Abstract
Acrolein, known as one of the most common reactive carbonyl species, is a toxic small molecule affecting human health in daily life. This study is focused on the scavenging abilities and mechanism of ferulic acid and some other phenolic acids against acrolein. Among the 13 phenolic compounds investigated, ferulic acid was found to have the highest efficiency in scavenging acrolein under physiological conditions. Ferulic acid remained at (3.04±1.89)% and acrolein remained at (29.51±4.44)% after being incubated with each other for 24 h. The molecular mechanism of the detoxifying process was also studied. Detoxifying products, namely 2-methoxy-4-vinylphenol (product 21) and 5-(4-hydroxy-3-methoxyphenyl)pent-4-enal (product 22), were identified though nuclear magnetic resonance (NMR) and gas chromatography-mass spectrometry (GC-MS), after the scavenging process. Ferulic acid showed significant activity in scavenging acrolein under physiological conditions. This study indicates a new method for inhibiting damage from acrolein.
Keywords: Acrolein, Reactive carbonyl species, Ferulic acid, Cytotoxicity, Oxidative stress
1. Introduction
Reactive carbonyl species (RCS), which share a reactive carbonyl group in structure, are toxic small molecules existing in humans and are related to some common chronic diseases. A type of very frequent structure of RCS is α,β-unsaturated aldehydes or ketones. As downstream products of reactive oxygen species (ROS), RCS play an important role in the oxidation process in cells. The cytotoxic RCS can cause many diseases by increasing oxidative stress in cells, and further lead to cell death or apoptosis. First, ROS cause lipid peroxidation and RCS generation, and then RCS consume antioxidants such as glutathione (Ålin et al., 1985; Mano et al., 2009), causing the increase of cell oxidative stress and subsequent ROS levels (Mano, 2012).
The representative RCS acrolein is a harmful unsaturated aldehyde compound, which has been demonstrated to be tightly associated with many diseases (Table S1) such as Alzheimer’s (Lovell et al., 2001; Luo and Shi, 2005), Parkinson’s (Shamoto-Nagai et al., 2007), diabetes (Suzuki and Miyata, 1999), and atherosclerosis (DeJarnett et al., 2014; Rom et al., 2017). Lovell et al. (2001) reported that acrolein concentration showed statistically significant difference in Alzheimer’s disease and the control brain, and it showed about eight times higher concentration than the control brain in the amygdala and hippocampus. Acrolein is also associated with cardiovascular disease such as atherosclerosis; levels of several markers of atherosclerosis in mice aortas showed a statistically significant increase after being incubated with acrolein (Rom et al., 2017). Acrolein can be generated from carbohydrates (Yaylayan and Keyhani, 2000) and fatty acids (Esterbauer et al., 1991) in an oxidative process when the temperature is too high, from incomplete combustion of cigarette, petrol, wood, and plastic (Esterbauer et al., 1991), and from endogenous lipid peroxidation and endogenous polyamine metabolism (Stevens and Maier, 2008). Also it was reported that acrolein can be generated because of autoxidation of fatty acids at human body temperature (Wang and Cui, 2015). In addition, the anticancer agent cyclophosphamide, which is related to bladder toxicity, can generate acrolein as a metabolite (Cox, 1979; Liu et al., 2012). Thus, the most common pathway for acrolein to enter human body is through cooked food (especially overheated oil) and cigarette smoke.
Some phenolic compounds were demonstrated to have RCS scavenging activity. For instance, curcumin was proved to be able to protect SK-N-SH cells against acrolein toxicity (Doggui et al., 2013); resveratrol and hesperetin can exert health benefits in part through neutralizing toxic acrolein in vivo (Wang et al., 2015); quercetin could also scavenge acrolein, crotonaldehyde, and (E)-2-pentenal during the frying process (Zamora et al., 2016).
