Abstract
Noncoding RNAs (ncRNAs) have played a critical role in cellular biological functions. Recently, some peptides or proteins originating from annotated ncRNAs were identified in organism development and various diseases. Here, we briefly review several novel peptides translated by annotated ncRNAs and related key functions. In addition, we summarize the potential mechanism of bifunctional ncRNAs and propose a specific “switch” triggering the transformation from the noncoding to the coding state under certain stimuli or cellular stress. The coding properties of ncRNAs and their peptide products may provide a novel horizon in proteomic research and can be regarded as a potential therapeutic target for the treatment of various diseases.
Keywords: Non-coding RNA, Biological function, Micropeptides, Cellular stress
Noncoding RNAs (ncRNAs) have played a critical role in cellular biological functions. Recently, some peptides or proteins originating from annotated ncRNAs were identified in organism development and various diseases. Here, we briefly review several novel peptides translated by annotated ncRNAs and related key functions. In addition, we summarize the potential mechanism of bifunctional ncRNAs and propose a specific “switch” triggering the transformation from the noncoding to the coding state under certain stimuli or cellular stress. The coding properties of ncRNAs and their peptide products may provide a novel horizon in proteomic research and can be regarded as a potential therapeutic target for the treatment of various diseases.
In eukaryotic genomes, the majority of DNA sequences without coding capability were transcribed into a large number of ncRNAs. These include housekeeping RNAs, such as transfer RNAs (tRNAs), ribosomal RNAs (rRNAs) and small nuclear RNAs (snRNAs), and non-housekeeping RNAs, such as microRNAs (miRNAs), long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) (Carninci et al., 2005). In the human genome, Hon et al. (2017) systematically assessed the diversity of lncRNAs using Functional Annotation of the Mammalian Genome 5 (FANTOM5) cap analysis and obtained a better understanding of the function of nearly 20 000 human lncRNAs through online databases. This can help researchers conduct functional studies of lncRNA candidates. Researchers believed that ncRNAs are “dark matters” and play an essential role in biological development and pathological processes, and many excellent reviews have summarized their cellular functions (Cech and Steitz, 2014; Dey et al., 2014; Xu et al., 2017).
Translation of messenger RNA (mRNA) is an essential process in biological regulation. Canonically, proteins were translated from eukaryotic mRNAs containing long open reading frame (ORF) by recognizing the m7GpppX cap structure at the 5' end of the transcript (Sonenberg and Hinnebusch, 2009). In addition, a ribosome can also initiate the translation process in a cap-independent manner, termed the internal ribosome entry site (IRES). IRES is an RNA regulatory element and is critical for the production of many human and viral proteins (Lozano et al., 2015). In many positive-strand RNA viruses or positive-sense single-stranded RNA ([+]ssRNA) viruses, the transcripts are naturally uncapped and rely heavily on the IRES-dependent manner for protein synthesis (den Boon et al., 2010). Because of the little extensive ORF which is fewer than 100 codons, ncRNAs display limited translation property (Eddy, 1999; Costa, 2005). However, with the advances of large-scale genome analysis, some realized that the “noncoding” is an unreliable annotation of these small ORFs (Aspden et al., 2014; Mumtaz and Couso, 2015). A large portion of identified ncRNAs with small ORFs have the capacity of coding peptides and play specific roles in Escherichia coli (Hemm et al., 2008), plants (Röhrig et al., 2002; Hanada et al., 2013; Okamoto et al., 2014), Drosophila (Kondo et al., 2007, 2010; Magny et al., 2013), zebrafish (Pauli et al., 2014), mice (Crappé et al., 2013; Anderson et al., 2015; Nelson et al., 2016), and the human (Slavoff et al., 2013; Anderson et al., 2015; Nelson et al., 2016). These studies indicated that many ncRNAs have coding features (Chew et al., 2013).
