Abstract
Background:
Intra-resuscitation antiarrhythmic drugs may improve resuscitation outcomes, in part by avoiding rearrest, a condition associated with poor out-of-hospital cardiac arrest (OHCA) outcomes. However, antiarrhythmics may also alter defibrillation threshold. The objective of this study was to investigate the relationship between rearrest and intra-resuscitation antiarrhythmic drugs in the context of the Resuscitation Outcomes Consortium (ROC) amiodarone, lidocaine, and placebo (ALPS) trial.
Hypothesis:
Rearrest rates would be lower in cases treated with amiodarone or lidocaine, versus saline placebo, prior to first return of spontaneous circulation (ROSC). We also hypothesized antiarrhythmic effects would be quantifiable through analysis of the prehospital electrocardiogram.
Methods:
We conducted a secondary analysis of the ROC ALPS trial. Cases that first achieved prehospital ROSC after randomized administration of study drug were included in the analysis. Rearrest, defined as loss of pulses following ROSC, was ascertained from emergency medical services records. Rearrest rate was calculated overall, as well as by ALPS treatment group. Multivariable logistic regression models were constructed to assess the association between treatment group and rearrest, as well as rearrest and both survival to hospital discharge and survival with neurologic function. Amplitude spectrum area, median slope, and centroid frequency of the ventricular fibrillation (VF) ECG were calculated and compared across treatment groups.
Results:
A total of 1144 (40.4%) cases with study drug prior to first ROSC were included. Rearrest rate was 44.0% overall; 42.9% for placebo, 45.7% for lidocaine, and 43.0% for amiodarone. In multivariable logistic regression models, ALPS treatment group was not associated with rearrest, though rearrest was associated with poor survival and neurologic outcomes. AMSA and median slope measures of the first available VF were associated with rearrest case status, while median slope and centroid frequency were associated with ALPS treatment group.
Conclusion:
Rearrest rates did not differ between antiarrhythmic and placebo treatment groups. ECG waveform characteristics were correlated with treatment group and rearrest. Rearrest was inversely associated with survival and neurologic outcomes.
Background
The administration of antiarrhythmic drugs during resuscitation of ventricular fibrillation (VF) or ventricular tachycardia (VT) out-of-hospital cardiac arrest (OHCA) follows the premise that modification of the arrhythmogenic myocardium can suppress recurrent VF episodes after initial return of spontaneous circulation (ROSC).1 In this sense, antiarrhythmic administration can be contextualized to the intermediate resuscitation outcome of rearrest, including the rhythm-specific subcategory of recurrent VF, previously associated with poor survival to hospital discharge and/or neurologic outcomes by our group and others.2–6 The mechanism of antiarrhythmics in obviating recurrent VF or rearrest during resuscitation is conceptually complicated by the known effect of some antiarrhythmic drugs to increase the defibrillation threshold, an effect that should reduce the probability of successful defibrillation.7–9 Even so, it was demonstrated almost two decades ago that both amiodarone and lidocaine can improve survival to hospital admission when administered for recurrent or refractory VF.10–11 Later, the Resuscitation Outcomes Consortium (ROC) conducted the Amiodarone-Lidocaine-Placebo Study (ALPS), a large randomized controlled trial of amiodarone, lidocaine or placebo for treatment of recurrent or refractory VF.12 In the general analysis, no significant differences were observed between treatment groups for the survival to discharge or neurologic outcomes, although subgroup analyses showed heterogeneity of treatment effect based on witness status. Congruent with earlier studies, survival to hospital admission was greater among the antiarrhythmic treatment arms.
The ALPS trial provides a jumping off point for further investigation into the mechanisms and constraints by which antiarrhythmics fit into the resuscitation process, including their relationship to rearrest and their role in defibrillation. In the present study we sought to examine the intersection of these questions by considering not only the incidence and outcomes of rearrest in the ALPS trial, but also a measurable effect of the study drugs on the myocardium through analysis of the electrocardiogram (ECG) during resuscitation. We hypothesized that amiodarone and lidocaine would decrease the probability of rearrest occurrence compared to placebo and that their action would be demonstrable in ECG waveform analysis.
Methods
Primary Clinical Trial
We conducted this retrospective study under existing Institutional Review Board approved protocols. The population, design, and results of the primary analysis have been reported elsewhere,17 as have the structural details of the ROC.18 Briefly, the ROC was a clinical research network including 10 sites across the US and Canada, as well as the data coordinating center (DCC) at the University of Washington in Seattle, conducting population level surveillance and clinical trials in the realm of OHCA and major trauma. In the ROC ALPS trial, patients were randomized to receive either amiodarone, lidocaine or placebo if they exhibited recurrent or refractory VF/VT after at least one defibrillation attempt and had intravenous or intraosseous vascular access established at the time of randomization. A second dose of study drug could be administered if the first resulted in continued recurrent or refractory VF/VT. As part of the standard data form, characteristics of the patient, emergency medical services (EMS) procedures and timing, and outcomes were abstracted from prehospital care reports, 911 dispatch records, electronic defibrillator data files and hospital records. Among the data points collected at this stage were prehospital rearrest (“occurred ever”) and the timing of each prehospital ROSC event throughout the case, identified by site-level ROC data abstractors.
