Abstract
With an increasing number of antimicrobial stewardship–related articles published each year, attempting to stay current is challenging. The Southeastern Research Group Endeavor (SERGE-45) identified antimicrobial stewardship–related peer-reviewed literature that detailed an actionable intervention for 2018. The top 13 publications were selected using a modified Delphi technique. These manuscripts were reviewed to highlight the actionable intervention used by antimicrobial stewardship programs to provide key stewardship literature for teaching and training as well as to identify potential intervention opportunities within one’s institution.
Keywords: antibiotics, antimicrobial stewardship, infectious diseases, metrics, resistance
Top antimicrobial stewardship-related peer-reviewed articles from 2018 that detail an actionable intervention were identified. This targeted literature compilation may be used for stewardship training and to identify potential stewardship program intervention opportunities within one’s institution or region.
Antimicrobial stewardship has become a common term in acute care facilities, sparking significant interest among physicians, pharmacists, and other health care professionals. Antimicrobial stewardship program (ASP) foundations, including leadership by infectious diseases (ID) pharmacists and physicians, have long been established and directed by clinical practice guidelines and regulatory bodies [1–3]. The focus of antimicrobial stewardship activities continues to move beyond the walls of inpatient institutions. Certificate programs targeting ID physicians and clinicians working in ambulatory or long-term care stewardship are now being offered [4–8]. In addition, formal recommendations and guidance for outpatient and nursing home ASP activities from the Centers for Disease Control and Prevention (CDC) and regulatory agencies are available [9–11]. In January 2020, new Joint Commission (TJC) standards for ambulatory care facilities that routinely prescribe antibiotics will go into effect [12]. Many questions on the optimal execution of antimicrobial stewardship activities still remain. Given the variability in institutional settings, local epidemiologic patterns, patient mix, and available resources, continued research on successful and optimal ASP interventions is needed [13].
The most successful work in antimicrobial stewardship has been the result of strong interprofessional collaborations, with research and scholarship being no exception. Members of the Southeastern Research Group Endeavor (SERGE-45), an interprofessional research network primarily composed of expert pharmacist stewards in the Southeastern United States, systematically compiled the top peer-reviewed publications from 2018 involving an ASP intervention. Table 1 provides a brief overview of the 13 selected articles (aka “Baker’s Dozen”), which are detailed herein [14–26]. Annual reviews using similar criteria have been previously published since 2016 [27, 28].
Table 1.
Summary of Included Studies
| Study Citation | Study Design | Intervention Summary | Primary and Key Secondary Outcomes |
|---|---|---|---|
| Yadav et al. 2018 [14] | Single-center, quasi-experimental study of incorporation of institutional EP for duration of therapy into preexisting ASP rounds | Institutional EP for duration of antimicrobial therapy developed and approved by hospital committees. EP reinforced on ASP rounds. Preexisting ASP rounds included prospective audit and feedback, restriction program, and de-escalation rounds. |
Primary outcomes: mean antimicrobial DOTs administered inpatient and prescribed outpatient for patients discharged with ICD-10 codes for UTI, SSTI, PNA, VAP in 12 months before and 12 months after implementation of EP • Change in mean DOTs: UTI, –1.4 (–2.3 to –0.6; P = .001); SSTI, –2.2 (–3.3 to –1.0; P < .001); PNA, –2.0 (–3.2 to –0.9; P = .001); VAP, –9.6 (–16.0 to –3.3; P = .003) Secondary outcomes: total antibiotic exposure (sum of total milligrams of antibiotics administered inpatient plus prescribed outpatient) • Change in antibiotic exposure: UTI, –3718 (–5185 to –2252; P < .001); SSTI, –5404 (–8227 to –2582; P < .001); PNA, –9430 (–12 028 to –6833; P < .001); VAP, –34 246 (–57 507 to –10 986; P = .004) |
| Thom et al. 2019 [15] | Multicenter, quasi-experimental, pre- and postintervention study | Provider-driven ATOs were implemented across 11 units located in 6 hospitals. Providers were prompted to complete paper ATO tool on antibiotic days 3–5 without study or stewardship input. |
No difference between hospital DOT per admission or total DOT per admission before or after controlling for study unit and season • Average hospital DOT 12.7 vs 12.2 and total DOT 18.9 vs 18.2 • Multivariable analysis showed no association between intervention and number of times regimen was modified or discontinued on antibiotic days 3–5 (OR, 1.0; 95% CI, 0.85–1.19) • Multivariable analysis showed that the ATO was inversely associated with receipt of inappropriate antibiotics on antibiotic days 3–5 (OR, 0.58; 95% CI, 0.48–0.69), as was having undergone a surgical procedure (OR, 0.70; 95% CI, 0.54–0.90) |
| Foolad et al. 2018 [16] | Multicenter, quasi-experimental study |
1) Update and dissemination of institution-specific CAP guidelines via pocket cards and hospital intranet sites. 2) Multiple educational sessions to prescribers and pharmacists regarding appropriate management of CAP, focusing on DOT, updates to the institution-specific guidelines, and the stewardship initiative. 3) Targeted prospective audit with feedback and intervention by ID pharmacists Monday–Friday. |
Decrease in median antibiotic DOT • Historical 9 (IQR, 7–10) days vs intervention 6 (IQR, 5–7) days; P < .001 Improvement in guideline-concordant therapy • Historical 5.6 % vs intervention 42%; P < .001 Decrease in median excess antibiotic days • Historical 3 (IQR 2–5) days vs intervention 1 (IQR 0–2) days; P < .001 No significant difference in clinical outcomes 30 days postdischarge, No. (%) • CDI: historical 0 (0) vs intervention 0 (0); P = not reported • Re-presented to emergency center or clinic with pneumonia: historical 20 (6.8) vs intervention 13 (4.4); P = .22 • Readmission with pneumonia: historical 21 (7.1) vs intervention 11 (3.8); P = .075 |