Recent advances in research on ribosome function revealed that translation can initiate at codons other than AUG (Kearse and Wilusz, 2017). For example, death-associated protein 5 (DAP5) is a member of the eukaryotic translation initiation factor 4G (eIF4G) family of translation initiation factors and plays an important role in IRES-mediated translation, which is only encoded from the initiation codon GUG in various species (Chang and Wang, 2004; Takahashi et al., 2005; Liberman et al., 2015). Some repeat sequences within RNA regions are typically considered noncoding. These can also initiate non-AUG translation and produce toxic proteins which are associated with neurodegenerative diseases. CAG and CUG repeat RNA translation is associated with spinocerebellar ataxia type 8 (SCA8) and myotonic dystrophy type 1 (DM1) (Zu et al., 2011). GGGGCC/CCCCGG repeats in C9orf72 (C9ALS/FTD) gene occur in amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (Ash et al., 2013; Mori et al., 2013). Previous studies suggested that these small ORFs in ncRNAs may translate in a conventional way from an AUG codon to a stop codon (Stammers et al., 2015; Magny et al., 2013). The non-AUG translation initiation of ncRNAs is rarely reported and calls for further research.
In cellular development, ncRNAs play critical roles in biological regulation such as cell proliferation, organ development, and immune response (Xie and Liu, 2015; Gore-Panter et al., 2016; Li et al., 2016; Liu and Liu, 2016). Some ncRNAs contain coding and noncoding properties and act as bifunctional regulators. The specific biological features of ncRNAs with coding potential are largely unknown. The transformation mechanisms between noncoding and coding conditions of a transcript are unclear. Here, we briefly review several novel peptides translated by ncRNAs and summarize the possible mechanisms of the transformation between the coding and the noncoding states.
Researchers conventionally believed that transcriptions with small ORFs are hardly translated and the polypeptides are unstable in cytoplasm, so these transcriptions are classified as ncRNAs (Basrai et al., 1997). Recently, the annotation of “noncoding” looks unreliable and the function of the small ORF is hindered by their sequence length (Aspden et al., 2014). The new experimental technique of ribosome profiling has found that there are a large number of ribosome-protected fragments (RPFs) associated with many previously annotated ncRNAs (Mumtaz and Couso, 2015; Calviello et al., 2016). These ncRNAs share similar characters with 5' leaders of coding mRNAs (Vandin et al., 2012) and contain small ORFs (Chu et al., 2015; Calviello et al., 2016). Both observations suggest the encoding potential of ncRNAs. Several research groups have identified some new peptides or proteins translated by specific ncRNAs and function in various organisms (Hemm et al., 2008; Pauli et al., 2014; Anderson et al., 2015; Nelson et al., 2016; Pueyo et al., 2016). Here, we have reviewed several novel identified products translated by ncRNAs and summarized them briefly in Table 1.
Table 1.
Several micropeptides encoded by noncoding RNAs (ncRNAs) and their corresponding functions
| Peptide | Size (kDa) | Species | Function | Reference |
| MLN | ~5 | Mouse | Suppressing SERCA activity in skeletal muscle | Anderson et al., 2015 |
| SCL | ~3 | Drosophila | Suppressing SERCA activity in cardiac muscle | Magny et al., 2013 |
| DWORF | ~4 | Mouse | Enhancing SERCA activity in skeletal muscle | Nelson et al., 2016 |
| SPAR | ~10 | Mouse | Suppressing mTORC1 activation in skeletal muscle | Matsumoto et al., 2017 |
| Myomixer | ~9 | Mouse | Promoting myoblast fusion during muscle development | Bi et al., 2017 |
| HOXB-AS3 | ~6 | Human | Suppressing colon cancer growth | Huang et al., 2017 |
| STORM | ~6 | Human | Reducing the interaction of SRP19 and 7SL RNA | Min et al., 2017 |
| Circ-ZNF609 encoded | 30–40 | Mouse, human | Unknown | Legnini et al., 2017 |
| CircMbl3 encoded | 37.04 | Drosophila | Unknown | Pamudurti et al., 2017 |
MLN: myoregulin; SCL: sarcolamban; DWORF: dwarf open reading frame; SPAR: small regulatory polypeptide of amino acid response; HOXB-AS3: HOXB cluster antisense RNA 3; STORM: stress-and tumor necrosis factor-α (TNF-α)-activated open reading frame (ORF) micropeptide; SERCA: sarcoplasmic reticulum Ca2+-ATPase; mTORC1: mechanistic target of rapamycin complex 1; SRP19: signal recognition particle 19
