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl KS, Walop W, Gidudu J, et al. Swelling at or near injection site: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine. 2007;25:5858–74. doi: 10.1016/j.vaccine.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 15.Kosen S, Andrijono A, Ocviyanti D, Indriatmi W. The cost-effectiveness of quadrivalent human papillomavirus vaccination in Indonesia. Asian Pac J Cancer Prev. 2017;18:2011–17. doi: 10.22034/APJCP.2017.18.7.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MSD Data on File. Pooled Data from Protocols 007,011,012,013,015,016 and 018 for elevated temperature days 1 to 5 following any vaccination visit [Google Scholar]
- 17.Naleway AL, Gold R, Drew L, et al. Reported adverse events in young women following quadrivalent human papillomavirus vaccination. J Womens Health. 2012;21:425–32. doi: 10.1089/jwh.2011.2895. [DOI] [PubMed] [Google Scholar]
- 18.Nicol AF, Andrade CV, Russomano FB, et al. HPV vaccines: a controversial issue? Braz J Med Biol Res. 2016;49:e5060. doi: 10.1590/1414-431X20155060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogawa Y, Takei H, Ogawa R, Mihara K. Safety of human papillomavirus vaccines in healthy young women: a meta-analysis of 24 controlled studies. J Pharm Health Care Sci. 2017;3:18. doi: 10.1186/s40780-017-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.School Immunization in Indonesia. 2014. Retrieved from http://www.who.int/immunization/programmes_systems/policies_strategies/school-based-immunization/en/index1.html .
- 21.Siegrist CA, Lewis EM, Eskola J, et al. Human papilloma virus immunizationin adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J. 2007;26:979–84. doi: 10.1097/INF.0b013e318149dfea. [DOI] [PubMed] [Google Scholar]
- 22.Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 23.Stillo M, Carrillo Santisteve P, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015;14:697–712. doi: 10.1517/14740338.2015.1013532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subelj M, Ucakar V, Kraigher A, Klavs I. Adverse events following school-based vaccination of girls with quadrivalent human papillomavirus vaccine in Slovenia 2009 to 2013. Euro Surveill. 2016;21 doi: 10.2807/1560-7917.ES.2016.21.14.30187. doi:10.2807/1560-7917.ES.2016.21.14.30187. [DOI] [PubMed] [Google Scholar]
- 25.Taddio A, Ipp M, Thivakaran S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. 2012;30:4807–12. doi: 10.1016/j.vaccine.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Vaccine Safety Basics (2104). [Webpage] Retrieved from: http://vaccinesafety-training.org/
- 27.Weekly Epidemiological Record 2017) WHO. 92:393–404. [Google Scholar]
- 28.WHO (2014). Report of the SAGE Working Group on Vaccine Hesitancy. The State of Vaccine Confidence. Retrieved from http://www.who.int/immunization/sage/meetings/2014/october/1_Report_WORKING_GROUP_vaccine_hesitancy_final.pdf .
- 29.WHO Vaccine Safety Basics e-Learning Course. Case Study C (2018) Retrieved from http://vaccine-safety-training.org/c-introduction.html .
- 30.World Health Organization (2017). Weekly epidemiological record: Human papilloma virus vaccines: WHO position paper, May. 2017;19:241–68. [Google Scholar]


