Abstract
Background:
Bone marrow hypoxia can promote leukemia progression in human cases of acute myeloid leukemia (AML). In addition, low oxygen tension is able to regulate the expression of different genes involved in malignancy. In this study, we hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF-A) genes were assessed as principal regulators of hypoxia in do novo AML patients.
Methods:
Peripheral blood and bone marrow samples were collected from 57 AML patients and 17 normal control subjects with informed consent. Expression of HIF1α and VEGF-A was then evaluated using quantitative real-time PCR (Q-Real time PCR) and data were analyzed with SPSS 16.
Result:
HIF1α and VEGF-A showed overexpression in AML patients compared to normal controls (P <0.0001 and P<0.005, respectively). The expression level of HIF1α was significantly higher in AML-M3 cases versus AML-non M3 cases. Furthermore, there was a positive correlation between HIF1α and VEGF-A (P <0.0001 and r = 0.497).
Conclusion:
Adding to the many studies on the role of hypoxia in solid tumors, our data indicate that HIF1a and VEGF-A overexpression also occurs in AML patients. We consider that this is possibly involved in leukemic cell growth and therefore could be a promising target for clinical control.
Keywords: Acute myeloid leukemia, hypoxia, HIF1α, VEGF
Introduction
Acute myeloid leukemia (AML) is a type of cancer that is defined by permeation of the bone marrow, blood, and other tissues by proliferative, clonal, abnormally differentiated, and occasionally poorly differentiated treated cells of the hematopoietic system (Mohamed et al., 2014). The dynamic interaction between leukemia cells and the bone marrow microenvironment is one of the characteristics of leukemia. It is well- known in solid tumors that the excessive proliferation of tumor cells causes a severe hypoxia, which can lead to tumor progression. Likewise In the hematologic malignancies, there is the notion that proliferation and accumulation of blasts lead to a hypoxic environment in the bone marrow microenvironment (Fiegl et al., 2009; Semenza, 2013). The adaptive cellular program in response to low oxygen availability is mainly triggered by hypoxia-inducible factors (HIFs). Hypoxia-inducible factors are heterodimers that composed of an alpha subunit and a beta subunit. Alpha subunits include HIF1α, HIF2α, HIF3α, and the beta subunit, including HIF1β. HIF1α directly regulates the transcription of more than one hundred genes involved in hematopoietic processes, angiogenesis, autophagy, cell survival and glucose metabolism (Kaelin and Ratcliffe, 2008). Previous studies have shown that HIF1α protein is involved in the development of a tumor, and the increased expression of these proteins is associated with poor prognosis (Keith et al., 2011). One of the most crucial mechanisms in cancer is angiogenesis that refers to the formation of new blood vessels from the previous arteries (Carmeliet, 2005). Studies have shown that tumor development and invasion in solid tumors are associated with angiogenesis, and recent evidence suggests that angiogenesis may be in the development of acute myeloid leukemia (Dong et al., 2007). In acute myeloid leukemia, angiogenesis directly contributes to the tumor progression by the provision of necessary factors for blast proliferation as well as through angiogenic factors (Albitar, 2001). Significant angiogenic factors include VEGF-A, BFGF, PDGF, FGF, in which among them VEGF-A is the most important. Furthermore the angiogenesis, VEGF-A has other functions in the progress of leukemia cells, which performs these functions by autocrine and paracrine loops. There are 4 receptors for VEGF have been recognized: Flt-1 (VEGFR-1), Flk-1/KDR (VEGFR-2), Flt-4 (VEGFR-3), and neuropilin-1 (NRP-1) (Haghi et al., 2017) When VEGF-A binds to its receptors on leukemia cells it stimulates proliferation in these cells and also increases survival by reducing apoptotic proteins (autocrine). Also, VEGF-A by binding to its receptors on the epithelial cells of the bone marrow in addition to stimulation of angiogenesis stimulates epithelial cells to secrete growth factors such as G-CSF, GM-CSF, IL-6, which play an important role in the proliferation of leukemic cells (Song et al., 2012). Expression levels of VEGF and its receptors are accepted as an indicators of angiogenesis in solid tumors. Different types of leukemia like solid tumors were also shown to have high microvessel density (MVD) in bone marrow (Lee et al., 2000). HIF1α and VEGF-A are considered as important factors in tumor progression in many solid tumors, as well as in some of the hematologic malignancies such as multiple myeloma, but in acute leukemia, the role of HIF1α and VEGF have not been well known, thus in this study we focused on the expression level of HIF1α and VEGF-A genes in AML patients. Our study showed alteration in expression level of these genes in AML patients.