In normal muscle tissue, sarcoplasmic reticulum Ca2+-ATPase (SERCA) regulates Ca2+ uptake into the sarcoplasmic reticulum (SR) and takes responsibility for subsequent contraction-relaxation cycles in cardiac and skeletal muscle. Phospholamban (PLN) and sarcolipin (SLN) regulate SERCA activity and modulate Ca2+ levels in SR (Stammers et al., 2015). Anderson et al. (2015) discovered a new micropeptide, named myoregulin (MLN), that directly interacts with SERCA and restrains Ca2+ into SR. MLN is coded by a specific RNA which previously was annotated as a putative lncRNA in skeletal muscle and robustly expressed in all adult skeletal muscle. Researchers observed that SR Ca2+ levels are obviously enhanced in MLN knockout mice, suggesting that MLN behaves similar fashion to SLN and PLN (Anderson et al., 2015). Calcium ATPase at 60A (Ca-P60A) is the invertebrate homolog of SERCA in Drosophila cardiac muscle and is modulated by micropeptide sarcolamban (SCL) which is encoded by a functional small ORF of almost 30 amino acids (aa) in a putative ncRNA (Magny et al., 2013). In addition, SCL, SLN, and PLN are putative homologs in both structure and function, regulating Ca2+ uptake at the SR by interacting with SERCA or Ca-P60A. Nelson et al. (2016) recently identified a peptide, named dwarf open reading frame (DWORF), which acts in an opposite way to SERCA inhibitors. DWORF promotes SR Ca2+ uptake by enhancing SERCA activity. In DWORF overexpression mice, peak Ca2+ transient amplitude and SR Ca2+ load are significantly increased while decreasing Ca2+ decay time in cardiomyocytes during each contraction-relaxation cycle of cardiac muscle. Recently, the discovery of a small regulatory polypeptide of amino acid response (SPAR), which is encoded by the lncRNA LINC00961, showed another surprising function of polypeptide in muscle tissue. SPAR specifically suppresses mechanistic target of rapamycin complex 1 (mTORC1) activation by interacting with the lysosomal vacuolar H+-ATPase (V-ATPase). Down-regulating SPAR expression, using clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 engineering, increases mTORC1 activity and promotes muscle regeneration in the mice model (Matsumoto et al., 2017). In addition, a muscle-specific peptide, called Myomixer, is translated by lncRNA (LOC101929726) and plays an essential role for skeletal muscle formation during development. Myomixer expresses in the plasma membrane where it promotes fibroblast-fibroblast fusion and fibroblast-myoblast fusion by interacting with Myomaker, a fusogenic membrane protein (Bi et al., 2017).
In colon cancer, a conserved 53-aa peptide was translated by lncRNA HOXB cluster antisense RNA 3 (HOXB-AS3) (Huang et al., 2017). Compared with normal patients, HOXB-AS3 peptide was down-regulated in colon cancer patients and indicated poor prognoses. Research further revealed that low expression of HOXB-AS3 peptide impacts colon cancer cell metabolism by regulating pyruvate kinase M (PKM) alternative splicing pattern (Huang et al., 2017). Eukaryotic translation initiation factor 4E (eIF4E) is one of one of the translation initiation factors and can be phosphorylated by mammalian Ste20-like kinase 1 (MST1). Phosphorylated eIF4E weakly interacts with 5' cap to inhibit translation of target mRNAs such as eukaryotic translation initiation factor 2 α (eIF2-α), eukaryotic translation elongation factor 2 (eEF2), and chaperonin containing t-complex polypeptide 1 (TCP1) subunit 2 (CCT2) mRNAs, but activates translation of linc00689 to produce a micropeptide named stress-and tumor necrosis factor-α (TNF-α)-activated ORF micropeptide (STORM). STORM shows high similarity with signal recognition particle 19 (SRP19) and acts as a competing peptide to inhibit SRP19 interaction with 7SL RNA (Min et al., 2017). It was recently found that circRNAs are able to encode peptide in a cap-independent way with an IRES. Circular (circ)-ZNF609 is a circRNA and plays a crucial role during myogenesis (Legnini et al., 2017). Bioinformatic analysis of the circ-ZNF609 sequence showed the existence of an ORF and two proteins originating from the translation of circ-ZNF609. Heat shock significantly increased the translation activation of circ-ZNF609 (Legnini et al., 2017). In Drosophila, Pamudurti et al. (2017) uncovered that circRNA Mbl1 is generated from the muscleblind locus and can encode a protein in cap-independent way. Meanwhile, the translation efficiency of circRNA Mbl1 is affected by starvation and a transcription factor, forkhead box O (FOXO) (Pamudurti et al., 2017). These findings indicated a possible coding mechanism of ncRNAs responding to cellular stress or stimulation.