Inclusion – Exclusion Criteria
Case data from the ROC ALPS trial from 10 ROC sites were included in this analysis. In order to assess the association between antiarrhythmic drugs and rearrest, we restricted our analyses to only those cases that achieved ROSC after initial randomization and administration of ALPS study drug. Cases achieving ROSC prior to randomization were excluded because their eventual randomization would be a consequence of a rearrest event according to the primary trial protocol, rather than the reverse. Additional cases were excluded for missing primary variables of interest. For the waveform analysis portion of the study, we included any case meeting the above criteria and having an available defibrillator download file through the ROC DCC. Because not all ROC sites participated in voluntary defibrillator file upload during the ALPS trial, signal data was not uniformly available from all sites.
Outcomes
The primary outcomes for the present study were rearrest (defined as any loss of pulses after ROSC during the course of prehospital care), survival to hospital discharge (defined as discharge from the hospital alive to home or a long-term care facility), and good neurologic function at hospital discharge, taken as Modified Rankin Scale (MRS) ≤ 3.15 Secondary outcomes included quantitative ECG measures at first analyzable VF, immediately prior to ROSC, and at onset of first rearrest. ROSC was definied as a palpable pulse for any length of time.
Quantitative Waveform Measures
ECG signal data corresponding to the first analyzable instance of VF, immediate pre-ROSC VF, and VF at the onset of first rearrest were extracted from defibrillator files, parsed and analyzed by two operators (MLS/ACK) using a combination of proprietary manufacturer software and custom MATLAB (R2016b) scripts. The most proximal 3 seconds of ECG to each of the 3 measurement points were used for calculation of amplitude spectrum area (AMSA), median slope (MS), and centroid frequency (CF) quantitative waveform measures (QWM). The calculation methodologies of these measures have been described extensively in the literature.16–18 In brief, QWM capture in a single numerical measure the coarseness or fineness of the VF ECG as a function of one or more objective characteristics of the waveform. These include derivations of the ECG time series, like MS, which is calculated from the instantaneous slope of the ECG, or frequency domain derivations like AMSA and CF, which both describe the distribution of signal power in the Fourier transformed ECG. In laboratory experiments, QWM progressively deteriorate as untreated VF goes on, progressively restore as VF is reperfused, and correlate with myocardial energy stores.19,20 In retrospective clinical studies, they have been correlated with defibrillation outcomes, as well as survival to hospital discharge.21–25
Analysis
Overall rearrest rate was calculated as the proportion of all cases with any rearrest and then stratified by ALPS treatment arm, as well as by ROC site. Case characteristics, including demographics, witness status, presenting ECG rhythm, event location, EMS arrival time, and bystander-treatment status were compared by rearrest status. The primary variable of interest was ALPS treatment arm(amiodarone, lidocaine or placebo). The primary analysis examined the association of ALPS treatment arms and rearrest, using a multivariable logistic regression model. In addition, we fit multivariable logistic regression models examining the association of rearrest with survival to hospital discharge and good neurologic function, both for all patients and restricted to those patients who went on to arrive at the emergency department (ED) with pulses, the latter intended to provide a more conservative estimate of rearrest’s impact on outcomes. In order to understand the implications of excluding patients who had ROSC prior to study drug administration, we conducted a sensitivity analysis that incorporated these patients into our primary comparisons, which are reported in the associated supplement.
In order to understand the mechanistic association between study drug and rearrest, QWM for the first available VF and immediate pre-ROSC VF were compared between cases with and without rearrest and across ALPS arms in separate 2-way ANOVA models. QWM for cases with rearrest were compared across ALPS arms in separate 1-way ANOVA models
Data management and analyses were conducted using S-Plus version 6.2.1 (TIBCO Software Inc. Palo Alto, California, USA), and Stata version 11 (StataCorp, College Station, Texas, USA). An alpha level of 0.05 was used as the criterion for statistical significance for all analyses.
Results
From the original ROC ALPS trial, 2381 cases qualified for inclusion in this study. In turn, 1144 (40.4%) achieved ROSC after initial ALPS study drug administration, qualifying for study inclusion. Characteristics of the study cohort are shown in Table 1. Five-hundred and three (44.0%) patients experienced at least one rearrest event prior to hospital arrival; rearrest rates ranged from 33.0% to 60.0% across the ROC sites. Due to the primary trial’s inclusion criteria, nearly all cases presented with an initial rhythm of VF/VT (99.0%), with no detectible difference in first rhythm classification between those with and without rearrest. Cases with rearrest were less likely to have the first EMS unit arrive in under 6 minutes (62.8% vs 69.3%; p = 0.023).
Table 1.