Materials and Methods
Study population
Samples obtained from 57 newly diagnosed AML patients prior to any treatment and also 17 Healthy individuals as negative control group at Talghani hospital. Informed consent was obtained from individual participant included in the study and the study was coincident with the 1964 Helsinki declaration.
RNA extraction and DNA synthesis
To determine mRNA expression level of HIF1α, VEGF-A, mononuclear cells were acquired using Ficoll density centrifugation, subsequent, the RNA extraction was carried out by RNeasy Kit (Qiagen, Germany) and following it, the integrity of RNA was measured by the NanoDrop (Thermo Scientific, Wilmington, North Carolina, USA). All samples showed high purity with OD 260/280 nm ratio >1.8. Subsequently, 1 µl of total RNA was transcribed into cDNA to a final volume of 20µl by means of a cDNA Synthesis Kit (Thermo Scientific, Qiagen, Hudson, NH, USA). Finally, an equal amount of control and patient cDNA used as a substrate for qRT-PCR amplification.
Real-Time PCR gene expression
Gene expression level was measured by SYBR green-based Real-Time PCR using a thermocycler (Rotor-Gene 6000, Bosch, Qiagen, Germany). The qRT-PCR assay was accomplished in 15 µl final reaction volume using 1.5 μL template target cDNA, 1.2 μL forward and reverse primer, 7.5 μL RealQ Plus 2x Master Mix Green- Low ROX (Ampliqon, Denmark), and 4.8 μL water to reach total volume. Primers were designed via oligo 7/56 software and their specificity was confirmed in NCBI Blast database. The primers pairs for the mention genes are available in table 1. Thermal cycling for each reaction (HIF1α, VEGF-A, and ABL) included an initial hold at 95°C for 10 minutes followed by 40 denaturation cycles at 95°C for 10 seconds and annealing/extension at 58°C (VEGF-A), 59°C (HIF1α) and 65°C (ABL) for 20 seconds. All the reactions were done in triplicated manner. A standard curve was obtained by four consecutive 1:10 dilutions of a positive sample (1, 0/1, 0/01 and 0/001). Expression level of target genes analyzed in comparison with ABL (housekeeping gene) by Livak method (2-ΔΔct) (Livak and Schmittgen, 2001; Schmittgen and Livak, 2008).
Table 1.
Real- Time PCR Oligonucleotide Primers
| Primer | Forward | Reverse |
|---|---|---|
| HIF1A | GCAGCAACGACACAGAAACT | TTCAGCGGTGGGTAATGGAG |
| VEGF-A | CTTGCCTTGCTGCTCTACC | CACACAGGATGGCTTGAAG |
| ABL | AGTCTCAGGATGCAGGTGCT | TAGGCTGGGGCTTTTTGTAA |
Statistical Analysis
Analysis of generated data was implemented using the SPSS Statistics 16.0. Applying both the Shapiro-Wilk and Kolmogorov-Smirnov tests revealed the normality distribution of HIF1α and VEGF-A relative expression in AML and control groups. In addition, the Mann-Whitney U and Spearman tests were used to determine the difference in HIFα and VEGF-A gene expression level between AML patients and normal control group, and the linear correlation between HIF1a and VEGF-A expression, respectively.
Results
Profile of Patient Sample Specifications
The samples analyzed in this study were obtained from 57 patients with de novo AML varying in gender and age that presented with malignancies with distinct morphologic features (Table 2). In this study, 57 patients were divided into two groups including AML-M3 and AML-nonM3. (21 cases AML-M3 patient and 36 cases AML-nonM3 patient)
Table 2.