| Musgrove et al. 2018 [17] | Multicenter, single pre- and postintervention, quasi-experimental study | Clinical microbiology laboratory changed wording in reports on non-pathogen-containing respiratory cultures to emphasize no Staphylococcus aureus, MRSA, or Pseudomonas aeruginosa. |
• Mortality: historical 7 (2.3) vs intervention 3 (1); P = .233 Primary outcome • De-escalation: 39% vs 73%; P < .001 Secondary outcomes • Discontinuation of anti-MRSA therapy: 37% vs 71%; P < .001 • Discontinuation of antipseudomonal therapy: 32% vs 70%; P < .001 • Acute kidney injury: 31% vs 14%; P = .003 • In-hospital, all-cause mortality: 30% vs 18%; P = .52 |
| García-Rodríguez et al. 2019 [18] | Single-center, quasi-experimental, pre- and postintervention study | A multidisciplinary antimicrobial stewardship team was implemented with prospective follow-up of meropenem use. An ID physician reviewed the EMR for each case and provided antibiotic treatment recommendations to the prescribers, with adherence to or rejection of the recommendations from the ID physician assessed at 24–48 hours postrecommendation. |
Improved rates in appropriate justification of meropenem use • Pre-intervention (2014) 47.3% vs postintervention (2017) 76.8%; P = .001 • Reduction in meropenem consumption (DDD/100 OBDs) • During 2015–2017, meropenem consumption decreased compared with 2012–2014 (RR, 0.67; 95% CI, 0.58–0.77; P < .001) |
| Kulwicki et al. 2019 [19] | Retrospective, single-center cohort analysis | Addition of an emergency medicine pharmacist into the ED to provide antimicrobial stewardship. Adherence to empiric treatment recommendations for CAP and community-acquired IAIs was examined pre-EMP and post-EMP. A secondary analysis was undertaken to examine adherence to these same guidelines in the early phases of implementation of an ASP compared with the established program. |
Significant difference in total appropriate empiric antibiotic selection with the EMP vs without the EMP • 78% vs 61%; P = .001 Significant difference in CAP treatment with the EMP vs without the EMP • 95% vs 79%; P = .005 Significant difference in community-acquired IAIs treatment with the EMP vs without the EMP • 62% vs 44%; P = .025 Significant difference in guideline-directed antibiotic prescribing in the established ASP period compared with the pre-ASP period • 82.5% vs 60%; P < .001 |
| Sacco et al. 2019 [20] | Single-center, quasi-experimental pre- and postintervention study | Following development of a validated risk stratification algorithm to guide testing and antibiotic use in patients with penicillin allergy. Health care professionals were educated on its use. The algorithm was intended to guide patient assessment and antibiotic selection. Data were assessed pre– and post–educational initiative. |
Antibiotic use • Cephalosporins +121.2%; P = .03 • Penicillins +256%; P = .04 • Vancomycin –67.2%; P = .04 • Fluoroquinolones –33.3%; P = .31 • Carbapenems –81.9%; P = .08 • Aztreonam –73.8%; P = .18 EMR documentation of type of adverse reaction to penicillin in the admission note • Pre 4.8% vs education 64.9%; P < .001 Use of the test-dose procedure • 8/27 patients Occurrence of adverse drug reactions • None Length of hospital stay • Pre 2.33 days vs education 2.07 days |
| Lee et al. 2018 [21] | Retrospective, single-center quasi-experimental cohort analysis | A fluoroquinolone restriction policy was implemented in 2005. Fluoroquinolone susceptibility was analyzed in a pre-implementation period (1998–2004) and a postimplementation period (2006–2016). Five Gram-negative organisms were included in the analysis: Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, P. aeruginosa, and Acinetobacter species. |
Fluoroquinolone use decreased from 173 DOT in the pre-implementation period to <60 DOT in the postimplementation period Fluoroquinolone susceptibility increased for: • Acinetobacter species (RR, 1.038; 95% CI, 1.005–1.072) • E. cloacae (RR, 1.028; 95% CI, 1.013–1.044) • P. aeruginosa (RR, 1.013; 95% CI, 1.006–1.020) Susceptibility did not change significantly for K. pneumoniae (RR, 1.002; 95% CI, 0.996–1.008) E. coli susceptibility continued to decline postimplementation (RR, 0.981; 95% CI, 0.975–0.987) |
| Keller et al. 2018 [22] | Single-center, prospective time series analysis | To reduce the ordering of urinalyses and urine cultures in patients without symptoms of a UTI, a series of interventions including the distribution of educational materials and implementation of CDS alerts in the EMR was implemented. CDS alerts were placed on all orders for urinalyses, urine cultures, and for antibiotics commonly used for treating UTIs (nitrofurantoin, trimethoprim-sulfamethoxazole, ciprofloxacin, cefazolin, cephalexin, and ceftriaxone). |
Primary outcome: Urinalysis orders did not significantly decrease • –10.2%; P = .24 Secondary outcome: Orders for urine cultures did significantly decrease • –6.3%; P < .001 Other results • Decrease in simultaneously ordering urinalyses and urine cultures (–5.8%; P < .001) • Decrease in urinalysis orders followed by antibiotic orders within 1–24 hours (–0.56%; P = .021) • Decrease in urine culture results followed by an antibiotic order within 24 hours (–0.24%; P = .036) |
| Lee et al. 2018 [23] | Prospective, multicenter pre/post chart audit | 15-minute education session to clinical staff focusing on the appropriate management of UTI and ASB, complimented by awareness posters and pocket cards summarizing UTI diagnostic criteria. |
Reduction in antibiotic prescriptions for ASB • Pre-intervention 45 of 50 (90%) vs postintervention 22 of 35 (63%); P = .003 Increase in proportion of residents presenting with localizing UTI symptoms • Pre-intervention 21 of 62 (34%) vs postintervention 22 of 50 (44%); P = .273 Reduction in health care costs • 64% reduction for pharmacy • 30% reduction for laboratory |
| Porter et al. 2018 [24] | Retrospective, single-center, before-and-after study | Conventional microbiology communication vs mRDT plus pharmacist-driven reporting protocol for positive blood cultures. |
Significant decrease in time to change in optimal therapy (50 vs 160 minutes; P = .0081) • Significant increase in percent changed to optimal therapy (41.4% vs 15.6%; P = .013) • Nonsignificant change in percent changed to effective therapy (17.2% vs 24.4%; P = .462) • Multivariate regression analysis showed that the intervention group was significantly less likely to have greater time-to-change value and more likely to be changed to optimal therapy (P < .01 for both) |