Descriptive Statistics by Rearrest and ROSC Status
| ROSC, Rearrest | ROSC, No Rearrest |
No ROSC | Overall | |
|---|---|---|---|---|
| N | 503 | 641 | 1237 | 2381 |
| Male, n (%) | 399 (79.3%) | 483 (75.4%) | 1028 (83.1%) | 1910 (80.2%) |
| Age | ||||
| Median (IQR) | 66 (19.0) | 63 (20.0) | 63 (20.0) | 64 (20.0) |
| <40 yrs, n (%) | 16 (3.2%) | 41 (6.4%) | 60 (4.9%) | 117 (4.9%) |
| 40 – 60 yrs, n (%) | 154 (30.6%) | 228 (35.6%) | 434 (35.1%) | 816 (34.3%) |
| ≥60 yrs, n (%) | 333 (66.2%) | 372 (58.0%) | 743 (60.1%) | 1448 (60.8%) |
| Witness Status | ||||
| Bystander, n (%) | 351 (69.8%) | 448 (69.9%) | 785 (63.5%) | 1584 (66.5%) |
| None, n (%) | 14 (2.8%) | 27 (4.2%) | 58 (4.7%) | 99 (4.2%) |
| None, n (%) | 138 (27.4%) | 166 (25.9%) | 394 (31.9%) | 698 (29.3%) |
| Bystander CPR, n (%) | 314 (62.4%) | 392 (61.2%) | 674 (54.5%) | 1380 (58.0%) |
| Initial rhythm | ||||
| VT/VF, n (%) | 497 (98.8%) | 635 (99.1%) | 1231 (99.5%) | 2363 (99.2%) |
| PEA, n (%) | 3 (0.6%) | 3 (0.5%) | 2 (0.2%) | 8 (0.3%) |
| Asystole, n (%) | 2 (0.4%) | 2 (0.3%) | 4 (0.3%) | 8 (0.3%) |
| No shock advised, n (%) | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | 2 (0.1%) |
| Episode location | ||||
| Public, n (%) | 341 (67.8%) | 423 (66.0%) | 869 (70.3%) | 1633 (68.6%) |
| Private, n (%) | 162 (32.2%) | 218 (34.0%) | 368 (29.7%) | 748 (31.4%) |
| First agency arrival time | ||||
| <6 minutes, n (%) | 316 (62.8%) | 444 (69.3%) | 760 (61.4%) | 1520 (63.8%) |
| ≥6 minutes, n (%) | 187 (37.2%) | 197 (30.7%) | 477 (38.6%) | 861 (36.2%) |
| ALPS Treatment Arm | ||||
| Placebo, n (%) | 155 (30.8%) | 206 (32.1%) | 472 (38.2%) | 833 (35.0%) |
| Lidocaine, n (%) | 192 (38.2%) | 228 (35.6%) | 366 (29.6%) | 786 (33.0%) |
| Amiodarone, n (%) | 156 (31.0%) | 207 (32.3%) | 399 (32.3%) | 762 (32.0%) |
| Number of Shocks, mean (SD) | 6.2 (3.5) | 4.6 (2.4) | 6.9 (4.2) | 6.2 (3.8) |
| Site | ||||
| A, n (%)1 | 3 (11.1%) | 5 (18.5%) | 19 (70.4%) | 27 |
| B, n (%)1 | 44 (11.7%) | 70 (18.6%) | 263 (69.8%) | 377 |
| C, n (%)1 | 40 (24.8%) | 48 (29.8%) | 73 (45.3%) | 161 |
| D, n (%)1 | 30 (19.5%) | 39 (25.3%) | 85 (55.2%) | 154 |
| E, n (%)1 | 3 (21.4%) | 2 (14.3%) | 9 (64.3%) | 14 |
| F, n (%)1 | 37 (17.6%) | 75 (35.7%) | 98 (46.7%) | 210 |
| G, n (%)1 | 31 (16.4%) | 49 (25.9%) | 109 (57.7%) | 189 |
| H, n (%)1 | 122 (30.3%) | 143 (35.5%) | 138 (34.2%) | 403 |
| I, n (%)1 | 108 (19.7%) | 124 (22.6%) | 317 (57.7%) | 549 |
| J, n (%)1 | 85 (28.6%) | 86 (29.0%) | 126 (42.4%) | 297 |
Abbreviations: ALPS: Amiodarone-Lidocaine-Placebo Study, CPR: cardiopulmonary resuscitation, IQR: interquartile range, PEA: pulseless electrical activity, ROSC: return of spontaneous circulation, VT/VF: ventricular tachycardia/ventricular fibrillation
Rearrest was not independently associated with ALPS treatment groups. In a multivariable logistic regression model with outcome rearrest (results shown in Table 2), age ≥ 60 and time-to-ROSC ≥ 30 minutes were directly related to rearrest, while non-cardiac etiology was inversely related to rearrest.
Table 2.
Logistic Regression Results for Outcome Rearrest
| N | ROSC in PH Rearrest 1144 |
|
|---|---|---|
| OR | 95% CI | |
| Treatment Arm | ||
| Placebo1 | ||
| Lidocaine | 1.13 | (0.85, 1.52) |
| Amiodarone | 1.01 | (0.75, 1.37) |
| Sex | ||
| Female1 | ||
| Male | 1.26 | (0.94, 1.69) |
| Age | ||
| <601 | ||
| ≥60 | 1.35 | (1.05, 1.73) |
| Witness Status | ||
| Bystander Witness1 | ||
| EMS Witness | 0.58 | (0.29, 1.17) |
| No Witness | 1.10 | (0.83, 1.45) |
| Bystander-initiated CPR | ||
| Not Administered1 | ||
| Administered | 1.03 | (0.79, 1.34) |
| Episode Location | ||
| Private1 | ||
| Public | 0.93 | (0.72, 1.21) |
| Etiology | ||
| Cardiac1 | ||
| Noncardiac | 0.10 | (0.01, 0.76) |
| Time to 1st Agency Arrival | ||
| <6 minutes1 | ||
| ≥6 minutes | 1.18 | (0.90, 1.55) |
| Time to ROSC | ||
| <30 minutes1 | ||
| ≥30 minutes | 1.54 | (1.16, 2.04) |
| CPR Fraction | ||
| <0.901 | ||
| ≥0.90 | 1.05 | (0.78, 1.41) |
| Site | ||
| A | 0.99 | (0.22, 4.54) |
| B1 | ||
| C | 1.25 | (0.70, 2.22) |
| D | 1.08 | (0.58, 2.03) |
| E | 2.30 | (0.35, 15.07) |
| F | 0.68 | (0.39, 1.20) |
| G | 0.94 | (0.51, 1.72) |
| H | 1.25 | (0.78, 2.00) |
| I | 1.28 | (0.80, 2.07) |
| J | 1.25 | (0.75, 2.08) |
Abbreviations: CI: Confidence interval, CPR: cardiopulmonary resuscitation, EMS: Emergency Medical Services, PH: prehospital, ROSC: return of spontaneous circulation
Cases with rearrest had a 24.3% rate of survival to hospital discharge and a 19.3% rate of good neurologic outcome compared to 57.0% and 44.4%, respectively, among those without rearrest, when using a prehospital ROSC criterion. After adjustment for common resuscitation covariates, rearrest was inversely related both to survival to hospital discharge (OR: 0.24; 95% CI: 0.18 – 0.31) and good neurologic outcome (OR: 0.30; 95% CI: 0.22 – 0.40), both among all cases and among the subset of all cases that had ROSC at ED arrival (See Table 3). In the same models, age, unwitnessed status, and time-to-ROSC greater than 30 minutes were inversely related to survival and good neurologic outcome, while EMS witness status, bystander cardiopulmonary resuscitation (CPR) administration and public location were associated with improved outcomes.