Profile of Specifications of Patients with de Novo AML from which Samples were Obtained
| Specification | %of Patient Samples* |
|---|---|
| SEX | |
| Male | 45 |
| Female | 55 |
| AGE(YEARS) | |
| Median(47) | |
| Range(1.4-89) | |
| BLAST | |
| Median(80) | |
| Range(20-96) | |
| FAB CLASSIFICATION | 36 |
| Aml-M3 | 64 |
| Aml-nonM3 | |
| SPECIMEN TYPE | 29 |
| PB | 71 |
| BM |
HIF1α and VEGF-A expression in AML patients and controls
HIF1α and VEGF-A expression levels were analyzed using real-time PCR in 57 de novo AML patients and 17 normal subjects. For validation of the DDCt method, the amplification efficiencies of reference and target genes were adjusted between 0.9-1.1 with using four serial dilutions of cDNA. As the control group, the expression levels of interested genes were evaluated in 12 BM and 5 PB samples of healthy volunteers. Subsequently, the Ct values obtained from HIF1α and VEGF-A were normalized against the internal reference gene, ABL, for both AML positive and normal control group samples. A statistical comparison was then made between the normalized values of AML-positive and normal control group samples, which revealed a significant difference between both HIF1α (P<0.0001) and VEGF-A (P<0.005) mRNA expression and healthy patients (Figure 1 A, and B). The average expression level (±SD) measured for AML-positive and normal control was 12.61 ± 0.38, 2.46 ± 0.78 for HIF1a, and 6.9 ± 0.59 and 2.01 ± 0.14 for VEGF-A, respectively. An expression level in the range of a 95% confidence interval that defined for the expression level for HIF1α and VEGF in the healthy population was considered at 1.75–3.16 and 1.48–2.52, respectively. According to the statistical analysis, 76% and 68% of AML positive patients indicated high HIF1α and VEGF expression, respectively and also, expression levels of 13% and 19% of AML patients for HIF1α and VEGF did not alter, respectively.furthermore, 11% and 13% of AML patients fell below the threshold of the ranges for HIF1α and VEGF were defined as low expression levels. In the current study, we had 21 AML-M3 patients and 36 AML-NonM3 patients. In AML-M3 patients, a more increase in the expression of gene HIF1a was observed (P<0.028) but the gene expression of VEGF-A was not significantly different between APL patients and Nonm3 patients (P<0.334) (Figure 2).
Figure 1.
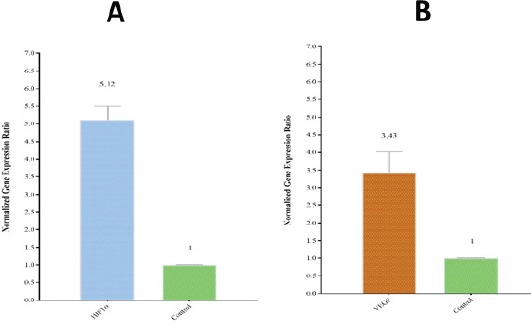
Relative Expression of HIF1α and VEGF-A in 57 AML Patients and 17 Healthy Patients was Measured from Ct Values and Normalized against a Reference Gene (ABL). A) A significant difference (P < 0.0001) between HIF1α expression in AML patients and healthy patients were identified. A relative HIF1α expression level of 12.61 ± 3.25 (SD) was measured in AML patients in comparison to 2.46 ± 0.33 (SD) in the normal control group. B) A significant difference (P < 0.005) between VEGF-A expression in AML patients and healthy patients were also identified. A relative VEGF-A expression level of 5.04 ± 0.76 (SD) was measured in AML patients in comparison to 2.003 ± 0.24 (SD) in the normal control group
Figure 2.