| Menichetti et al. 2018 [25] | Retrospective cohort comparing those who received ID consult plus intervention vs intervention alone | Restricted use of voriconazole, posaconazole, caspofungin, anidulafungin, micafungin, liposomal amphotericin B, and lipid complex amphotericin B to ID, intensive care, and hematology, plus ID consultation. |
Primary outcomes • In-hospital, 30-day mortality 20% with ID consult vs 37% without; P = .011 Secondary outcomes • Antibiotic consumption (DDD/100 bed-days): increases in fluconazole (3.1 to 4.3), echinocandins (0.22 to 0.35); decreases in voriconazole (0.25 to 0.18), and amphotericin (0.06 to 0.04) • Antibiotic cost: increased by €207 000 during study period |
| Claeys et al. 2018 [26] | Retrospective, single-center, observational study | Validation of a theoretical Verigene GNB treatment algorithm based on institutional antibiogram data, evidence-based management, and ASP practice. |
Significant theoretical decrease in cases receiving appropriate antibiotic therapy vs standard care (88.4% vs 78.1%; P = .014) • Strong level of agreement between reviewers regarding algorithm recommendations (ĸ = .855) • 14.4% appropriate de-escalation and 5.3% appropriate escalation • 4.8% inappropriate de-escalation and 16% unnecessary escalation |
Abbreviations: ASB, asymptomatic bacteriuria; ASP, antimicrobial stewardship program; ATO, antibiotic time-out; CAP, community-acquired pneumonia; CDI, Clostridioides difficile infection; CDS, clinical decision support; CI, confidence interval; DDD, defined daily dose; DOT, days of therapy; ED, emergency department; EMP, emergency medicine pharmacist; EMR, electronic medical record; EP, expected practice; GNB, Gram-negative bacteremia; IAI, intra-abdominal infection; ICD-10, International Classification of Diseases, Tenth Revision; ID, infectious diseases; IQR, interquartile range; mRDT, molecular rapid diagnostic technology; MRSA, methicillin-resistant Staphylococcus aureus; OBD, occupied bed-days; OR, odds ratio; PNA, pneumonia; RR, rate ratio; SSTI, skin and soft tissue infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
METHODS
Using a modified Delphi technique (detailed previously), members of the SERGE-45 network identified antimicrobial stewardship publications from 2018 considered to be significant using the following inclusion criteria: (1) published in 2018, including electronic, “early-release” publications, and (2) must include an actionable intervention [29]. An actionable intervention was defined as a stewardship strategy that was implemented in practice and resulted in measurable outcomes. Clinical practice guidelines, official statements, review articles, and articles without an actionable intervention were excluded.
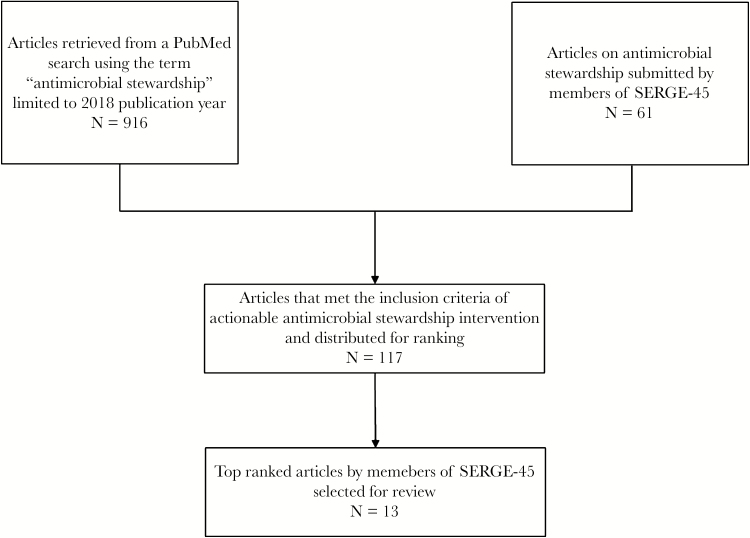
A PubMed search using “antimicrobial stewardship” for 2018 revealed 916 potential publications. EBC and PBB screened abstracts to ensure that all relevant articles were considered. In addition, a total of 61 publications were also submitted by authors for potential inclusion, and comments were provided electronically to E.B.C., C.M.B., and P.B.B. A total of 117 articles were distributed to the SERGE-45 network for ranking using SurveyMonkey based on contribution and/or application to ASP [30]. A teleconference among E.B.C., C.M.B., and P.B.B. reviewed the final ranking and established final consensus on the top 13 articles based on number of votes received for each article; all articles are described herein. Figure 1 depicts the flowchart of database and article selection, and Table 1 is a summary of the selected articles.
Figure 1.
Strategy for identification of top antimicrobial stewardship publications.
RESULTS
Expected Practice and Duration of Therapy
Yadav and colleagues sought to determine the impact of an institutional “expected practice” (EP) for antimicrobial duration of therapy on total days of therapy (DOT) administered inpatient and prescribed at discharge for common infections at a large academic medical center in Los Angeles, California [14]. The EP document, developed by a interdisciplinary group, listed many common infections seen in the inpatient and outpatient settings and referenced shorter courses of therapy with supporting evidence. The EP was endorsed by the Pharmacy & Therapeutics Committee and Medical Executive Committee. Providers were asked to explicitly justify longer antimicrobial durations in the medical record when deemed necessary for optimal patient care.
Implementation included a memo to clinicians and incorporation of EP into ASP rounds. Total DOTs and total antimicrobial exposure (defined as total mg of antibiotic administered + antibiotic prescribed at discharge) were compared among patients discharged from the facility in the 12 months before and after implementation of the EP, modeled as a function of the ASP. Patients were included if International Classification of Diseases, Tenth Revision (ICD-10), codes corresponding to targeted infectious processes were within the top 20 discharge diagnoses. Covariates in the model included age, gender, insurance status, in-hospital and expected mortality, and severity of illness. Significant decreases were observed in average DOT and antimicrobial exposure for all infection types. Mortality was assessed as a measure of safety for shorter courses of therapy and was similar across both time periods for each infection type. Use of the procalcitonin assay, which was implemented at the same time as the EP, was associated with longer durations of therapy. The authors attributed this to confounding by indication, as clinicians likely ordered procalcitonin for cases of greater complexity.
ASPs may consider EP an effective way to translate shorter durations of therapy into new institutional standards of care.
Antibiotic Time-outs and Duration of Therapy
The CDC and TJC recommend the use of interventions such as antibiotic time-outs (ATOs) or prospective audit and feedback (PAF) to improve antibiotic prescribing [3, 11]. ATOs may occur as part of standard practice without ASP involvement, prompting providers to have a structured conversations regarding the appropriateness of antibiotic regimens and durations.