As a phenolic compound, ferulic acid (4-hydroxy-3-methoxy cinnamic acid) is distributed widely in nature, and has diverse benefits for health such as being antioxidative and anti-inflammatory (Amro et al., 2002; Srinivasan et al., 2007; Huang et al., 2017; Baskaran et al., 2018). It has been shown to have positive effects on many diseases such as Alzheimer’s, cancer, cardiovascular diseases, and diabetes (Srinivasan et al., 2007; Kumar and Pruthi, 2014; Mancuso and Santangelo, 2014). It is worth mentioning that the antioxidant function of ferulic acid is shown through not only inhibiting radicals like ROS or RCS (Picone et al., 2009; Cassano et al., 2010; Trombino et al., 2013), but also its effects on some enzymes of the cell stress response (Joshi et al., 2006; Calabrese et al., 2008; Fetoni et al., 2010; Catino et al., 2016). Ferulic acid can be found in fruits, vegetables, cereals, and some traditional medicinal plants such as Angelica sinensis (Oliv.) Diels (Sheu et al., 1987; Balasubashini et al., 2003). Herein, based on the research method of previous work on scavenging ROS of our group (He et al., 2009; Li et al., 2015), we systematically studied the scavenging reaction between ferulic acid and acrolein under physiological conditions, along with the mechanism and structure-activity relationship of this detoxifying process.
2. Materials and methods
2.1. Chemicals
Ferulic acid, caffeic acid, pyrogallic acid, vanillin, isoeugenol, cinnamic acid, chlorogenic acid, L-tyrosine, and 2,4-dinitrophenylhydrazine (DNPH) were purchased from Aladdin Industrial Inc., Shanghai, China. Trans-4-coumaric acid, sinapic acid, galllic acid, 3-methoxycinnamic acid, 3-hydroxycinnamic acid, 2-methoxy-4-vinylphenol, glutathione (GSH), and ferulic acid methyl ester were purchased from Energy Chemical Industrial Inc., Shanghai, China. Resveratrol was purchased from Sigma, St. Louis, MO, USA. Deionized H2O used for reaction and high-performance liquid chromatography (HPLC) experiments was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). Methanol, acetonitrile, and formic acid for HPLC analysis were of chromatographic grade and purchased from Merck, Darmstadt, Germany. All other reagents were purchased at the highest commercial quality and used without further purification.
2.2. Reaction between acrolein and phenolic compounds
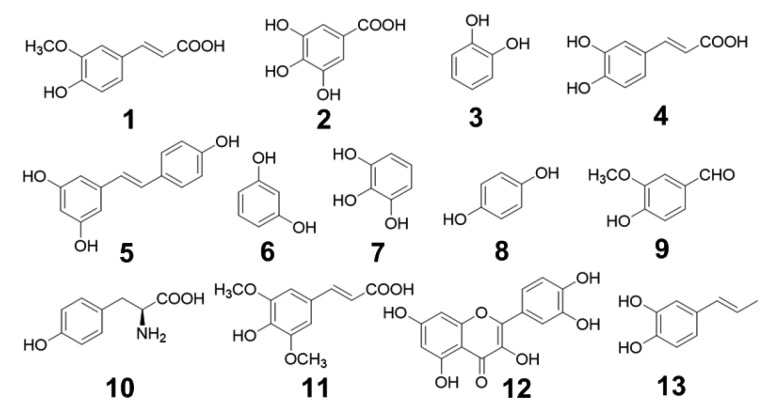
Phenolic compounds shown in Fig. 1 and acrolein were dissolved in dimethyl sulfoxide (DMSO)/phosphate buffer (pH 7.4) (1:99, v/v) to a concentration of 20 mmol/L. Phenolic compound solution (0.5 mL) and acrolein solution (0.5 mL) were incubated at 37 °C for 24 h. Remaining phenolic compounds were detected by HPLC (Varian ProStar 210) equipped with a detector (Varian ProStar 325), an automatic sampler (Varian ProStar 410), and a C18 column (Elite Hypersil ODS2, 5 μm, 4.6 mm×250 mm), with remaining compounds incubated in the same conditions, without acrolein, as control. Mobile phases were composed of water (mobile phase A) and methanol (mobile phase B). The flow rate was 0.8 mL/min, and the injection volume was 20 μL. The linear gradient for the detection of the remaining phenolic compounds was 0–40 min, 30%–70% B; 40–50 min, 70%–90% B; 50–60 min, 90% B. Detection wavelength was set at 256 nm.
Fig. 1.