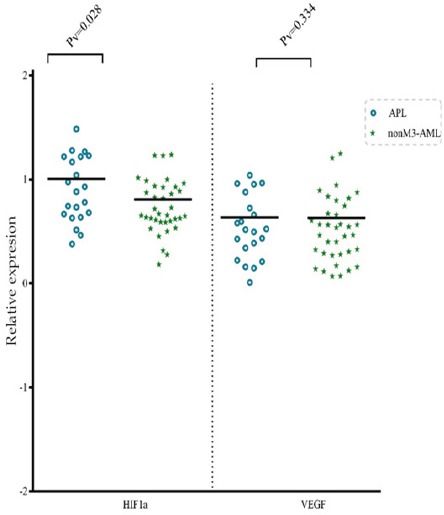
Expression of HIF1α and VEGF Genes in Patients with APL
Correlation between HIF1a and VEGF-A Expression Levels
Statistical analysis was applied to determine whether expression of HIF1α and VEGF-A was dependent and related.(linear correlation) The analysis determined that there is a positive and significant correlation between HIF1α and VEGF-A (P <0.0001 and r = 0.497) in AML patients, thus suggesting dependence between their expression.
Figure 3.
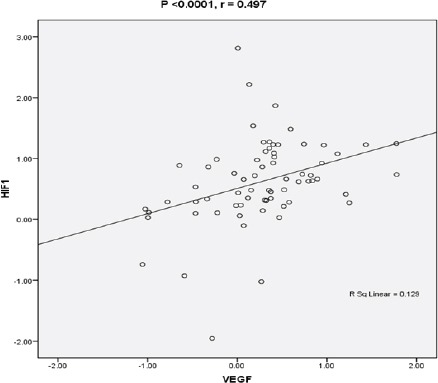
Statistical Analysis by Means of Spearman test Reveals Dependence and Relation between the Expression of HIF1α and VEGF-A. A) Correlation between HIF1a and VEGF-A in 57 AML patients was determined to be positive and significant (P <0.0001 r = 0.497
Discussion
Acute myeloid leukemia is a heterogeneous malignancy characterized by the extreme and uncontrollable proliferation of leukemic cells and their accumulation in the bone marrow. Although significant progress has been made in the treatment of this leukemia, overall survival is still poor and a significant percentage of patients are resistant to treatment.(De Kouchkovsky and Abdul-Hay, 2016) Bone marrow microenvironment is an important factor in the progression and resistance to therapy in leukemia (Ayala et al., 2009). One of the significant factors in the bone marrow microenvironment is the oxygen pressure that in the bone marrow is physiologically three times lower than the oxygen demand for cells in the culture medium (Harrison et al., 2002). Hif1α is the most important molecule; since it can respond to the hypoxia (Semenza, 2003). Increasing the expression of the HIF1α protein in many solid tumors is considered as a poor prognostic marker that results in resistance to treatment and recurrence of the neoplasm (Birner et al., 2000; Chi et al., 2006; Bangoura et al., 2007). But in leukemia, the HIF1α function is more complicated and there are different results about the expression or absence of HIF1α protein. We examined the expression of genes involved in hypoxia pathway in AML patients. We investigated the expression of the HIF1α and VEGF-A gene in newly diagnosed AML patients. A previous study by Mortensen et al., (1998) showed that the bone marrow microenvironment alters during the development of acute myeloid leukemia in rats and one of this alteration seems to be a reduction in oxygen pressure. Although direct measurement of oxygen pressure in bone marrow is very difficult, Fiegl et al., (2009)showed that oxygen pressure in bone marrow aspirates of AML patients is very comparable to those of normal people. Also by using a mouse model Jensen et al., (2000) also discovered, some of the hypoxia was created in the bone marrow microenvironment during the development of promyelocytic leukemia. Our results showed that expression of HIF1α gene increased compared to control group. (P<0.0001). In a study by Hatfield et al., (2010) on primary human AML cells and AML cell line, it was shown that increased expression of HIF1α protein in leukemia cells is significant under hypoxic conditions and also, a study was conducted by Deeb et al., (2011) on 84 patients with normal karyotype AML, which they found increased HIF1α protein in the nucleus and cytoplasm of leukemia cells in these patients, that these studies coincide with our study. Our study suggests that one of the characteristics that leukemic environment obtains during malignancy could be a reduction of oxygen pressure and, consequently, hypoxia. Albeit, the cause of hypoxia in leukemia has not yet been definitely determined, but an elucidation that could be presented for this phenomenon is likely to be rapid and extensive proliferation as well as the accumulation of leukemic cells in the bone marrow that increase the consumption of oxygen. The previous study on patients with multiple myeloma revealed hypoxia in the bone marrow of these patients (Colla et al., 2010). In our research, the key protein regulator of hypoxia