Table 3.
Logistic Regression Results Outcome Survival
| ROSC at ED | ROSC in PH | |||||||
|---|---|---|---|---|---|---|---|---|
| Survival | MRS≤3 | Survival | MRS≤3 | |||||
| N | 853 | 851 | 1135 | 1133 | ||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Rearrest | ||||||||
| No Rearrest1 | ||||||||
| Prehospital Rearrest | 0.55 | (0.39, 0.77) | 0.69 | (0.48, 0.97) | 0.24 | (0.18, 0.31) | 0.30 | (0.22, 0.40) |
| Sex | ||||||||
| Female1 | ||||||||
| Male | 1.36 | (0.95, 1.95) | 1.39 | (0.96, 2.00) | 1.23 | (0.88, 1.72) | 1.32 | (0.93, 1.87) |
| Age | ||||||||
| <601 | ||||||||
| ≥60 | 0.33 | (0.24, 0.45) | 0.35 | (0.26, 0.48) | 0.38 | (0.28, 0.50) | 0.38 | (0.29, 0.50) |
| Witness Status | ||||||||
| Bystander Witness1 | ||||||||
| EMS Witness | 5.03 | (1.99, 12.75) | 5.90 | (2.38, 14.60) | 4.02 | (1.82, 8.88) | 5.37 | (2.44, 11.81) |
| No Witness | 0.61 | (0.43, 0.86) | 0.64 | (0.44, 0.91) | 0.57 | (0.41, 0.78) | 0.60 | (0.43, 0.84) |
| Bystander-initiated CPR | ||||||||
| Not Administered1 | ||||||||
| Administered | 1.60 | (1.14, 2.23) | 1.44 | (1.03, 2.02) | 1.58 | (1.16, 2.14) | 1.50 | (1.09, 2.06) |
| Episode Location | ||||||||
| Private1 | ||||||||
| Public | 1.54 | (1.11, 2.14) | 1.63 | (1.19, 2.26) | 1.52 | (1.13, 2.03) | 1.57 | (1.17, 2.10) |
| Etiology | ||||||||
| Cardiac1 | ||||||||
| Noncardiac | 0.59 | (0.17, 2.06) | 0.76 | (0.22, 2.60) | 0.57 | (0.17, 1.92) | 0.74 | (0.22, 2.45) |
| Time to 1st Agency Arrival | ||||||||
| <6 minutes1 | ||||||||
| ≥6 minutes | 1.01 | (0.71, 1.43) | 1.07 | (0.75, 1.51) | 1.03 | (0.75, 1.41) | 1.09 | (0.79, 1.50) |
| Time to ROSC | ||||||||
| <30 minutes1 | ||||||||
| ≥30 minutes | 0.37 | (0.26, 0.55) | 0.44 | (0.29, 0.65) | 0.32 | (0.23, 0.46) | 0.37 | (0.25, 0.53) |
| CPR Fraction | ||||||||
| <0.901 | ||||||||
| ≥0.90 | 1.37 | (0.95, 1.97) | 1.26 | (0.88, 1.81) | 1.31 | (0.94, 1.82) | 1.26 | (0.90, 1.76) |
| Mean Compression Rate | ||||||||
| <1001 | ||||||||
| [100–120] | 0.99 | (0.58, 1.68) | 0.94 | (0.56, 1.59) | 1.19 | (0.74, 1.90) | 1.07 | (0.66, 1.72) |
| >120 | 0.97 | (0.51, 1.83) | 0.89 | (0.47, 1.67) | 1.13 | (0.64, 2.01) | 1.03 | (0.57, 1.84) |
| Site | ||||||||
| A | 0.58 | (0.11, 3.01) | 0.28 | (0.03, 2.77) | 0.94 | (0.18, 4.92) | 0.43 | (0.04, 4.23) |
| B1 | ||||||||
| C | 1.04 | (0.50, 2.16) | 1.04 | (0.50, 2.18) | 1.22 | (0.65, 2.31) | 1.23 | (0.62, 2.44) |
| D | 0.50 | (0.23, 1.08) | 0.68 | (0.31, 1.53) | 0.65 | (0.32, 1.31) | 0.84 | (0.39, 1.81) |
| E | 2.36 | (0.20, 27.84) | 0.20 | (0.02, 2.19) | 5.99 | (0.48, 74.12) | 0.31 | (0.03, 3.66) |
| F | 0.77 | (0.39, 1.55) | 1.03 | (0.51, 2.06) | 0.89 | (0.48, 1.63) | 1.08 | (0.57, 2.04) |
| G | 0.61 | (0.29, 1.30) | 0.92 | (0.43, 1.95) | 0.59 | (0.30, 1.13) | 0.89 | (0.44, 1.77) |
| H | 0.62 | (0.34, 1.12) | 0.99 | (0.55, 1.78) | 0.90 | (0.53, 1.51) | 1.30 | (0.75, 2.24) |
| I | 0.53 | (0.29, 0.97) | 1.28 | (0.70, 2.34) | 0.69 | (0.41, 1.16) | 1.46 | (0.85, 2.53) |
| J | 0.61 | (0.32, 1.16) | 1.35 | (0.70, 2.58) | 0.88 | (0.49, 1.56) | 1.70 | (0.94, 3.11) |
Abbreviations: CPR: cardiopulmonary resuscitation, ED: emergency department, MRS: modified Rankin scale, PH: prehospital, ROSC: return of spontaneous circulation