Phenolic compounds used for acrolein-scavenging activity selection
2.3. Monitoring the reaction between acrolein and ferulic acid by HPLC
Ferulic acid and acrolein were dissolved in DMSO/phosphate buffer (pH 7.4) (1:99, v/v) to a concentration of 20 mmol/L. Ferulic acid solution (0.5 mL) and acrolein solution (0.5 mL) were incubated at 37 °C for 0, 0.5, 1, 2, 4, 8, 12, and 24 h, and phosphate buffer was incubated with acrolein solution as control. Remaining acrolein was derivatized by 2,4-DNPH. 2,4-DNPH crystal (130 mg) and 1 mol/L HCl (6 mL) were dissolved in acetonitrile and diluted to 100 mL. Reaction mixture (100 μL) was incubated with 600 μL 2,4-DNPH solution for 1 min. DNPH derivatives of adducts were detected by an HPLC system in the same way as described above. Mobile phases were composed of 0.1% formic acid in water (mobile phase A) and acetonitrile (mobile phase B). The flow rate was 0.8 mL/min, and the injection volume was 20 μL. The linear gradient for the detection of the derivatives was 0–10.0 min, 35%–70% B; 10.0–17.5 min, 70%–85% B; 17.5–20.0 min, 85% B. Detection wavelength was set at 365 nm.
2.4. Detection of levels of GSH incubated with acrolein and ferulic acid in vitro
GSH and ferulic acid were dissolved in the DMSO/phosphate buffer (pH 7.4) (1:99, v/v) to a concentration of 30 μmol/L, and acrolein was dissolved in DMSO/phosphate buffer (pH 7.4) (1:99, v/v) to a concentration of 10 μmol/L. GSH solution (0.3 mL) acrolein solution (0.3 mL), and ferulic acid solution or phosphate buffer (0.3 mL) were incubated at 37 °C for 0, 30, and 90 min. GSH solution (0.3 mL) was incubated with phosphate buffer (0.6 mL) as control. The mixture was used to determine levels of GSH by GSH/oxidized GSH (GSSG) Assay Kit (Beyotime, China) (Vandeputte et al., 1994; Martirosyan et al., 2006).
2.5. Structural elucidation of reaction products between acrolein and ferulic acid
Ferulic acid (5 mmol) was dissolved in 100 mL DMSO/phosphate buffer (pH 7.4) (1:99, v/v) and incubated at 37 °C for 24 h. Acrolein (5 mmol) was added to the solution each time for three times. The reaction mixture was extracted with 100 mL of ethyl acetate three times. The organic phase was evaporated and isolated by silica gel column with petroleum ether and ethyl acetate as the mobile phase.
Product 21 (Fig. S1) was identified by gas chromatography-mass spectrometry (GC-MS; Thermo Fisher Scientific, HP-5 capillary column). A gradient temperature program (150–300 °C, 2 min initial hold, 15 °C/min ramp rate, and hold until the end of run) was used. The ionization potential was 70 eV, and the temperature of the ion source was 200 °C.
Nuclear magnetic resonance (NMR) analyses of product 21 (Fig. S2) and product 22 (Fig. S3) were carried out on a Bruker 400 MHz instrument with CDCl3 as solvent and tetra-methylsilane (TMS) as internal standard. The spectra are included in Fig. S3.
2.6. Study of structure–reactivity relationship of reaction between ferulic acid and acrolein
Cinnamic acid derivatives shown in Fig. 2 and acrolein were dissolved in DMSO/phosphate buffer (pH 7.4) (1:99, v/v) to a concentration of 20 mmol/L. Cinnamic acid (0.5 mL) derivative solutions and acrolein solution (0.5 mL) were incubated at 37 °C for 24 h. Reaction mixtures were extracted with equal ethyl acetate for three times. Products were identified by GC-MS as described above.
Fig. 2.
Cinnamic acid derivatives used for studying structure–reactivity relationship
2.7. Statistical analysis
Samples were analyzed in triplicate. Triplicate analyses were expressed as the mean±standard deviation (SD). Independent-samples t-test was used to determine statistical significance by SPSS 22.0 software (SPSS Inc., IBM, Chicago, IL, USA). Differences were considered to be significant at P<0.05.