Thom and colleagues performed a quasi-experimental study pre- and postimplementation of an ATO across 11 units (including adult and pediatric general and intensive care wards) located in 6 different hospitals in Maryland to measure the impact of a provider-driven ATO [15]. Pre-intervention data were collected during a 6-month baseline period, and postintervention data were collected for 9 months after implementation of the paper ATO tool that prompted care teams on antibiotic days 3–5 without input from the study or stewardship team. Primary outcomes were hospital antibiotic DOT per patient admission and total antibiotic DOT per patient admission, including antibiotic prescriptions at discharge. Secondary outcomes included antibiotic appropriateness and proportion of cases in which there was a modification or discontinuation of the regimen within 3–5 calendar days of onset. There was no difference between hospital DOT per admission or total DOT per admission in the pre- vs postimplementation groups, before and after controlling for unit and seasonal differences. Multivariable analysis showed no association between ATO intervention and number of times antibiotic regimens were modified or discontinued on days 3–5.
The findings of this study contribute to growing evidence supporting the impact of ASP input on improving antimicrobial utilization and overall patient outcomes. Further studies are needed to investigate the impact of additional adjunctive methods with ASP feedback on antibiotic use.
Optimizing the Treatment of Community-Acquired Pneumonia
The 2007 Infectious Diseaes Society of America (IDSA)/American Thoracic Society (ATS) guidelines for community-acquired pneumonia (CAP) recommend that patients be treated for a minimum of 5 days, afebrile for 48–72 hours, and have no more than 1 CAP-associated sign of clinical instability before discontinuation of therapy [31]. Despite these recommendations, patients continue to receive longer courses of therapy, increasing the risk of adverse events and antimicrobial resistance.
Foolad and colleagues conducted a multicenter, pre–post quasi-experimental study assessing the impact of a multifaceted prospective stewardship intervention on antimicrobial DOT and clinical outcomes in patients admitted with CAP to the medicine service at 3 large academic medical centers in Ann Arbor, Michigan, Milwaukee, Wisconsin, and New Orleans, Louisiana [16]. Interventions included (1) dissemination of institution-specific CAP guidelines and pocket cards, (2) educational sessions to prescribers and pharmacists on the appropriate management of CAP, focusing on DOT, and (3) targeted PAF and intervention by ID pharmacists Monday–Friday. Notably, patients admitted to the ICU were excluded. The primary objective was CAP antimicrobial DOT pre- and postintervention. Secondary clinical outcomes evaluated included mortality, readmission or presentation to a health care facility for pneumonia, and incidence of Clostridioides difficile infection, all at 30 days postdischarge. Six hundred patients were included in the study, 307 in the historical group and 293 in the intervention group. Decreases in median antibiotic DOT and improvement in guideline-concordant therapy were demonstrated postintervention. There were no significant differences in secondary clinical outcomes within 30 days of discharge.
The authors note that this was the largest study to date assessing the impact of ASP interventions on antibiotic DOT and clinical outcomes in patients with CAP. It was conducted at 3 large academic institutions and required dedicated ASP pharmacist time and resources to perform PAF, which may limit generalizability. It is also difficult to assess which intervention had the greatest impact, as they were implemented concurrently, and the number of interventions performed by the ASP pharmacists was not reported.
Microbiology Reports and Antibiotic Prescribing for Pneumonia
Antimicrobial prescribing patterns are directly influenced by clinicians’ interpretation of microbiology results and reports [32]. Musgrove and colleagues conducted a quasi-experimental study to compare de-escalation rates before and after changing respiratory culture reports across a 4-hospital health system in Detroit, Michigan [17]. The intervention, in combination with previously established antimicrobial stewardship practices (eg, syndrome-specific treatment guidelines, PAF), modified wording on non-pathogen-containing respiratory cultures to specifically note absence of Staphylococcus aureus, methicillin-resistant S. aureus (MRSA), or Pseudomonas aeruginosa. In addition, in-person education was provided to intensive care unit providers and pharmacists, which was supplemented by a 1-page educational handout. One hundred five patients receiving inpatient treatment with anti-MRSA (vancomycin or linezolid) and antipseudomonal (cefepime, piperacillin/tazobactam, meropenem, or aztreonam) therapy for respiratory infections were included in both the 6-month pre- and postintervention groups. De-escalation and discontinuation of unnecessary anti-MRSA and antipseudomonal therapy occurred significantly more often in the postintervention group, resulting in an average decrease of 2 DOTs. After adjusting for disease severity, the revised wording on respiratory cultures was associated with 5.5-fold increased odds of de-escalation. Fewer patients in the postintervention group experienced acute kidney injury, but no difference was observed in intensive care unit or hospital length of stay (LOS), or in-hospital, all-cause-mortality.
This study reinforces the importance of microbiology reports in achieving ASP goals. In addition, the results of this study demonstrate that simple ASP interventions can result in significant improvements in antimicrobial prescribing.
Optimizing the Use of Meropenem
García-Rodríguez and colleagues performed a quasi-experimental pre/postintervention study to evaluate the impact of meropenem ASP recommendations on rates of appropriate justification of treatment, antibiotic consumption measures, infection-related and all-cause mortality, and incidence of multidrug-resistant hospital-acquired bloodstream infections [18]. Additional clinical and economical comparisons were described between the groups of patients with and without acceptance of ASP recommendations when meropenem did not fulfill justification criteria.
This study describes a resource-limited approach by a multidisciplinary team to improve meropenem utilization at a single 350-bed teaching hospital in Spain from 2015 to 2017. Local guidelines for empiric antibiotic treatment were developed and made accessible on every hospital desktop computer. In addition, active surveillance was performed 6 hours weekly by an ID physician who reviewed each case and provided recommendations to prescribers in 1 of the following ways: face to face, telephone, or through the electronic medical record (EMR). During the last 4 months of 2014, patient cases with meropenem were reviewed retrospectively as the pre-intervention study group for comparison. Overall, in the pre-intervention period, 47.3% of the 150 patients receiving meropenem were considered justified based on study criteria for appropriate treatment, which included severe sepsis, history of extended spectrum beta-lactamase (ESBL) colonization, or hospital-acquired infection in which broad-spectrum antibiotics were necessary. There were 852 patients who received meropenem treatment during the intervention period, with 61% of cases considered justified or appropriate. Of the 330 cases that were not considered justified, the prescribers accepted 82% of the ID physician recommendations. Acceptance of intervention was associated with shorter duration of antibiotic treatment and inpatient days. The study further compared patients with and without acceptance of ASP recommendations and found that pulmonary and abdominal infections were associated with lower acceptance rates. Overall, there was a 33% decrease in meropenem consumption when comparing the pre-intervention years (2012–2014) with the intervention years (2015–2017).