Results of QWM analyses are summarized in Figure 2. Electronic defibrillator files were available for 424 (37%) of ALPS cases identified as having ROSC prior to study drug administration. In two-way ANOVA models, CF (p = 0.0270) during the first available VF signal and both AMSA (p = 0.0277) and MS (p = 0.0203) during the immediate pre-ROSC VF signal differed by rearrest case status. In the same models, there were no significant associations between ALPS treatment group and the QWM during the first available VF signal, however treatment group was associated with MS (p = 0.0235), and CF (p = 0.0449) in the immediate pre-ROSC VF signal. Neither ALPS treatment group nor rearrest status were significantly associated with QWM of the first rearrest VF signal in those cases that exhibited a rearrest. Mean QWM in general were highest in cases without rearrest and among those cases in the ALPS placebo arm.
Figure 2. Immediate Pre-ROSC Quantitative Waveform Measures Stratified by Rearrest and ALPS Treatment Arm –
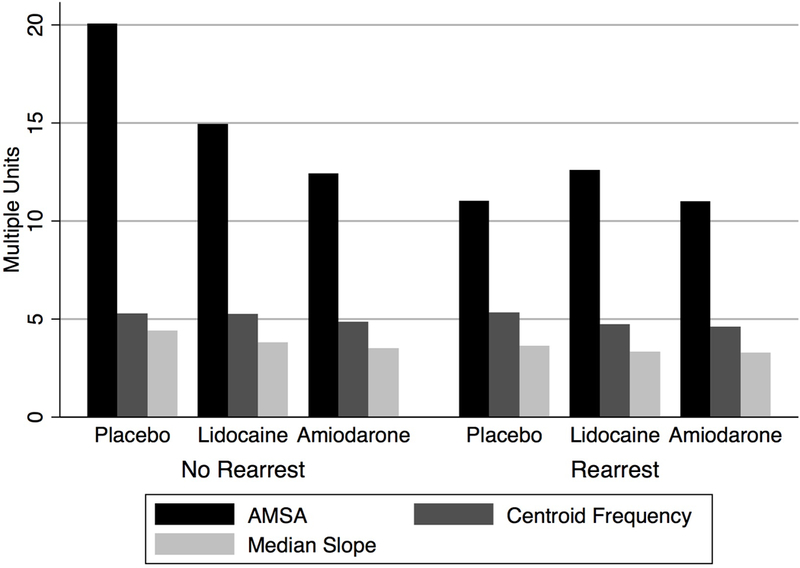
QWM are shown for each ALPS trial treatment arm and stratified by rearrest status for the VF signal immediately prior to defibrillation resulting in ROSC. Actual QWM magnitudes are expressed on a single y-axis, though units are not individually indicated.
Abbreviations: ALPS: Amiodarone-Lidocaine-Placebo Study, AMSA: amplitude spectrum area, QWM: quantitative waveform measures, ROSC: return of spontaneous circulation, VF: ventricular fibrillation
Discussion
The results of this study indicate that rearrest was not associated with ALPS treatment group, but QWM of the VF ECG were associated with either rearrest, ALPS treatment group, or both. Moreover, rearrest was related to survival to hospital discharge and good neurologic function. The disconnect between these findings leaves a complex picture, but one that nonetheless may inform the results of the primary ALPS trial analyses, the phenomenon of rearrest, and the effects of intra-resuscitation antiarrhythmic administration.
The intervention at the center of the ROC ALPS trial, theoretically intersects with the process of rearrest by reducing the likelihood of downstream lethal arrhythmia. As observed in this study, the intervention did not affect the likelihood of rearrest. In the primary analysis of the ALPS trial, both amiodarone and lidocaine were associated with a greater probability of admission to hospital, and lidocaine was associated with a greater probability of arriving at hospital with pulses, compared to placebo.12 Either outcome could potentially be interpreted as an incomplete surrogate for rearrest, with the important limitation that rearrest is reversible prior to hospital arrival and admission, and therefore would not be uniformly captured by these surrogates. On the other hand, rearrest in the present study was ascertained only in cases that had been randomized to amiodarone, lidocaine or placebo prior to first ROSC, so inference regarding the effect of each on downstream rearrest is better than purely associational. Still, in light of the findings of the trial, one might expect to see differential rearrest rates here.