3. Results and discussion
3.1. Acrolein-scavenging activity of phenolic compounds
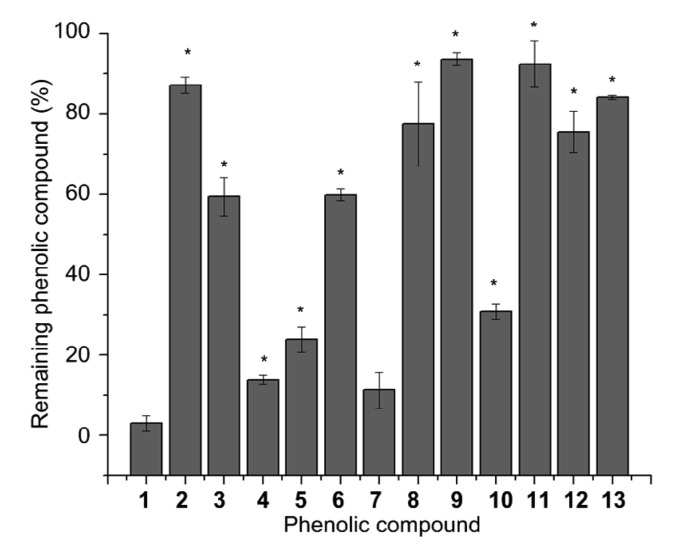
At the initial step, scavenging activity to acrolein of 13 phenolic compounds was detected with the remaining phenolic compounds after being incubated with acrolein. The results are summarized in Fig. 3, which suggests that ferulic acid (compound 1) is a probable highly-efficient acrolein-scavenging reagent. The average remaining ferulic acid was (3.04±1.89)%. Some other phenolic compounds such as p-benzenediol (compound 8) showed no significant quenching activity. In addition, phenolic compounds with similar structural characteristics to cinnamic acid, such as compounds 1, 4, and 10, left less residue after incubating with acrolein. The other compound, with a lower residue amount of resveratrol (compound 5), also bears a styrene moiety. This may suggest that this kind of phenolic compound has higher reactivity with acrolein. The reaction process of acrolein and ferulic acid was then monitored and the results are summarized in Fig. 4. The level of remaining acrolein decreased quickly and then smoothly in 24 h. Compared with resveratrol, which was reported to have significant acrolein-scavenging activity (Wang et al., 2015), the acrolein-scavenging activity of ferulic acid was at the same level, with higher efficiency in the initial hours but lower efficiency in the following period. This result suggested that acrolein can be effectively scavenged by ferulic acid under physiological conditions.
Fig. 3.
Remaining levels of phenolic compounds (1–13) after incubated with acrolein at 37 °C for 24 h
Data are expressed as mean±standard deviation (SD), n=3. * P<0.05, vs. ferulic acid (compound 1)
Fig. 4.
Comparison between variational concentrations of remaining acrolein during incubation with ferulic acid (FA) and resveratrol (Res) in 24 h
Data are expressed as mean±standard deviation (SD), n=3. * P<0.05, FA vs. Res
3.2. Protective function of ferulic acid to GSH against acrolein in vitro
GSH plays an important role in the endogenous antioxidation process in cells. In order to clarify expression of ferulic acid in simulative cellular environment against acrolein, levels of GSH incubated with acrolein with or without ferulic acid were detected. Results showed that ferulic acid could significantly inhibit decreasing of levels of GSH when incubated with acrolein under simulative physiological conditions in 90 min (Fig. 5). This result supported the conclusion of scavenging activity against acrolein in simulative cellular environment of ferulic acid.
Fig. 5.
Levels of glutathione (GSH; 30 μmol/L) incubated with acrolein (10 μmol/L) with or without ferulic acid (30 μmol/L) for 0, 30, and 90 min
“−” means that acrolein (ACR) or ferulic acid (FA) was absent, and “+” means that it was present. Data are expressed as mean±standard deviation (SD), n=3. * P<0.05, vs. 0 min
3.3. Identification of reaction products of acrolein and ferulic acid
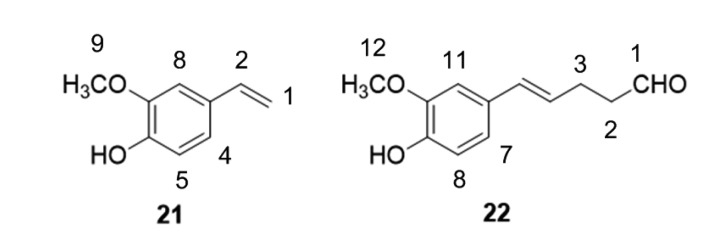
Then the reaction between ferulic acid and acrolein was enlarged to 5 mmol, aiming to get a deeper understanding of the scavenging process. Two ferulic acid–acrolein reaction products were isolated by silica gel columns and identified by GC-MS and NMR analyses, respectively. The GC-MS result of product 21 was the same as 2-methoxy-4-vinylphenol standard and indicated that product 21 was the decarboxylation product 2-methoxy-4-vinylphenol. The structure of product 22 was identified as shown in Fig. 6 by the analysis of NMR data. NMR data of products 21 and 22 are shown below.