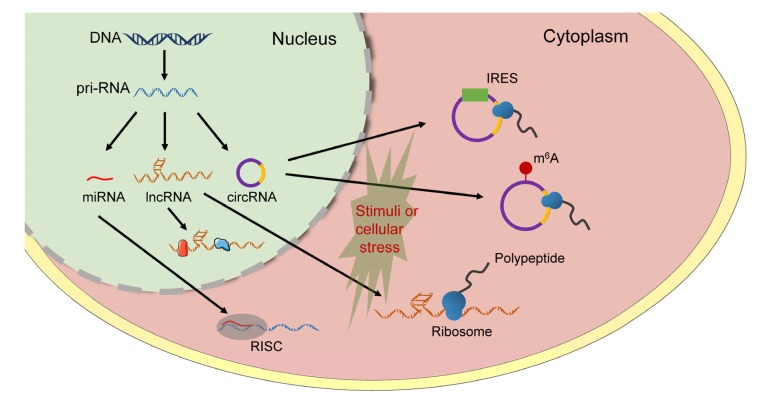
Bifunctional ncRNAs containing coding and noncoding properties exist in diverse genomes and transcriptomes. Newly developed gene scanning techniques, such as ribosome profiling and RNA binding protein immunoprecipitation (RIP)-sequence, have revealed the abundant existence of ncRNAs containing small ORF and associated with ribosome possessing coding. These had been neglected previously. However, when does this form of translation driven by ncRNAs occur? Are there any existing associations between encoded peptides or proteins and cellular circumstances? If the peptides or proteins were translated after specific cellular stress or stimulation, how are these cases regulated? Are these encoded peptides or proteins driven from canonical cap-dependent or cap-independent translation? All these remain largely unanswered questions and require further exploration. Here, we propose a hypothesis that, in biological and pathological processes, there might exist a transformation mechanism between the coding and noncoding properties of ncRNAs. Generally, ncRNAs regulate the expression of other genes in post-transcriptional modification. In certain circumstances, it may trigger this “switch” by modifying ncRNAs sequences to activate the translation activity of ncRNAs (Fig. 1).
Fig. 1.
Schematic showing the translation activity of noncoding RNAs (ncRNAs)
In nucleus, ncRNA was transcribed from primary RNA (pri-RNA). The major function of microRNAs (miRNAs) was assembled into RNA-inducing silencing complex (RISC) to regulate the expression of target messenger RNA (mRNA). For long noncoding RNAs (lncRNAs), some of them can target proteins and display different functions in cellular development. Other lncRNAs with small open reading frame (ORF) may combine with the ribosome showing coding potential. It has been reported that internal ribosome entry site (IRES) and N6-methyladenosine (m6A) motif were essential for certain circular RNA (circRNA) translation. In some specific circumstances or stimuli, such as tumorigenesis, heat shock, and starvation, certain ncRNAs with coding potential may interact with the ribosome and encode polypeptides in the cytoplasm
IRESs are located within the 5'-untranslated regions (UTRs) mostly and have complex structures, such as stem loops and pseudoknots, mediating translation when cap-dependent translation is compromised under some conditions (Komar and Hatzoglou, 2011). CircRNA with IRES can be spliced functionally and translated into protein (Wang and Wang, 2015). Yang et al. (2017) found the protein translation of circRNA can be initiated though N6-methyladenosine (m6A) motif and catalyzed by m6A writers, methyltransferase-like 3 (METTL3) and METTL14. This indicated the general effect of m6A on circRNA translation. In addition to specific ncRNA structure, sequence modification, the phosphorylation of eIF4E (Min et al., 2017) and certain stimuli can promote the favored translation of ncRNAs, such as neoplastic environment (Huang et al., 2017), hot shock (Legnini et al., 2017), and starvation (Pamudurti et al., 2017), as summarized above.