The strength of this study is that it can be replicated in settings where targeting a specific antibiotic is needed and an ID physician is available for intervention. Despite these results, it remains important to consider syndrome-specific interventions that may result in advantageous declines in antibiotic utilization and avoid compensatory increases in other broad-spectrum antibiotics.
Emergency Medicine Pharmacist and Antibiotic Prescribing for CAP and Intra-abdominal Infections
In the United States, approximately 16% of all patients who visit the emergency department (ED) each year receive an antibiotic, but many are either inappropriate or unnecessary [33, 34]. ASPs can help improve antibiotic prescribing practices, including in the ED, and pharmacists play an important role in the provision of ASPs [35].
Kulwicki and colleagues conducted a retrospective cohort analysis to determine the effect of an emergency medicine pharmacist (EMP) on the selection of appropriate empiric antibiotics for the treatment of CAP and community-acquired intra-abdominal infections (IAIs) in the ED, compared with having no EMP, in Grand Rapids, Michigan [19]. Determination of appropriate antibiotics was based on following institutional protocols derived from IDSA guidelines, in conjunction with local antimicrobial resistance patterns. A secondary objective was to examine empiric antibiotic prescribing for these 2 disease states in the ED during a period of early ASP (2014) compared with an established ASP (2016). Three-hundred twenty patients were included (185 in the EMP group and 135 in the no-EMP group). Appropriate empiric prescribing occurred more often in the EMP group compared with the no-EMP group. Treatment of both CAP and community-acquired IAIs was more likely to be appropriate in the EMP group compared with the non-EMP group. Further, guideline-directed antibiotic prescribing significantly improved from the early ASP period to the established ASP period.
Overall, the results of this study demonstrate the positive impact of having an EMP as a steward extender for ASPs.
Use of an Inpatient Penicillin Allergy Assessment Protocol
Allergy to penicillin is one of the most frequently reported and documented allergies. Over 30 million patients have reported an allergy to penicillin, and as many as 90% of these allergies are inaccurate [36]. Carrying this label has an impact on ASP, as it leads to increased prescribing of broad-spectrum or second-line agents, as well as increased LOS and overall costs [36]. One intervention used to assess patients with a history of IgE-mediated allergy is penicillin skin testing (PST); however, logistics and access to PST can be limited [37].
Sacco and colleagues performed a single-center, quasi-experimental pre- and postintervention study in Jacksonville, Florida, to assess the effects on antibiotic prescribing after education and implementation of a validated algorithm that categorizes patients based on risk stratification [20]. Providers were educated by an allergist on penicillin allergies and given a standardized algorithm to help with taking a proper history and antibiotic selection. In the pre- and postintervention cohort of patients admitted to the general medicine ward with a reported penicillin allergy, there were 42 and 57 patients, respectively. Documentation of allergy reaction history on admission improved from 4.8% pre-intervention to 64.9% postintervention (P < .001). Penicillin and cephalosporin usage increased by 256% and 121%, respectively, whereas vancomycin, fluoroquinolone, carbapenem, and aztreonam usage decreased.
Although only a single center with limited sample size, this study demonstrated that education and standardization of prescribing can affect antibiotic selection in patients who present with a penicillin allergy to facilities with limited resources to routinely perform PST.
Fluoroquinolone Use and Pre-authorization Policy
Fluoroquinolone use in the United States has steadily increased in the past decade, with a concomitant increase in resistance, particularly among Gram-negative organisms [38, 39]. ASPs can improve fluoroquinolone use and lead to improvements in susceptibility.
Lee and colleagues conducted a retrospective, quasi-experimental study to examine fluoroquinolone susceptibility before (pre-intervention period 1998–2005) and after (postintervention period 2006–2016) implementation of a policy that required ASP approval for empiric fluoroquinolone use at a large academic medical center in Birmingham, Alabama [21]. Susceptibility patterns of 5 Gram-negative organisms were analyzed: Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, P. aeruginosa, and Acinetobacter species. Inpatient use of fluoroquinolones increased steadily beginning in 1998, peaking in 2004 with 173 DOT per 1000 patient-days. The fluoroquinolone prior authorization policy was implemented in October 2005 and was successful in reducing fluoroquinolone use. Between 2006 and 2016, fluoroquinolone use was <60 DOT per 1000 patient-days. In the postintervention period, fluoroquinolone susceptibility significantly increased (P < .0001) for Acinetobacter species, E. cloacae, and P. aeruginosa. No significant change was noted for K. pneumoniae. The susceptibility for E. coli continued to decline, albeit not as dramatically as in the pre-intervention period. Both E. coli and K. pneumoniae are often community-onset pathogens, and unrestricted fluoroquinolone use in the community setting would likely contribute to the lack of significant impact on susceptibilities.
One limitation of this study was that the data were from a single academic center. In addition, a control unit or hospital could not be used for comparison because the fluoroquinolone restriction was universally applied. The results of this study demonstrate the effectiveness of a fluoroquinolone restriction policy in decreasing overall use and improvement in susceptibility of hospital-acquired Gram-negative organisms.
Asymptomatic Bacteriuria and Clinical Decision Support
Asymptomatic bacteriuria (ASB) is a common medical condition that seldom requires treatment [40]. Obtaining urinalyses and urine cultures in patients without signs or symptoms of a urinary tract infection (UTI) can lead to unnecessary antibiotics, which in turn leads to increasing resistance [41, 42].
Keller and colleagues implemented a combination of interventions to reduce urine testing for ASB that included provider education and clinical decision support (CDS) alerts in the EMR at a large academic medical center in Baltimore, Maryland [22]. In August 2015, educational materials were placed on hospital-wide screen savers and disseminated through a newsletter email. The CDS tools included informational messages recommending not to test for UTIs in patients without symptoms and recommending against treating ASB. These messages appeared on all EMR orders for urinalysis, urine culture, and for antibiotics commonly used for treating UTIs. The authors performed a prospective time series analysis utilizing a pre-intervention phase (September 2014–June 2015) and a postintervention phase (September 2015–June 2016). Orders for urinalyses did not decrease significantly but orders for urine cultures significantly decreased. Additionally, in the postintervention phase, there was a decrease in simultaneously ordering urinalyses and urine cultures (–5.8%), a decrease in urinalysis orders followed by antibiotic orders within 1–24 hours (–0.56%), and a decrease in urine culture results followed by an antibiotic order within 24 hours (–0.24%).