Fig. 6.
Structures of reaction products of acrolein and ferulic acid
3.3.1 Product 21
1H NMR (400 MHz, DMSO): 9.09 (1H, C-9, s), 7.04 (1H, C-8, d, J=2.0 Hz), 6.85 (1H, C-4, dd, J=2.0 and 8.0 Hz), 6.73 (1H, C-5, d, J=8.0 Hz), 6.60 (1H, C-2, m), 5.63 (1H, C-1, d, J=19.6 Hz), 5.06 (1H, C-1, d, J=10.8 Hz), 3.79 (3H, C-9, s).
3.3.2 Product 22
1H NMR (400 MHz, CDCl3): 9.81 (1H, C-1, s), 6.81–6.85 (3H, C-7, C-8, C-11, each s), 6.34 (1H, C-5, d, J=16.0 Hz), 6.00 (1H, C-4, dt, J=6.8 and 16.0 Hz), 5.67 (1H, hydrogen, s), 3.89 (3H, C-12, s), 2.60 (2H, C-2, t, J=6.8 Hz), 2.50 (2H, C-2, m). 13C NMR (100 MHz, chloroform-d1): 202.10 (C-1), 146.62 (C-10), 145.17 (C-9), 130.91 (C-5), 129.85 (C-6), 125.84 (C-4), 119.68 (C-7), 114.41 (C-8), 108.01 (C-11), 55.88 (C-12), 43.47 (C-2), 25.47 (C-3). High-resolution mass spectrometry (HRMS; electrospray ionization (ESI), m/z) result was calculated for C12H13O3 (M-H)− 205.0870, found 205.0880.
The structure of product 22 is unique compared with the reaction products of other phenolic compounds and acrolein. For example, in the reaction between resveratrol and acrolein, the adduction site of acrolein is near to phenolic hydroxyl at the benzene ring (Wang et al., 2015), while the adduction site of acrolein is at terminal alkene in the reaction between ferulic acid and acrolein. Most reactions between phenolic acids and RCS are the same as that of resveratrol (Wang et al., 2015; Zamora et al., 2016).
3.4. Mechanism study of reaction of ferulic acid and acrolein
In order to investigate mechanism of the reaction of ferulic acid and acrolein, several reactions among these reactants and possible intermediate products were studied (Fig. 7). Products were identified by GC-MS. When product 21 was incubated with acrolein, almost 11% product 21 transformed to product 22, while product 22 cannot transform to product 21 no matter whether there was acrolein in the solutions. In addition, when ferulic acid was incubated without acrolein, a little part of ferulic acid transformed into product 21 but most remained, while there was no ferulic acid remaining when incubated with acrolein. The results indicated that ferulic acid was assumed to have lost a carboxyl group and formed a styrene derivatize through both ways with or without impact by acrolein first, and then acrolein underwent Michael addition with the styrene derivatize (Kovalev et al., 1990; Jung and Kim, 2002). When acrolein was not added to the solution, only a trace amount of decarboxylation product was detected. A possible mechanism of the reaction between ferulic acid and acrolein is shown in Fig. 8.
Fig. 7.
Test of whether products 21 and 22 were interconvertible with or without acrolein (ACR)
Fig. 8.