More interestingly, the transformation from protein-coding mRNA to ncRNA also exists. Williamson et al. (2017) recently found that ultraviolet (UV) irradiation induces a switch from a long protein-coding activating signal cointegrator 1 complex subunit 3 (ASCC3) mRNA to a short alternative last exon (ALE) transcript isoform which functions as lncRNA. The UV-induced ASCC3 short isoform regulates transcription recovery and plays an antagonistic role compared with long ASCC3 isoform. The coding pattern transition of mRNA can be regulated by alternative splicing. Heterogeneous nuclear ribonucleoprotein E1 (hnRNP E1) acts as a splicing factor and directly binds to exon 12 of phosphatase 1 nuclear targeting subunit (PNUTS) pre-mRNA, and then generates a noncoding transcription of PNUTS (Grelet et al., 2017). In breast cancer, the expression of lncRNA-PNUTS is increased and regulates epithelial-mesenchymal transition (EMT) by binding miR-205 competitively (Grelet et al., 2017). Genome screening and sequence technique have uncovered a large number of diverse transcriptions arranging from animalcules to mammals. Coding and noncoding RNAs (cncRNAs) are a specific group of RNAs, possessing both the coding and noncoding properties, and this is considered as bifunctional RNAs in transcriptomes. The functions and sources of cncRNAs in cellular development have been reviewed previously (Nam et al., 2016; Sampath and Ephrussi, 2016). These findings and facts strongly indicate that there is a “coordinator” regulating the transformation between alternative roles of ncRNAs.
Tumorigenesis is a complicated process involving many steps. In this process, tumor cells are often subjected to a severe microenvironment such as hypoxia, nutrient deprivation, and other stress induced by rapid cell division and defective tumor vasculature (Leprivier et al., 2015). To manage these stresses, the canonical cap-dependent mRNA translation is depressed in order to keep an energetic balance but some proteins, such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF), translated though cap-independent means, are increased (Leprivier et al., 2015). The study of ncRNAs encoding activity in tumorigenesis may initiate a promising field and benefit clinical tumor diagnosis and therapy.
In summary, transcriptomes and genomes are far more complicated than previously thought. Novel functions and features of ncRNAs are continually identified. Among these newly identified functions and features, the protein-coding property may be a prominent but mysterious one. The functions of polypeptides encoded by some earlier annotated ncRNAs are largely unknown. In addition, the potential mechanisms and effects of bifunctional ncRNAs need further research in cellular development and disease. This may provide promising biological insights and prospective medical therapies.
Footnotes
Contributors: Gui-zhen ZHENG, Wei LI, and Zhi-yong LIU conceived the study and researched the literature. Gui-zhen ZHENG and Zhi-yong LIU wrote and revised the manuscript. All authors have read and approved the final manuscript and, therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines: Gui-zhen ZHENG, Wei LI, and Zhi-yong LIU declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Anderson DM, Anderson KM, Chang CL, et al. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell. 2015;160(4):595–606. doi: 10.1016/j.cell.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ash PEA, Bieniek KF, Gendron TF, et al. Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron. 2013;77(4):639–646. doi: 10.1016/j.neuron.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspden JL, Eyre-Walker YC, Phillips RJ, et al. Extensive translation of small open reading frames revealed by Poly-Ribo-Seq. Elife, 3:e03528. 2014 doi: 10.7554/eLife.03528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basrai MA, Hieter P, Boeke JD. Small open reading frames: beautiful needles in the haystack. Genome Res. 1997;7(8):768–771. doi: 10.1101/gr.7.8.768. [DOI] [PubMed] [Google Scholar]
- 5.Bi PP, Ramirez-Martinez A, Li H, et al. Control of muscle formation by the fusogenic micropeptide myomixer. Science. 2017;356(6335):323–327. doi: 10.1126/science.aam9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calviello L, Mukherjee N, Wyler E, et al. Detecting actively translated open reading frames in ribosome profiling data. Nat Methods. 2016;13(2):165–170. doi: 10.1038/nmeth.3688. [DOI] [PubMed] [Google Scholar]
- 7.Carninci P, Kasukawa T, Katayama S, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309(5740):1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 8.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Chang KJ, Wang CC. Translation initiation from a naturally occurring non-AUG codon in Saccharomyces cerevisiae. J Biol Chem. 2004;279(14):13778–13785. doi: 10.1074/jbc.M311269200. [DOI] [PubMed] [Google Scholar]