Our results also show that amiodarone and lidocaine were associated with lower QWM, specifically MS and CF, in the time between study drug administration and ROSC, the expected effect if both were raising the defibrillation threshold. Interestingly, the same relationship was not observed for AMSA, which did not differ by rearrest status. The reason for this disparity is unclear. Each quantitative waveform measure captures a different feature or combination of features of the VF signal. It may be that these measures truly capture different phenomena that differentiate cases by ALPS treatment arm, or it could be that signal quality characteristics influenced the sensitivity of these measures. AMSA and CF have related derivations, both beginning with a Fourier transform, while MS might be considered a loose mathematical approximation of both, wherein it expresses the distribution of a property of the VF waveform that varies roughly in proportion to frequency. However, unlike CF and MS, AMSA incorporates a weighted sum of signal power, rather than a central tendency. In continuous analysis of prolonged VF, these measures track together on very similar trajectories under uniform recording conditions.20
QWM were also correlated in this study with rearrest occurrence. Immediate pre-ROSC AMSA and MS were correlated with rearrest, as was the CF of first available VF signal. To clarify, the VF immediately preceding successful defibrillation and first ROSC showed different characteristics between those cases that would go on to have or not have a subsequent rearrest. On average, QWM were higher (i.e., better) in cases without rearrest. As a general picture of myocardial condition, QWM may correlate with a healthier heart, given their relationship to defibrillation success, ROSC, and survival. It then fits this picture that lower QWM would foreshadow the potential for downstream rearrest. Interestingly, we also found that QWM did not differ between study drugs at the time of rearrest occurrence. One potential implication of this finding is that the mechanistic effect of study drug did not persist among those cases that had a rearrest event, expressing both as rearrest and similar QWM. It is difficult to assert this with certainty, in part because a definitive comparison would involve QWM analysis of equivalent time points among cases without rearrest – an impossibility because there would be no VF to analyze.
Lastly, in the present study rearrest was once again found to be a strong predictor of poor resuscitation outcomes, including survival to hospital discharge and neurologic function, confirming recent findings from the ROC Continuous Chest Compressions trial.6,26 In the prior study, no association was found between definitively classified compression-to-ventilation ratio and rearrest occurrence, an association that we had hypothesized was rooted in additional no-flow time created by regular cessation of chest compressions for delivery of ventilations.
This study has several important limitations. First, the phenomenon of rearrest has been simplified to allow investigation of the association between rearrest and antiarrhythmic administration. Cases that may have had ROSC and rearrest prior to administration of study drug were not considered in the primary analysis, although a sensitivity analysis was performed to determine whether their exclusion resulted in dramatically different results. Second, we only considered first rearrest, not subsequent occurrence. It may be that the classification of rearrest as a whole-case status obscures an additional, important dimension of the relationship between rearrest and antiarrhythmics. Even so, it should be appreciated that previous findings demonstrating the negative impact of rearrest on prognosis used a similar whole-case definition. Third, QWM were only available in a subset of cases and analyzed as a secondary mechanistic aim, and consequently could not be factored into the larger multivariable analyses at the center of the study. The large amount of missing signal data may have introduced unanticipated biases, which likely would relate to inter-site differences in patient and treatment characteristics. Importantly, the true relationship between amiodarone, lidocaine and placebo and the immediate pre-ROSC ECG is ultimately not known, since the signal characteristics immediately prior to drug administration, characteristics of resuscitation in the intervening time, and other factors would have to be considered, an endeavor out of the scope of this secondary analysis. An on-going study of the general relationship between QWM and the resuscitation process throughout the ALPS study is currently underway and may reveal the full picture in the near future.
Conclusions
In this secondary analysis of a very specific subset of cases, rearrest rate did not differ between the amiodarone, lidocaine or placebo groups of the ROC ALPS trial, indicating that the relationship between antiarrhythmics and resuscitation outcomes may be more complicated than simple obviation of rearrest. However, some QWM of the VF ECG did differ between ALPS treatment groups, as well as by rearrest case status. More work is needed to understand the significance of the VF waveform in the context of rearrest.
Supplementary Material
Figure 1. Survival and Good Neurologic Outcome Stratified by Rearrest –
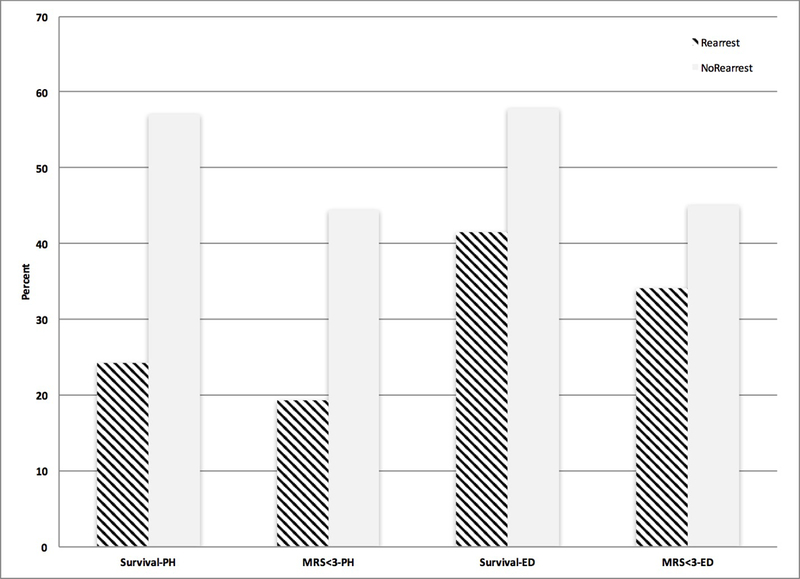
Resuscitation outcomes survival to hospital discharge and MRS <=3 stratified by rearrest status (rearrest = striped) are shown for all patients with prehospital ROSC (left), as well as the subset of patients with pulses at ED arrival (right).