This study has a number of limitations, including short duration (<1 year) and a single center, and appropriateness of each antibiotic-based documentation of symptoms was not assessed. Overall, this study demonstrated that the use of educational materials and CDS may reduce the number of urine cultures ordered and antibiotics prescribed for the treatment of ASB.
Asymptomatic Bacteriuria and Antibiotic Use in the Long-term Care Setting
Unnecessary antimicrobial use in long-term care (LTC) residents related to ASB has been identified as a major area of opportunity for improvement and has led to efforts such as the “Symptom-Free Pee, Let It Be” campaign [43]. The best approach to increasing appropriate ASB management in the LTC setting is not known, and great interest exists in identifying viable methods for tackling the problem, particularly in resource-limited organizations.
Lee and colleagues undertook an evaluation of an educational intervention related to ASB in patients at 7 LTC facilities in Regina, Saskatchewan, Canada, with the primary outcome of percentage of residents who received inappropriate antibiotic treatment for ASB [23]. There was a pre-assessment period and a postassessment period of 5 weeks each, and the intervention took place during the 2 weeks in between. The intervention was designed to include feedback and monitoring, shaping knowledge, natural consequences, and comparison of behavior. The intervention included educational sessions that incorporated information on ASB treatment guidelines, local findings from the pre-intervention audit, and diagnostic criteria for UTIs. Educational posters were displayed after the 15-minute sessions, and pocket cards were distributed. Educational efforts were focused toward clinical staff, which was primarily made up of nursing staff. Intervention demonstrated a decrease in ABS antibiotic prescribing from 90% to 63%
One important limitation to this study is that only 15% of the clinical staff were present for an educational session and physicians were not included. Additionally, the study period was relatively short, with long-term impact unknown. However, this resource- and time-limited effort was effective in improving ASB management at 7 different LTC facilities.
Multiplex Polymerase Chain Reaction Blood Culture Results vs Conventional Microbiology Methods
Rapid identification of organisms and timely optimization of therapy are important to limit morbidity and mortality, decrease use of broad-spectrum agents, and improve clinical response [44–47]. With recent advancements in molecular rapid diagnostic technology (mRDT), organisms can be identified faster than the conventional 48–72 hours. Pharmacists are optimally placed to aid in correct interpretation and application of these results.
Porter and colleagues performed a single-center, retrospective, before-and-after study comparing time with change in antimicrobial therapy between a conventional microbiology protocol (December 2014–November 2015) and multiplex polymerase chain reaction with pharmacist-driven reporting protocol (April 2016–March 2017) at a community hospital in Savannah, Georgia [24]. Conventional protocol included results being communicated to a nurse, who would then notify the provider. The intervention group consisted of pharmacists utilizing a protocol developed and approved by the ASP subcommittee to notify the team, make recommendations, and enter accepted orders into the EMR. The primary outcome of time to change in antimicrobial therapy was measured from time of call with results to time of antimicrobial change, with only changes within 24 hours and initial calls being included. Secondary outcomes further divided results by time to change from suboptimal to optimal therapy or from ineffective to effective therapy. Change to optimal therapy included escalation to vancomycin for MRSA and discontinuation of vancomycin when clinically unnecessary. Patients in the intervention group (77/118) had decreased median time-to-change values for effective therapy (50 vs 160 minutes; P = .0081), and a higher percentage were changed to optimal therapy (41.4% vs 15.6%; P = .013). Additionally, there was a significant decrease in vancomycin utilization for coagulase-negative Staphylococcus spp. in the intervention group (69.3% vs 10%; P < .01).
Lack of improvement in clinical outcomes with mRDT without ASP intervention has been previously established. This study provides evidence for clinical benefits with mRDT and pharmacist involvement in resource-limited institutions, enabling front-line pharmacists to provide direct recommendations, with additional backup by ASP pharmacists through approved protocols. Additionally, analysis of immediate changes only may more closely represent the impact of ASP and the protocol.
ASP With or Without ID Consults and Candidemia
With the persistently high rates of associated mortality, programs have been targeting candidemia for antifungal stewardship interventions [48–51]. Menichetti and colleagues conducted a single-center retrospective study evaluating patients receiving an ID consultation in combination with an antifungal stewardship program vs those who did not at a large academic medical center in Italy [25]. The intervention consisted of antifungal restriction for most agents outside of fluconazole, requiring authorization via ID consult. ID consults were at the discretion of the primary provider and were completed within 24–36 hours of the request by 2 senior ID physicians. Education regarding awareness and appropriate treatment of candidemia based on published guidelines was additionally provided during the study period. The primary outcome was impact of the antifungal stewardship program with or without ID consultation on in-hospital 30-day mortality associated with candidemia. Secondary outcomes included mortality risk factors, antifungal consumption, and cost. From 2012 to 2014, 276 patients were included (76 with ID consult, 200 without). In-hospital 30-day mortality was 20% for patients with an ID consult and 37% for those without (P = .011). Of note, 26% of patients in the group without ID consult received no antifungal treatment. On univariate analysis, age >65 years and admission to an internal medicine ward were associated with higher risk of death, whereas ID consult, late-onset candidemia, and nonalbicans Candida species were protective. In multivariate analysis, ID consult, nonalbicans Candida species, and age remained significant. During the study period, fluconazole and echinocandin use increased, whereas voriconazole and amphotericin decreased.
Limitations include the small sample size, retrospective single-center design, and lack of information on source control. Further study on the impact of antifungal stewardship on patient outcome metrics would be beneficial in extrapolating these data to other institutions.
mRDT for Gram-Negative Bacteremia
Timely, appropriate antibiotic therapy is key for improved morbidity and mortality in Gram-negative bacteremia (GNB). The Verigene Blood Culture Gram-Negative (BC-GN) rapid diagnostic test can quickly identify 8 common target organisms and 6 resistance determinants with 97.1% sensitivity and 99.5% specificity [52, 53]. It is important to pair these with ASP involvement.