Possible mechanism of reaction between ferulic acid and acrolein (ACR)
3.5. Study of structure–reactivity relationship of reaction between ferulic acid and acrolein
Various cinnamic acid derivatives sharing similar structural characteristics with ferulic acid were tested for reaction products after being incubated with acrolein. Results are shown in Table 1. Several facts are summarized: (1) ferulic acid and sinapic acid, which have methoxyl group in C-3 and hydroxyl group in C-4 of the benzene ring, showed high reactivity in this reaction; (2) cinnamic acid derivatives with hydroxyl group in C-4 but not methoxyl group in C-3 such as caffeic acid and 4-hydroxycinnamic acid could lose a carboxyl group while they could not form an adduct with acrolein; (3) cinnamic acid derivatives without hydroxyl group in C-4 such as cinnamic acid showed no reaction with acrolein; (4) esters of cinnamic acid derivatives showed no reaction with acrolein. The structures of adducts are shown in Fig. S4. These facts indicate that: (1) hydroxyl group in C-4 was necessary for generation of decarboxylation product in this reaction; (2) methoxyl group in C-3 could increase reactivity of cinnamic acid derivatives and enhance the yield of phenolic compounds-acrolein adducts.
Table 1.
Reaction product yield of cinnamic acid derivatives with acrolein (yields obtained from gas-phase chromatography)
| Compound | Decarboxylation product (%) | Adduct (%) |
| 1 | 10.2±0.5 | 34.8±0.9 |
| 4 | 97.1±1.2 | 1.5±0.2 |
| 11 | 45.8±3.3 | 42.8±1.1 |
| 14 | 22.5±1.5 | 0 |
| 15–20 | 0 | 0 |
Data are expressed as mean±standard deviation (SD), n=3
4. Conclusions
As a widely distributed phenolic acid, ferulic acid showed significant activity in scavenging the toxic molecule acrolein under physiological conditions. Also it was reported that ferulic acid can protect cells against neurotoxicity (Mhillaj et al., 2018). These results demonstrated that plant food including some Chinese herbal medicine which contains high levels of ferulic acid is beneficial to human health especially for those who have a dietary habit with fried food and cigarettes. However, there is a serious limitation of ferulic acid in use in the therapeutic setting because of its low bioavailability (Barone et al., 2009). According to a report by Trombino et al. (2013), it is possible to solve this problem through preparing a novel liposoluble formulation of ferulic acid. The hydroxyl group in C-4 of ferulic acid is necessary for decarboxylation process of this reaction, and the methoxyl group in C-3 can accelerate the adduction reaction between product 21 and acrolein.
List of electronic supplementary materials
GC-MS of product 21
1H NMR results of product 21
NMR results of product 22
Structures of compounds 23–25
Diseases proved to be related to acrolein
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 21327010 and 21372199)
Contributors: Zhi-hao TAO performed the experimental research and data analysis, wrote and edited the manuscript. Chang LI contributed to the study design, data analysis, writing and editing of the manuscript. Xiao-fei XU contributed to the study design and data analysis of the manuscript. Yuan-jiang PAN contributed to the study design, writing and editing of the manuscript. All authors read and approved the final manuscript and, therefore, had full access to all the data in the study and take responsibility for the integrity and security of the data.
Electronic supplementary materials: The online version of this article (https://doi.org/10.1631/jzus.B1900211) contains supplementary materials, which are available to authorized users
Compliance with ethics guidelines: Zhi-hao TAO, Chang LI, Xiao-fei XU, and Yuan-jiang PAN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Ålin P, Danielson UH, Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- 2.Amro B, Aburjai T, Al-Khalil S. Antioxidative and radical scavenging effects of olive cake extract. Fitoterapia. 2002;73(6):456–461. doi: 10.1016/s0367-326x(02)00173-9. [DOI] [PubMed] [Google Scholar]
- 3.Balasubashini MS, Rukkumani R, Menon VP. Protective effects of ferulic acid on hyperlipidemic diabetic rats. Acta Diabetol. 2003;40(3):118–122. doi: 10.1007/s00592-003-0099-6. [DOI] [PubMed] [Google Scholar]