Abbreviations: ED: emergency department, MRS: modified Rankin Scale, PH: prehospital
Acknowledgments
We owe our thanks to the EMS personnel of the participating agencies of the Resuscitation Outcomes Consortium.
Drs. Salcido and Menegazzi received support from NHLBI grants (K12HL109068, R01HL117979, R21HL135369). Dr. Salcido received grants from the Henry L. Hillman Foundation and a small grant from the Laerdal Foundation and Zoll Foundation for unrelated work.
The ROC is supported by a series of cooperative agreements to nine regional clinical centers and one Data Coordinating Center (5U01 HL077863-University of Washington Data Coordinating Center, HL077866-Medical College of Wisconsin, HL077867-University of Washington, HL077871-University of Pittsburgh, HL077872-St. Michael’s Hospital, HL077873-Oregon Health and Science University, HL077881-University of Alabama at Birmingham, HL077885-Ottawa Hospital Research Institute, HL077887-University of Texas SW Medical Ctr/Dallas, HL077908-University of California San Diego) from the National Heart, Lung and Blood Institute in partnership with the U.S. Army Medical Research & Material Command, The Canadian Institutes of Health Research (CIHR) - Institute of Circulatory and Respiratory Health, Defence Research and Development Canada, the Heart, Stroke Foundation of Canada and the American Heart Association. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung and Blood Institute or the National Institutes of Health.
References
- 1.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O’Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: Adult Advanced Cardiovascular Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2015. November 3;132(18 Suppl 2):S444–64. [DOI] [PubMed] [Google Scholar]
- 2.van Alem AP, Post J, Koster RW. VF recurrence: characteristics and patient outcome in out-of-hospital cardiac arrest. Resuscitation. 2003. November;59(2):181–8. [DOI] [PubMed] [Google Scholar]
- 3.Berdowski J, ten Haaf M, Tijssen JG, Chapman FW, Koster RW. Time in recurrent ventricular fibrillation and survival after out-of-hospital cardiac arrest. Circulation. 2010. September 14;122(11):1101–8. [DOI] [PubMed] [Google Scholar]
- 4.Lerner EB, O’Connell M, Pirrallo RG. Rearrest after prehospital resuscitation. Prehosp Emerg Care 2011. Jan-Mar;15(1):50–4. [DOI] [PubMed] [Google Scholar]
- 5.Salcido DD, Sundermann ML, Koller AC, Menegazzi JJ. Incidence and outcomes of rearrest following out-of-hospital cardiac arrest. Resuscitation. 2015. January;86:19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salcido DD, Schmicker RH, Buick JE, Cheskes S, Grunau B, Kudenchuk P, Leroux B, Zellner S, Zive D, Aufderheide TP, Koller AC, Herren H, Nuttall J, Sundermann ML, Menegazzi JJ; Resuscitation Outcomes Consortium Investigators. Compression-to-ventilation ratio and incidence of rearrest-A secondary analysis of the ROC CCC trial. Resuscitation. 2017. June;115:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echt DS, Black JN, Barbey JT, Coxe DR, Cato E. Evaluation of antiarrhythmic drugs on defibrillation energy requirements in dogs. Sodium channel block and action potential prolongation. Circulation. 1989. May;79(5):1106–17. [DOI] [PubMed] [Google Scholar]
- 8.Hohnloser SH, Dorian P, Roberts R, Gent M, Israel CW, Fain E, Champagne J, Connolly SJ. Effect of amiodarone and sotalol on ventricular defibrillation threshold: the optimal pharmacological therapy in cardioverter defibrillator patients (OPTIC) trial. Circulation. 2006. July 11;114(2):104–9. [DOI] [PubMed] [Google Scholar]
- 9.Silva AF, Bonatti R, Batatinha J, Nearing BD, Zeng D, Belardinelli L, Verrier RL. The Selective Late Sodium Current Inhibitor, Eleclazine, Unlike Amiodarone, Does Not Alter Defibrillation Threshold or Dominant Frequency of Ventricular Fibrillation. J Cardiovasc Pharmacol. 2016. December 28. [DOI] [PubMed] [Google Scholar]
- 10.Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with lidocaine for shock-resistant ventricular fibrillation. N Engl J Med. 2002. March 21;346(12):884–90. [DOI] [PubMed] [Google Scholar]
- 11.Kudenchuk PJ, Cobb LA, Copass MK, Cummins RO, Doherty AM, Fahrenbruch CE, Hallstrom AP, Murray WA, Olsufka M, Walsh T. Amiodarone for resuscitation after out-of-hospital cardiac arrest due to ventricular fibrillation. N Engl J Med. 1999. September 16;341(12):871–8. [DOI] [PubMed] [Google Scholar]
- 12.Kudenchuk PJ, Brown SP, Daya M, Rea T, Nichol G, Morrison LJ, Leroux B, Vaillancourt C, Wittwer L, Callaway CW, Christenson J, Egan D, Ornato JP, Weisfeldt ML, Stiell IG, Idris AH, Aufderheide TP, Dunford JV, Colella MR, Vilke GM, Brienza AM, Desvigne-Nickens P, Gray PC, Gray R, Seals N, Straight R, Dorian P; Resuscitation Outcomes Consortium Investigators.. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. N Engl J Med. 2016. May 5;374(18):1711–22. [DOI] [PubMed] [Google Scholar]