In a retrospective, single-center, observational study at a large academic medical center in Baltimore, Maryland, Claeys and colleagues developed a GNB treatment algorithm based on institution-specific antibiogram data and evidence-based practice [26]. A cutoff value of at least 88% susceptible, based on the reported susceptibility of piperacillin/tazobactam, was utilized for Gram-negative organisms without a resistance mechanism identified by Verigene BC-GN. Empiric therapy with meropenem was utilized in immunocompromised or critically ill patients where the antibiogram data showed higher rates of third-generation cephalosporin resistance with E. coli and Klebsiella spp. ASP pharmacists determined definitions for standard care (empiric) vs algorithm-based (optimal and targeted) antibiotic therapy and independently evaluated the appropriateness of standard care vs theoretical receipt of algorithm-based therapy. Allergy history or reconciliation was not considered for this assessment. Out of 144 patients with Verigene BC-GN target organisms, there was a moderate level of agreement between the reviewers regarding the appropriateness of standard care antibiotics and a strong level of agreement for algorithm recommendations. In vitro susceptibility was higher with algorithm-recommended therapy (92.1% vs 77.8%), and significantly more cases would have received appropriate therapy (88.4% vs 78.1%). Although 14.4% of cases were appropriately de-escalated, 4.8% were inappropriately de-escalated; related risk factors included polymicrobial GNB, central line source, Acinetobacter spp., Enterobacter spp., and/or OXA+ resistance determinants.
The strengths of this study include validation of a GNB treatment algorithm based on institution-specific antibiogram data, Verigene BC-GN results, and ASP input. This combination showed the potential for increase in patients receiving timely, appropriate therapy with theoretical, retrospective validation. ASPs interested in this implementation strategy must note that 100% adherence to the algorithm may cause unnecessary escalation or inappropriate de-escalation and should customize their algorithm according to their institutional data and practices.
DISCUSSION
Novel antimicrobial stewardship interventions continue to move practice and research forward for clinicians and ASP stakeholders. The scholarship highlighted in this review demonstrates a continued commitment to novel models of stewardship interventions, value assessment of mRDT implementation, and integration of stewardship into areas outside the traditional inpatient walls of an academic medical center (eg, community hospitals, LTC facilities). As the quantity of stewardship publications increases, it is important that the quality and scientific rigor of research increases as well [13]. For many inpatient institutions, antimicrobial stewardship is not a new concept; thus scholarship demonstrating sustainability is important. Clinicians and scholars should ensure that stewardship training includes skills development on research study design, methods, and data analysis. Mentoring by and collaboration with established scholars will aid new stewards in executing high-quality scholarship and promote generalizability of results. Prospective, interventional stewardship studies conducted across multiple centers outside the umbrella of a single health system would provide the quality evidence needed to establish best practices and efficient models to optimize antimicrobial therapy.
Acknowledgments
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Dellit TH, Owens RC, McGowan JE Jr, et al. Infectious Diseases Society of America; Society for Healthcare Epidemiology of America Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis 2007; 44:159–77. [DOI] [PubMed] [Google Scholar]
- 2. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016; 62:e51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Core elements of hospital antibiotic stewardship programs Available at: https://www.cdc.gov/antibiotic-use/healthcare/implementation/core-elements.html. Accessed 17 July 2019. [DOI] [PMC free article] [PubMed]
- 4. Society of Infectious Diseases Pharmacists. Antimicrobial stewardship certificate programs Available at: https://www.sidp.org/Stewardship-Certificate. Accessed 17 July 2019.
- 5. Making a Difference in Infectious Diseases. Antimicrobial stewardship programs Available at: https://mad-id.org/antimicrobial-stewardship-programs. Accessed 17 July 2019.
- 6. Luther VP, Shnekendorf R, Abbo LM, et al. Antimicrobial stewardship training for infectious diseases fellows: program directors identify a curriculum need. Clin Infect Dis 2018; 67:1285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Society for Healthcare Epidemiology of America. Antibiotic stewardship training course Available at: https://sheaspring.org/program/certificate-tracks/#SHEAAS. Accessed 17 July 2019.
- 8. Infectious Diseases Society of America. Antimicrobial stewardship curriculum for fellows Available at: https://my.idsociety.org/idsanews/home/april-2018/antimicrobialstewardship042018. Accessed 17 July 2019.
- 9. Centers for Disease Control and Prevention. Core elements of outpatient antibiotic stewardship Available at: https://www.cdc.gov/antibiotic-use/community/improving-prescribing/core-elements/core-outpatient-stewardship.html. Accessed 17 July 2019.
- 10. Centers for Disease Control and Prevention. Core elements of antibiotic stewardship for nursing homes Available at: https://www.cdc.gov/longtermcare/prevention/antibiotic-stewardship.html. Accessed 17 July 2019.
- 11. The Joint Commission. Approved: New antimicrobial stewardship standard Available at: https://www.jointcommission.org/assets/1/6/New_Antimicrobial_Stewardship_Standard.pdf. Accessed 17 July 2019.
- 12. The Joint Commission. Antimicrobial stewardship in ambulatory health care Available at: https://www.jointcommission.org/assets/1/18/R3_23_Antimicrobial_Stewardship_AMB_6_14_19_FINAL.pdf. Accessed 17 July 2019.