pathway has explicitly increased, although conditions in solid tumors may not be similar to acute leukemia, but, so far only a few cases have shown relatively similar results in AML patients (Demock et al., 2008). Therefore, it could be declared that hypoxia is one of the alterations in the leukemic environment to progress. As has already been stated, one of the effects of expressing the HIF1α protein activating the NOTCH1 pathway which results in the dissemination and invasion of leukemia, as well as by activating the Wnt pathway, it maintains leukemia stem cells and resistance to treatment (Zou et al., 2013; Giambra et al., 2015). However, the contradiction in the being oncogenesis or tumor-suppressor of HIF1α has been controversial in previous studies. In this regard, a study by Gao et al., (2015) on transgenic mice concluded that inactivation of the HIF1α gene by siHIF1α inhibited tumor growth. While Velasco-Hernandez et al., (2014), by performing tests on mouse models, found results contrary to our results, which showed that HIF1α acts as a tumor suppressor and inactivation of it makes tumor progression. Given this contradiction, we decided to study the HIF1α downstream for oncogenesis, so we chose the VEGF-A gene that discussed below. Considering the intrinsic differences between Aml-m3 and Nonm3-Aml at the line of involvement, pathophysiology, monitoring, diagnostic approaches and therapeutic protocols, we decided to survey the expression of the HIF1α gene between the two groups of patients. The results of this study showed that the expression of HIF1α in people with APL significantly increased compared to Non M3-Aml groups. Thus, the previous study, which was conducted by Coltella et al., (2014) on the APL cell line confirms our results. The reason why the expression of the HIF1α gene in Aml-m3 group has increased in comparison with Nonm3-Aml group can be likely due to oncogene protein PML-RARa. Since, in Aml-m3, the oncogene protein PML-RARa acts as a transcription factor, it can probably increase the expression of the HIF1A gene (Percio et al., 2014). As mentioned, one of the critical pathways that can be regulated by HIF1α is angiogenesis the importance of angiogenesis and angiogenic factors in solid tumors have been well understood recently, but there is less information about the role of angiogenesis and angiogenic factors in acute leukemia. An increase in vascularity of the bone marrow has been illustrated in people with leukemia (Hussong et al., 2000; Padro et al., 2000). We demonstrated the expression of VEGF-A gene in Aml patients was higher than the control group (P<0.005). Also, the results obtained from the previous study by Hou et al., (2008) on primary human AML cells are similar and consistent with our results but they didn’t show VEGF-A correlation with HIF1α. Also, a study by Mirzaei el al., (2017) showed that increasing the OPN protein in AML cell lines by increasing the expression of VEGF-A protein promotes anti-angiogenesis therapies. As demonstrated, angiogenic factors play a significant role in the progression, dissemination, and metastasis of solid tumors. In this regard, there are reports suggesting an increase in vascularity of bone marrow in AML patients (Hussong et al., 2000). In addition, an increase in serum level of VEGF-A in patients with Aml (Aguayo et al., 2002) and expression of VEGF-A receptors on leukemic cells (Bellamy et al., 1999) indicate the importance of VEGF-A in the pathogenesis of AML. While HIF1α can affect VEGF, it is a key factor in the pathway for angiogenesis, thus simultaneously increasing the expression of two HIF1α and VEGF-A genes suggests that the increase of HIF1α in AML patients has a goal beyond the control of unbreakable cell proliferation and don’t induce a deterrent corrective mechanism, but the increase in both of these can serve the development of leukemia. Studies are intended to provide a deeper understanding of use this pathway as diagnostic and therapeutic objectives.