- 10.Chew GL, Pauli A, Rinn JL, et al. Ribosome profiling reveals resemblance between long non-coding RNAs and 5' leaders of coding RNAs. Development. 2013;140(13):2828–2834. doi: 10.1242/dev.098343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu Q, Ma J, Saghatelian A. Identification and characterization of sORF-encoded polypeptides. Crit Rev Biochem Mol Biol. 2015;50(2):134–141. doi: 10.3109/10409238.2015.1016215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa FF. Non-coding RNAs: new players in eukaryotic biology. Gene. 2005;357(2):83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 13.Crappé J, van Criekinge W, Trooskens G, et al. Combining in silico prediction and ribosome profiling in a genome-wide search for novel putatively coding sORFs. BMC Genomics, 14:648. 2013 doi: 10.1186/1471-2164-14-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Boon JA, Diaz A, Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8(1):77–85. doi: 10.1016/j.chom.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5(4):e944014. doi: 10.4161/21541272.2014.944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eddy SR. Noncoding RNA genes. Curr Opin Genet Dev. 1999;9(6):695–699. doi: 10.1016/S0959-437X(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 17.Gore-Panter SR, Hsu J, Barnard J, et al. PANCR, the PITX2 adjacent noncoding RNA, is expressed in human left atria and regulates PITX2c expression. Circ Arrhythm Electrophysiol. 2016;9(1):e003197. doi: 10.1161/circep.115.003197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grelet S, Link LA, Howley B, et al. A regulated PNUTS mRNA to lncRNA splice switch mediates EMT and tumour progression. Nat Cell Biol. 2017;19(9):1105–1115. doi: 10.1038/ncb3595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanada K, Higuchi-Takeuchi M, Okamoto M, et al. Small open reading frames associated with morphogenesis are hidden in plant genomes. Proc Natl Acad Sci USA. 2013;110(6):2395–2400. doi: 10.1073/pnas.1213958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemm MR, Paul BJ, Schneider TD, et al. Small membrane proteins found by comparative genomics and ribosome binding site models. Mol Microbiol. 2008;70(6):1487–1501. doi: 10.1111/j.1365-2958.2008.06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hon CC, Ramilowski JA, Harshbarger J, et al. An atlas of human long non-coding RNAs with accurate 5' ends. Nature. 2017;543(7644):199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang JZ, Chen M, Chen D, et al. A peptide encoded by a putative lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell. 2017;68(1):171–184e176. doi: 10.1016/j.molcel.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Kearse MG, Wilusz JE. Non-AUG translation: a new start for protein synthesis in eukaryotes. Genes Dev. 2017;31(17):1717–1731. doi: 10.1101/gad.305250.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle. 2011;10(2):229–240. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo T, Hashimoto Y, Kato K, et al. Small peptide regulators of actin-based cell morphogenesis encoded by a polycistronic mRNA. Nat Cell Biol. 2007;9(6):660–665. doi: 10.1038/ncb1595. [DOI] [PubMed] [Google Scholar]
- 26.Kondo T, Plaza S, Zanet J, et al. Small peptides switch the transcriptional activity of shavenbaby during Drosophila embryogenesis. Science. 2010;329(5989):336–339. doi: 10.1126/science.1188158. [DOI] [PubMed] [Google Scholar]
- 27.Legnini I, di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37e9. doi: 10.1016/j.molcel.2017.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leprivier G, Rotblat B, Khan D, et al. Stress-mediated translational control in cancer cells. Biochim Biophys Acta. 2015;1849(7):845–860. doi: 10.1016/j.bbagrm.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 29.Li ZG, Hao S, Yin HQ, et al. Autophagy ameliorates cognitive impairment through activation of PVT1 and apoptosis in diabetes mice. Behav Brain Res. 2016;305:265–277. doi: 10.1016/j.bbr.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Liberman N, Gandin V, Svitkin YV, et al. DAP5 associates with eIF2β and eIF4AI to promote internal ribosome entry site driven translation. Nucleic Acids Res. 2015;43(7):3764–3775. doi: 10.1093/nar/gkv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu AF, Liu SR. Noncoding RNAs in growth and death of cancer cells. In: Song EW , editor. The Long and Short Non-coding RNAs in Cancer Biology. Springer, Singapore; 2016. pp. 137–172. [DOI] [PubMed] [Google Scholar]