- 13. Pagels CM, McCreary EK, Rose WE, Dodds Ashley ES, Bookstaver PB, Dilworth TJ. Designing antimicrobial stewardship initiatives to enhance scientific dissemination. J Am Coll Clin Pharm. In press. [Google Scholar]
- 14. Yadav K, Masuda E, Minejima E, Spellberg B. Expected practice as a novel antibiotic stewardship intervention. Open Forum Infect Dis 2018; 6(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thom KA, Tamma PD, Harris AD, et al. Impact of a prescriber-driven antibiotic time-out on antibiotic use in hospitalized patients. Clin Infect Dis 2019; 68:1581–4. [DOI] [PubMed] [Google Scholar]
- 16. Foolad F, Huang AM, Nguyen CT, et al. A multicentre stewardship initiative to decrease excessive duration of antibiotic therapy for the treatment of community-acquired pneumonia. J Antimicrob Chemother 2018; 73:1402–7. [DOI] [PubMed] [Google Scholar]
- 17. Musgrove MA, Kenney RM, Kendall RE, et al. Microbiology comment nudge improves pneumonia prescribing. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. García-Rodríguez JF, Bardán-García B, Peña-Rodríguez MF, et al. Meropenem antimicrobial stewardship program: clinical, economic, and antibiotic resistance impact. Eur J Clin Microbiol Infect Dis 2019; 38:161–70. [DOI] [PubMed] [Google Scholar]
- 19. Kulwicki BD, Brandt KL, Wolf LM, et al. Impact of an emergency medicine pharmacist on empiric antibiotic prescribing for pneumonia and intra-abdominal infections. Am J Emerg Med 2019; 37:839–44. [DOI] [PubMed] [Google Scholar]
- 20. Sacco KA, Cochran BP, Epps K, et al. Inpatient β-lactam test-dose protocol and antimicrobial stewardship in patients with a history of penicillin allergy. Ann Allergy Asthma Immunol 2019; 122:184–8. [DOI] [PubMed] [Google Scholar]
- 21. Lee RA, Scully MC, Camins BC, et al. Improvement of Gram-negative susceptibility to fluoroquinolones after implementation of a pre-authorization policy for fluoroquinolone use: a decade-long experience. Infect Control Hosp Epidemiol 2018; 39:1419–24. [DOI] [PubMed] [Google Scholar]
- 22. Keller SC, Feldman L, Smith J, et al. The use of clinical decision support in reducing diagnosis of and treatment of asymptomatic bacteriuria. J Hosp Med 2018; 13:392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee C, Phillips C, Vanstone JR. Educational intervention to reduce treatment of asymptomatic bacteriuria in long-term care. BMJ Open Qual 2018; 7:e000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Porter AM, Bland CM, Young HN, et al. Comparison of pharmacist-directed management of multiplex PCR blood culture results with conventional microbiology methods on effective and optimal therapy within a community hospital. Antimicrob Agents Chemother 2018; 63:pii:e01575–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Menichetti F, Bertolino G, Sozio E, et al. GISA (Italian Group for Antimicrobial Stewardship) Candidemia Study Group Impact of infectious diseases consultation as a part of an antifungal stewardship programme on candidemia outcome in an Italian tertiary-care, university hospital. J Chemother 2018; 30:304–9. [DOI] [PubMed] [Google Scholar]
- 26. Claeys KC, Schlaffer KE, Heil EL, et al. Validation of an antimicrobial stewardship-driven Verigene blood-culture Gram-negative treatment algorithm to improve appropriateness of antibiotics. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cluck DB, Bland CM, Chahine EB, et al. A baker’s dozen of top antimicrobial stewardship publications in 2016 Available at: https://www.preprints.org/manuscript/201903.0146/v1. Accessed 17 July 2019.
- 28. Chastain DB, Cluck DB, Stover KR, et al. A baker’s dozen of top antimicrobial stewardship intervention publications in 2017. Open Forum Infect Dis 2019; 6(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. Santa Monica, CA: RAND Corporation; 2001. [Google Scholar]
- 30. SurveyMonkey. Available at: https://www.surveymonkey.com/. Accessed 17 July 2019.
- 31. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America; American Thoracic Society Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 2007; 44(Suppl 2):S27–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cunney RJ, Smyth EG. The impact of laboratory reporting practice on antibiotic utilisation. Int J Antimicrob Agents 2000; 14:13–9. [DOI] [PubMed] [Google Scholar]
- 33. Niska R, Bhuiya F, Xu J. National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Rep 2010; 6:1–31. [PubMed] [Google Scholar]
- 34. Chin MH, Wang LC, Jin L, et al. Appropriateness of medication selection for older persons in an urban academic emergency department. Acad Emerg Med 1999; 6:1232–42. [DOI] [PubMed] [Google Scholar]
- 35. Trinh TD, Klinker KP. Antimicrobial stewardship in the emergency department. Infect Dis Ther 2015; 4:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones BM, Avramovski N, Concepcion AM, et al. Clinical and economic outcomes of penicillin skin testing as an antimicrobial stewardship initiative in a community health system. Open Forum Infect Dis 2019; 6(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: assessing tools for antimicrobial stewardship. J Allergy Clin Immunol 2017; 140:154–61.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linder JA, Huang ES, Steinman MA, et al. Fluoroquinolone prescribing in the United States: 1995 to 2002. Am J Med 2005; 118:259–68. [DOI] [PubMed] [Google Scholar]
- 39. Scheld WM. Maintaining fluoroquinolone class efficacy: review of influencing factors. Emerg Infect Dis 2003; 9:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nicolle LE, Gupta K, Bradley SF, et al. Clinical practice guideline for the management of asymptomatic bacteriuria: 2019 update by the Infectious Diseases Society of Americaa. Clin Infect Dis 2019; 68:e83–110. [DOI] [PubMed] [Google Scholar]
- 41. Cai T, Mazzoli S, Mondaini N, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat? Clin Infect Dis 2012; 55:771–7. [DOI] [PubMed] [Google Scholar]
- 42. Cai T, Nesi G, Mazzoli S, et al. Asymptomatic bacteriuria treatment is associated with a higher prevalence of antibiotic resistant strains in women with urinary tract infections. Clin Infect Dis 2015; 61:1655–61. [DOI] [PubMed] [Google Scholar]
- 43. Association of Medical Microbiology and Infectious Disease Canada. Symptom-free pee: let it be Available at: https://www.ammi.ca/?ID=127. Accessed 17 July 2019.
- 44. Huang AM, Newton D, Kunapuli A, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis 2013; 57:1237–45. [DOI] [PubMed] [Google Scholar]
- 45. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34:1589–96. [DOI] [PubMed] [Google Scholar]
- 46. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock. Crit Care Med 2016; 45:486–552. [DOI] [PubMed] [Google Scholar]
- 47. Timbrook TT, Morton JB, McConeghy KW, et al. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:15–23. [DOI] [PubMed] [Google Scholar]
- 48. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 49. López-Medrano F, Juan RS, Lizasoain M, et al. A non-compulsory stewardship programme for the management of antifungals in a university-affiliated hospital. Clin Microbiol Infect 2013; 19:56–61. [DOI] [PubMed] [Google Scholar]
- 50. Ramos A, Pérez-Velilla C, Asensio A, et al. Antifungal stewardship in a tertiary hospital. Rev Iberoam Micol 2015; 32:209–13. [DOI] [PubMed] [Google Scholar]
- 51. Rac H, Wagner JL, King ST, et al. Impact of an antifungal stewardship intervention on optimization of candidemia management. Ther Adv Infect Dis 2018; 5:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bork JT, Leekha S, Heil EL, et al. Rapid testing using the Verigene Gram-negative blood culture nucleic acid test in combination with antimicrobial stewardship intervention against Gram-negative bacteremia. Antimicrob Agents Chemother 2015; 59:1588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luminex Corporation. Luminex Corporation. Verigene Gram-negative blood culture test Available at: https://www.luminexcorp.com/clinical/infectious-disease/verigene-bloodstream-infection-tests/gram-negative-blood-culture/. Accessed 18 July 2019.