In conclusion, briefly, our study suggests that increasing HIF1α in AML patients not only does not prevent the development of malignancy but also assists the development of AML, and, therefore, it is more relevant as an oncogene and also, the increasing correlation between the expression of two HIF1α and VEGF-A genes proposes that the increase is correlated with the aim of developing malignancy. Thus, due to the lack of obvious improvement of treatment protocols in the last five decades, it seems necessary to be considered as a suitable target for diagnosis, monitoring, and treatment in future research.
References
- 1.Aguayo A, Kantarjian HM, Estey EH, et al. Plasma vascular endothelial growth factor levels have prognostic significance in patients with acute myeloid leukemia but not in patients with myelodysplastic syndromes. Cancer. 2002;95:1923–30. doi: 10.1002/cncr.10900. [DOI] [PubMed] [Google Scholar]
- 2.Albitar M. Angiogenesis in acute myeloid leukemia and myelodysplastic syndrome. Acta Haematol. 2001;106:170–6. doi: 10.1159/000046613. [DOI] [PubMed] [Google Scholar]
- 3.Ayala F, Dewar R, Kieran M, et al. Contribution of bone microenvironment to leukemogenesis and leukemia progression. Leukemia. 2009;23:2233–41. doi: 10.1038/leu.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangoura G, Liu ZS, Qian Q, et al. Prognostic significance of HIF-2alpha/EPAS1 expression in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3176–82. doi: 10.3748/wjg.v13.i23.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy WT, Richter L, Frutiger Y, et al. Expression of vascular endothelial growth factor and its receptors in hematopoietic malignancies. Cancer Res. 1999;59:728–33. [PubMed] [Google Scholar]
- 6.Birner P, Schindl M, Obermair A, et al. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000;60:4693–6. [PubMed] [Google Scholar]
- 7.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–6. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 8.Chi JT, Wang Z, Nuyten DS, et al. Gene expression programs in response to hypoxia: cell type specificity and prognostic significance in human cancers. PLoS Med. 2006;3:e47. doi: 10.1371/journal.pmed.0030047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colla S, Storti P, Donofrio G, et al. Low bone marrow oxygen tension and hypoxia-inducible factor-1alpha overexpression characterize patients with multiple myeloma: role on the transcriptional and proangiogenic profiles of CD138(+) cells. Leukemia. 2010;24:1967–70. doi: 10.1038/leu.2010.193. [DOI] [PubMed] [Google Scholar]
- 10.Coltella N, Percio S, Valsecchi R, et al. HIF factors cooperate with PML-RARαto promote acute promyelocytic leukemia progression and relapse. EMBO Mol Med. 2014:e201303065. doi: 10.1002/emmm.201303065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Kouchkovsky I, Abdul-Hay M. Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 2016;6:e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deeb G, Vaughan MM, McInnis I, et al. Hypoxia-inducible factor-1alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res. 2011;35:579–84. doi: 10.1016/j.leukres.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Dong X, Han ZC, Yang R. Angiogenesis and antiangiogenic therapy in hematologic malignancies. Crit Rev Oncol Hematol. 2007;62:105–18. doi: 10.1016/j.critrevonc.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Fiegl M, Samudio I, Clise-Dwyer K, et al. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–12. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao XN, Yan F, Lin J, et al. AML1/ETO cooperates with HIF1alpha to promote leukemogenesis through DNMT3a transactivation. Leukemia. 2015;29:1730–40. doi: 10.1038/leu.2015.56. [DOI] [PubMed] [Google Scholar]