- 4.Barone E, Calabrese V, Mancuso C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology. 2009;10(2):97–108. doi: 10.1007/s10522-008-9160-8. [DOI] [PubMed] [Google Scholar]
- 5.Baskaran X, Vigila AVG, Zhang S, et al. A review of the use of pteridophytes for treating human ailments. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2018;19(2):85–119. doi: 10.1631/jzus.B1600344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabrese V, Calafato S, Puleo E, et al. Redox regulation of cellular stress response by ferulic acid ethyl ester in human dermal fibroblasts: role of vitagenes. Clin Dermatol. 2008;26(4):358–363. doi: 10.1016/j.clindermatol.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Cassano R, Trombino S, Cilea A, et al. L-lysine pro-prodrug containing trans-ferulic acid for 5-amino salicylic acid colon delivery: synthesis, characterization and in vitro antioxidant activity evaluation. Chem Pharm Bull (Tokyo) 2010;58(1):103–105. doi: 10.1248/cpb.58.103. [DOI] [PubMed] [Google Scholar]
- 8.Catino S, Paciello F, Miceli F, et al. Ferulic acid regulates the Nrf2/heme oxygenase-1 system and counteracts trimethyltin-induced neuronal damage in the human neuroblastoma cell line SH-SY5Y. Front Pharmacol, 6:305. 2016 doi: 10.3389/fphar.2015.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox PJ. Cyclophosphamide cystitis–Identification of acrolein as the causative agent. Biochem Pharmacol. 1979;28(13):2045–2049. doi: 10.1016/0006-2952(79)90222-3. [DOI] [PubMed] [Google Scholar]
- 10.DeJarnett N, Conklin DJ, Riggs DW, et al. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3(4):e000934. doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doggui S, Belkacemi A, Paka GD, et al. Curcumin protects neuronal-like cells against acrolein by restoring Akt and redox signaling pathways. Mol Nutr Food Res. 2013;57(9):1660–1670. doi: 10.1002/mnfr.201300130. [DOI] [PubMed] [Google Scholar]
- 12.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 13.Fetoni AR, Mancuso C, Eramo SLM, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169(4):1575–1588. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 14.He S, Jiang LY, Wu B, et al. Pallidol, a resveratrol dimer from red wine, is a selective singlet oxygen quencher. Biochem Biophys Res Commun. 2009;379(2):283–287. doi: 10.1016/j.bbrc.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Huang RT, Huang Q, Wu GL, et al. Evaluation of the antioxidant property and effects in Caenorhabditis elegans of Xiangxi flavor vinegar, a Hunan local traditional vinegar. J Zhejiang Univ-Sci B (Biomed & Biotechnol) 2017;18(4):324–333. doi: 10.1631/jzus.B1600088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi G, Perluigi M, Sultana R, et al. In vivo protection of synaptosomes by ferulic acid ethyl ester (FAEE) from oxidative stress mediated by 2,2-azobis(2-amidino-propane)dihydrochloride (AAPH) or Fe2+/H2O2: insight into mechanisms of neuroprotection and relevance to oxidative stress-related neurodegenerative disorders. Neurochem Int. 2006;48(4):318–327. doi: 10.1016/j.neuint.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Jung SH, Kim JH. Efficient method for β-conjugate addition of α,β-unsaturated lactones and esters. Bull Korean Chem Soc. 2002;23(3):365–366. doi: 10.5012/bkcs.2002.23.3.365. [DOI] [Google Scholar]
- 18.Kovalev IP, Kolmogorov YN, Yinogradov MG, et al. The linear dimerization of vinyl ketones catalyzed by the [RhCl(C2H4)2]2-GeCl2 system. Russ Chem Bull. 1990;39(5):1070–1071. doi: 10.1007/bf00961723. [DOI] [Google Scholar]
- 19.Kumar N, Pruthi V. Potential applications of ferulic acid from natural sources. Biotechnol Rep. 2014;4:86–93. doi: 10.1016/j.btre.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Xu XF, Tao ZH, et al. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015;173:218–223. doi: 10.1016/j.foodchem.2014.09.165. [DOI] [PubMed] [Google Scholar]
- 21.Liu F, Li XL, Lin T, et al. The cyclophosphamide metabolite, acrolein, induces cytoskeletal changes and oxidative stress in Sertoli cells. Mol Biol Rep. 2012;39(1):493–500. doi: 10.1007/s11033-011-0763-9. [DOI] [PubMed] [Google Scholar]