- 13.Kudenchuk PJ, Brown SP, Daya M, Morrison LJ, Grunau BE, Rea T, Aufderheide T, Powell J, Leroux B, Vaillancourt C, Larsen J, Wittwer L, Colella MR, Stephens SW, Gamber M, Egan D, Dorian P; Resuscitation Outcomes Consortium Investigators. Resuscitation Outcomes Consortium-Amiodarone, Lidocaine or Placebo Study (ROC-ALPS): Rationale and methodology behind an out-of-hospital cardiac arrest antiarrhythmic drug trial. Am Heart J. 2014. May;167(5):653–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis DP, Garberson LA, Andrusiek DL, Hostler D, Daya M, Pirrallo R, Craig A, Stephens S, Larsen J, Drum AF, Fowler R. A descriptive analysis of Emergency Medical Service Systems participating in the Resuscitation Outcomes Consortium (ROC) network. Prehosp Emerg Care. 2007. Oct-Dec;11(4):369–82. [DOI] [PubMed] [Google Scholar]
- 15.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002. September;33(9):2243–6. [DOI] [PubMed] [Google Scholar]
- 16.Brown CG, Dzwonczyk R. Signal analysis of the human electrocardiogram during ventricular fibrillation: frequency and amplitude parameters as predictors of successful countershock. Ann Emerg Med. 1996. February;27(2):184–8. [DOI] [PubMed] [Google Scholar]
- 17.Marn-Pernat A, Weil MH, Tang W, Pernat A, Bisera J. Optimizing timing of ventricular defibrillation. Crit Care Med 2001. December;29(12):2360–5. [DOI] [PubMed] [Google Scholar]
- 18.Neurauter A, Eftestøl T, Kramer-Johansen J, Abella BS, Sunde K, Wenzel V, Lindner KH, Eilevstjønn J, Myklebust H, Steen PA, Strohmenger HU. Prediction of countershock success using single features from multiple ventricular fibrillation frequency bands and feature combinations using neural networks. Resuscitation. 2007. May;73(2):253–63. [DOI] [PubMed] [Google Scholar]
- 19.Salcido DD, Menegazzi JJ, Suffoletto BP, Logue ES, Sherman LD. Association of intramyocardial high energy phosphate concentrations with quantitative measures of the ventricular fibrillation electrocardiogram waveform. Resuscitation. 2009. August;80(8):946–50. [DOI] [PubMed] [Google Scholar]
- 20.Salcido DD, Kim YM, Sherman LD, Housler G, Teng X, Logue ES, Menegazzi JJ. Quantitative waveform measures of the electrocardiogram as continuous physiologic feedback during resuscitation with cardiopulmonary bypass. Resuscitation. 2012. April;83(4):505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ristagno G, Li Y, Fumagalli F, Finzi A, Quan W. Amplitude spectrum area to guide resuscitation-a retrospective analysis during out-of-hospital cardiopulmonary resuscitation in 609 patients with ventricular fibrillation cardiac arrest. Resuscitation. 2013. December;84(12):1697–703. [DOI] [PubMed] [Google Scholar]
- 22.Ristagno G, Mauri T, Cesana G, Li Y, Finzi A, Fumagalli F, Rossi G, Grieco N, Migliori M, Andreassi A, Latini R, Fornari C, Pesenti A; Azienda Regionale Emergenza Urgenza Research Group.. Amplitude spectrum area to guide defibrillation: a validation on 1617 patients with ventricular fibrillation. Circulation. 2015. February 3;131(5):478–87. [DOI] [PubMed] [Google Scholar]
- 23.Schoene P, Coult J, Murphy L, Fahrenbruch C, Blackwood J, Kudenchuk P, Sherman L, Rea T. Course of quantitative ventricular fibrillation waveform measure and outcome following out-of-hospital cardiac arrest. Heart Rhythm. 2014. February;11(2):230–6. [DOI] [PubMed] [Google Scholar]
- 24.Indik JH, Conover Z, McGovern M, Silver AE, Spaite DW, Bobrow BJ, Kern KB. Association of amplitude spectral area of the ventricular fibrillation waveform with survival of out-of-hospital ventricular fibrillation cardiac arrest. J Am Coll Cardiol. 2014. September 30;64(13):1362–9. [DOI] [PubMed] [Google Scholar]
- 25.Hulleman M, Salcido DD, Menegazzi JJ, Souverein PC, Tan HL, Blom MT, Koster RW. Predictive value of amplitude spectrum area of ventricular fibrillation waveform in patients with acute or previous myocardial infarction in out-of-hospital cardiac arrest. Resuscitation. 2017. November;120:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nichol G, Leroux B, Wang H, Callaway CW, Sopko G, Weisfeldt M, Stiell I, Morrison LJ, Aufderheide TP, Cheskes S, Christenson J, Kudenchuk P, Vaillancourt C, Rea TD, Idris AH, Colella R, Isaacs M, Straight R, Stephens S, Richardson J, Condle J, Schmicker RH, Egan D, May S, Ornato JP; ROC Investigators.. Trial of Continuous or Interrupted Chest Compressions during CPR. N Engl J Med. 2015. December 3;373(23):2203–14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