- 16.Giambra V, Jenkins CE, Lam SH, et al. Leukemia stem cells in T-ALL require active Hif1alpha and Wnt signaling. Blood. 2015;125:3917–27. doi: 10.1182/blood-2014-10-609370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haghi A, Mohammadi S, Heshmati M, et al. Anti-vascular endothelial growth factor effects of sorafenib and arsenic trioxide in acute myeloid leukemia cell lines. Asian Pac J Cancer Prev. 2017;18:1655–61. doi: 10.22034/APJCP.2017.18.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison JS, Rameshwar P, Chang V, et al. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield KJ, Bedringsaas SL, Ryningen A, et al. Hypoxia increases HIF-1alpha expression and constitutive cytokine release by primary human acute myeloid leukaemia cells. Eur Cytokine Netw. 2010;21:154–64. doi: 10.1684/ecn.2010.0204. [DOI] [PubMed] [Google Scholar]
- 20.Hou HA, Chou WC, Lin LI, et al. Expression of angiopoietins and vascular endothelial growth factors and their clinical significance in acute myeloid leukemia. Leuk Res. 2008;32:904–12. doi: 10.1016/j.leukres.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Hussong JW, Rodgers GM, Shami PJ. Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood. 2000;95:309–13. [PubMed] [Google Scholar]
- 22.Jensen PO, Mortensen BT, Hodgkiss RJ, et al. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif. 2000;33:381–95. doi: 10.1046/j.1365-2184.2000.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2011;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CG, Heijn M, di Tomaso E, et al. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–70. [PubMed] [Google Scholar]
- 26.Mirzaei A, Ghaffari SH, Nikbakht M, et al. OPN b and c isoforms doubtless veto anti-angiogenesis effects of curcumin in combination with conventional AML regiment. Asian Pac J Cancer Prev. 2017;18:2591–9. doi: 10.22034/APJCP.2017.18.9.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohamed AM, Thenoz M, Solly F, et al. How mRNA is misspliced in acute myelogenous leukemia (AML)? Oncotarget. 2014;5:9534–45. doi: 10.18632/oncotarget.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortensen BT, Jensen PO, Helledie N, et al. Changing bone marrow micro-environment during development of acute myeloid leukaemia in rats. Br J Haematol. 1998;102:458–64. doi: 10.1046/j.1365-2141.1998.00801.x. [DOI] [PubMed] [Google Scholar]
- 29.Padro T, Ruiz S, Bieker R, et al. Increased angiogenesis in the bone marrow of patients with acute myeloid leukemia. Blood. 2000;95:2637–44. [PubMed] [Google Scholar]
- 30.Percio S, Coltella N, Grisanti S, et al. A HIF-1 network reveals characteristics of epithelial-mesenchymal transition in acute promyelocytic leukemia. Genome Med. 2014;6:84. doi: 10.1186/s13073-014-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salarpour F, Goudarzipour K, Mohammadi MH, et al. Evaluation of CCAAT/enhancer binding protein (C/EBP) alpha (CEBPA) and runt-related transcription factor 1 (RUNX1) expression in patients with de novo acute myeloid leukemia. Ann Hum Genet. 2017;81:276–83. doi: 10.1111/ahg.12210. [DOI] [PubMed] [Google Scholar]
- 32.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J Clin Invest. 2013;123:3664–71. doi: 10.1172/JCI67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song G, Li Y, Jiang G. Role of VEGF/VEGFR in the pathogenesis of leukemias and as treatment targets (Review) Oncol Rep. 2012;28:1935–44. doi: 10.3892/or.2012.2045. [DOI] [PubMed] [Google Scholar]
- 35.Velasco-Hernandez T, Hyrenius-Wittsten A, Rehn M, et al. HIF-1alpha can act as a tumor suppressor gene in murine acute myeloid leukemia. Blood. 2014;124:3597–607. doi: 10.1182/blood-2014-04-567065. [DOI] [PubMed] [Google Scholar]
- 36.Zou J, Li P, Lu F, et al. Notch1 is required for hypoxia-induced proliferation, invasion and chemoresistance of T-cell acute lymphoblastic leukemia cells. J Hematol Oncol. 2013;6:3. doi: 10.1186/1756-8722-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