- 32.Lozano G, Trapote A, Ramajo J, et al. Local RNA flexibility perturbation of the IRES element induced by a novel ligand inhibits viral RNA translation. RNA Biol. 2015;12(5):555–568. doi: 10.1080/15476286.2015.1025190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magny EG, Pueyo JI, Pearl FMG, et al. Conserved regulation of cardiac calcium uptake by peptides encoded in small open reading frames. Science. 2013;341(6150):1116–1120. doi: 10.1126/science.1238802. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto A, Pasut A, Matsumoto M, et al. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature. 2017;541(7636):228–232. doi: 10.1038/nature21034. [DOI] [PubMed] [Google Scholar]
- 35.Min KW, Davila S, Zealy RW, et al. eIF4E phosphorylation by MST1 reduces translation of a subset of mRNAs, but increases lncRNA translation. Biochim Biophys Acta. 2017;1860(7):761–772. doi: 10.1016/j.bbagrm.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Mori K, Weng SM, Arzberger T, et al. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339(6125):1335–1338. doi: 10.1126/science.1232927. [DOI] [PubMed] [Google Scholar]
- 37.Mumtaz MAS, Couso JP. Ribosomal profiling adds new coding sequences to the proteome. Biochem Soc Trans. 2015;43(6):1271–1276. doi: 10.1042/bst20150170. [DOI] [PubMed] [Google Scholar]
- 38.Nam JW, Choi SW, You BH. Incredible RNA: dual functions of coding and noncoding. Mol Cells. 2016;39(5):367–374. doi: 10.14348/molcells.2016.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson BR, Makarewich CA, Anderson DM, et al. A peptide encoded by a transcript annotated as long noncoding RNA enhances SERCA activity in muscle. Science. 2016;351(6270):271–275. doi: 10.1126/science.aad4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamoto M, Higuchi-Takeuchi M, Shimizu M, et al. Substantial expression of novel small open reading frames in Oryza sativa. Plant Signal Behav. 2014;9(2):e27848. doi: 10.4161/psb.27848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pamudurti NR, Bartok O, Jens M, et al. Translation of circRNAs. Mol Cell. 2017;66(1):9–21e7. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pauli A, Norris ML, Valen E, et al. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343(6172):1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pueyo JI, Magny EG, Sampson CJ, et al. Hemotin, a regulator of phagocytosis encoded by a small ORF and conserved across metazoans. PLoS Biol. 2016;14(3):e1002395. doi: 10.1371/journal.pbio.1002395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Röhrig H, Schmidt J, Miklashevichs E, et al. Soybean ENOD40 encodes two peptides that bind to sucrose synthase. Proc Natl Acad Sci USA. 2002;99(4):1915–1920. doi: 10.1073/pnas.022664799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sampath K, Ephrussi A. CncRNAs: RNAs with both coding and non-coding roles in development. Development. 2016;143(8):1234–1241. doi: 10.1242/dev.133298. [DOI] [PubMed] [Google Scholar]
- 46.Slavoff SA, Mitchell AJ, Schwaid AG, et al. Peptidomic discovery of short open reading frame-encoded peptides in human cells. Nat Chem Biol. 2013;9(1):59–64. doi: 10.1038/nchembio.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136(4):731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stammers AN, Susser SE, Hamm NC, et al. The regulation of sarco(endo)plasmic reticulum calcium-ATPases (SERCA) Can J Physiol Pharmacol. 2015;93(10):843–854. doi: 10.1139/cjpp-2014-0463. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi K, Maruyama M, Tokuzawa Y, et al. Evolutionarily conserved non-AUG translation initiation in NAT1/p97/DAP5 (EIF4G2) Genomics. 2005;85(3):360–371. doi: 10.1016/j.ygeno.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Vandin F, Clay P, Upfal E, et al. Discovery of mutated subnetworks associated with clinical data in cancer. In: Altman RB Dunker AK, Hunter L et al., editors. Pacific Symp on Biocomputing 2012. Kohala Coast, Hawaii, USA; 2012. pp. 55–66. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y, Wang ZF. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21(2):172–179. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williamson L, Saponaro M, Boeing S, et al. UV irradiation induces a non-coding RNA that functionally opposes the protein encoded by the same gene. Cell. 2017;168(5):843–855e13. doi: 10.1016/j.cell.2017.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie N, Liu G. ncRNA-regulated immune response and its role in inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2015;309(10):L1076–L1087. doi: 10.1152/ajplung.00286.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu ZJ, Yan YL, Qian L, et al. Long non-coding RNAs act as regulators of cell autophagy in diseases. Oncol Rep. 2017;37(3):1359–1366. doi: 10.3892/or.2017.5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Y, Fan XJ, Mao MW, et al. Extensive translation of circular RNAs driven by N6-methyladenosine. Cell Res. 2017;27(5):626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zu T, Gibbens B, Doty NS, et al. Non-ATG-initiated translation directed by microsatellite expansions. Proc Natl Acad Sci USA. 2011;108(1):260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]