- 22.Lovell MA, Xie CS, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22(2):187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Shi RY. Acrolein induces oxidative stress in brain mitochondria. Neurochem Int. 2005;46(3):243–252. doi: 10.1016/j.neuint.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Mancuso C, Santangelo R. Ferulic acid: pharmacological and toxicological aspects. Food Chem Toxicol. 2014;65:185–195. doi: 10.1016/j.fct.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 25.Mano J. Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol Biochem. 2012;59:90–97. doi: 10.1016/j.plaphy.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Mano J, Miyatake F, Hiraoka E, et al. Evaluation of the toxicity of stress-related aldehydes to photosynthesis in chloroplasts. Planta. 2009;230(4):639–648. doi: 10.1007/s00425-009-0964-9. [DOI] [PubMed] [Google Scholar]
- 27.Martirosyan A, Leonard S, Shi XL, et al. Actions of a histone deacetylase inhibitor NSC3852 (5-nitroso-8-quinolinol) link reactive oxygen species to cell differentiation and apoptosis in MCF-7 human mammary tumor cells. J Pharmacol Exp Ther. 2006;317(2):546–552. doi: 10.1124/jpet.105.096891. [DOI] [PubMed] [Google Scholar]
- 28.Mhillaj E, Catino S, Miceli FM, et al. Ferulic acid improves cognitive skills through the activation of the heme oxygenase system in the rat. Mol Neurobiol. 2018;55(2):905–916. doi: 10.1007/s12035-017-0381-1. [DOI] [PubMed] [Google Scholar]
- 29.Picone P, Bondi ML, Montana G, et al. Ferulic acid inhibits oxidative stress and cell death induced by Ab oligomers: improved delivery by solid lipid nanoparticles. Free Radic Res. 2009;43(11):1133–1145. doi: 10.1080/10715760903214454. [DOI] [PubMed] [Google Scholar]
- 30.Rom O, Korach-Rechtman H, Hayek T, et al. Acrolein increases macrophage atherogenicity in association with gut microbiota remodeling in atherosclerotic mice: protective role for the polyphenol-rich pomegranate juice. Arch Toxicol. 2017;91(4):1709–1725. doi: 10.1007/s00204-016-1859-8. [DOI] [PubMed] [Google Scholar]
- 31.Shamoto-Nagai M, Maruyama W, Hashizume Y, et al. In parkinsonian substantia nigra, α-synuclein is modified by acrolein, a lipid-peroxidation product, and accumulates in the dopamine neurons with inhibition of proteasome activity. J Neural Transm (Vienna) 2007;114(12):1559–1567. doi: 10.1007/s00702-007-0789-2. [DOI] [PubMed] [Google Scholar]
- 32.Sheu SJ, Ho YS, Chen YP, et al. Analysis and processing of Chinese herbal drugs; VI. The study of Angelicae radix. Planta Med. 1987;53(4):377–378. doi: 10.1055/s-2006-962742. [DOI] [PubMed] [Google Scholar]
- 33.Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40(2):92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med. 1999;38(4):309–314. doi: 10.2169/internalmedicine.38.309. [DOI] [PubMed] [Google Scholar]
- 36.Trombino S, Cassano R, Ferrarelli T, et al. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf B Biointerfaces. 2013;109:273–279. doi: 10.1016/j.colsurfb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Vandeputte C, Guizon I, Genestie-Denis I, et al. A microtiter plate assay for total glutathione and glutathione disulfide contents in cultured/isolated cells: performance study of a new miniaturized protocol. Cell Biol Toxicol. 1994;10(5-6):415–421. doi: 10.1007/bf00755791. [DOI] [PubMed] [Google Scholar]
- 38.Wang WX, Qi YJ, Rocca JR, et al. Scavenging of toxic acrolein by resveratrol and hesperetin and identification of adducts. J Agric Food Chem. 2015;63(43):9488–9495. doi: 10.1021/acs.jafc.5b03949. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Cui P. Reactive carbonyl species derived from omega-3 and omega-6 fatty acids. J Agric Food Chem. 2015;63(28):6293–6296. doi: 10.1021/acs.jafc.5b02376. [DOI] [PubMed] [Google Scholar]
- 40.Yaylayan VA, Keyhani A. Origin of carbohydrate degradation products in L-alanine/D-[13C]glucose model systems. J Agric Food Chem. 2000;48(6):2415–2419. doi: 10.1021/jf000004n. [DOI] [PubMed] [Google Scholar]
- 41.Zamora R, Aguilar I, Granvogl M, et al. Toxicologically relevant aldehydes produced during the frying process are trapped by food phenolics. J Agric Food Chem. 2016;64(27):5583–5589. doi: 10.1021/acs.jafc.6b02165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GC-MS of product 21
1H NMR results of product 21
NMR results of product 22
Structures of compounds 23–25
Diseases proved to be related to acrolein