ABSTRACT
Background
The nitrogen isotope ratio (NIR) is a promising index of traditional food intake for an Alaska Native (Yup'ik) population, which can be measured in blood and hair. However, the NIR has not been calibrated to high-quality measures of Yup'ik traditional food intake.
Objectives
Our primary objective was to examine associations between intakes of Yup'ik traditional food groups, including fish, marine mammals, birds, land mammals, berries, greens, and total traditional foods, and the NIR. In an exploratory analysis, we also examined whether NIR analyzed sequentially along hair could reflect dietary seasonality.
Methods
We recruited 68 participants from 2 Yup'ik communities in the Yukon Kuskokwim region of Southwest Alaska (49% female, aged 14–79 y). Participants completed 4 unscheduled 24-h food recalls over the period peak of RBC and hair synthesis preceding a specimen collection visit. The NIR was measured in RBCs ( n = 68), a proximal hair section (n = 58), and sequential segments of hair from individuals in the upper 2 quartiles of traditional food intake having hair >6 cm in length, plus 2 low subsistence participants for reference (n = 18). Diet–biomarker associations were assessed using Pearson's correlation and linear regression.
Results
Intakes of fish, marine mammals, berries, and greens were significantly associated with the NIR. The strongest dietary association was with total traditional food intake (R2 = 0.62), which indicated that each 1‰ increase in the RBC NIR corresponded to 8% of energy from traditional foods. Hair NIR appeared to fluctuate seasonally in some individuals, peaking in the summertime.
Conclusions
Findings support the use of the RBC and hair NIR to assess total traditional food intake in a Yup'ik population. Analyses of sequential hair NIR provided evidence of seasonality in traditional food intake, although seasonal variations were modest relative to interindividual variation.
Keywords: circumpolar health, traditional food, dietary seasonality, nitrogen stable isotope ratio, biomarker evaluation
Introduction
Traditional foods, defined as those hunted or gathered from the surrounding environment, have great cultural and nutritional importance for native people throughout the circumpolar north (1). The Yup'ik people of the Yukon-Kuskokwim Delta region of Southwest Alaska still consume about 20% of energy from traditional foods population-wide (2), with older adults consuming significantly more (2–5). Yup'ik traditional foods include predominantly fish, fish roe, seal oil, and seal meat, which have been estimated to contribute 70–80% of traditional food energy (2, 4). Yup'ik traditional foods also include other locally harvested game including birds (e.g., ducks, geese, cranes, ptarmigan), land mammals (e.g., caribou, moose), and plants (berries and greens), although plants are a minor contributor at <3% of traditional food energy (2, 6–8). There is considerable interest in understanding how adherence to a traditional dietary pattern relates to health in Yup'ik and other Alaska Native people (9–12). Investigating that question requires tools to assess traditional food intake easily, accurately, and reliably.
Several key studies have evaluated the diet of Alaska Native people using self-reported methods (2, 6, 13, 14). However, some types of instruments, particularly food records or repeated 24-h recalls, can be challenging to administer in remote, rural environments, and all self-reported methods have potential for bias and errors (15, 16). Dietary biomarkers are unbiased measures (15) that have the potential to be more accurate and easier to collect than self-reported measures of diet. The nitrogen isotope ratio (15N/14N; hereafter, “NIR”) is a biomarker that is elevated in many of the foods comprising the Yup'ik traditional diet because it increases up the food chain and, thus, is particularly elevated in marine mammals and fish (4). The NIR is strongly associated with the marine n–3 fatty acids EPA and DHA (17–19), and more moderately associated with traditional food intake based on food records (4). Our group has used the NIR as a proxy for dietary intake of traditional Yup'ik foods, and from these studies has demonstrated associations with improved vitamin D status (11, 20), blood pressure (17, 21, 22), blood lipid profiles (17, 21, 23), and adipokine profiles (17). These studies have built a strong evidence base supporting the critical importance of traditional foods to health in this population. However, the NIR has not been calibrated to high-quality measures of Yup'ik traditional food intake that reflect diet over the same time period as the biomarker, an aspect of its validation that is essential for data interpretation.
An additional complication of assessing dietary intake among Yup'ik people is that consumption of traditional foods may vary with seasonal availability (24). Seasonal changes in dietary intake can be difficult to measure using blood biomarkers, as they require repeated participant visits for sample collection. However, several studies have shown a strong relation between the NIR of blood and hair, with correlations exceeding 0.9 and a slope of 1 (19, 25). Hair can provide valuable information on seasonal or annual dietary shifts because it accretes dietary information over time (26–28). Thus, stable isotope analysis along hair from long-haired individuals could provide high-resolution data enabling reconstruction of seasonal diets, as it has done in other studies of ancient and modern populations (29–31). However, the extent to which the NIR varies seasonally within individuals in the Yup'ik population has not been examined.
This study had 2 aims. The first aim was to examine associations of intakes of traditional food groups and total traditional foods with the NIR in a Yup'ik study population in Southwest Alaska. To maximize the validity of our dietary measures, we conducted intensive dietary assessment via 4 weekly 24-h recalls (32–34), spanning the time of peak RBC synthesis preceding the blood draw from which the RBC NIR was measured. A second, exploratory aim was to investigate seasonal and annual patterns of traditional food intake by measuring the NIR sequentially along longer (>6 cm) hair samples from individuals in the validation study who were in the upper 2 quartiles of traditional food intake. Because human hair grows at a rate of ∼1 cm/mo (35), we expected that 6 cm would capture dietary intake over a period of roughly 6 mo.
Methods
Participant recruitment and study procedures
Data are from the Center for Alaska Native Health Research Negem Nallunailkutaa (“The Foods’ Marker”) study (36, 37). This study was approved by the University of Alaska Fairbanks Institutional Review Board and the Yukon-Kuskokwim Health Corporation Human Studies Committee and Executive Board. Between 2008 and 2010, 70 participants aged 14–79 y were recruited from 2 coastal Yup'ik communities in Southwest Alaska, here designated “A” and “B.” At entry into the study, participants completed a demographic questionnaire and the first of four 24-h-dietary recall interviews. Three more dietary interviews were conducted over the next 4 wk, as described elsewhere (36). Specimen collection was timed so that the mean age of RBCs (1.5 mo) would coincide with the midpoint of the period over which dietary interviews were conducted (38).
Collection of 24-h-dietary recall interviews, RBC, and hair samples took place in community A from November to December 2008 and in community B from March to April 2009. Blood samples were obtained from 68 of the 70 participants (97%) and hair samples were obtained from 58 of those 68 participants (85%), because hair collection was restricted to individuals with hair >2 cm in length. For the segmental analysis of hair NIR, we selected participants whose hair was >6 cm in length and who were in the upper 2 quartiles of either traditional food intake (>17% of energy) or RBC NIR (>8.85‰). This included 16 participants (28% of participants with hair samples). We also selected 2 participants with lower traditional food intake for sequential analysis, 1 male and 1 female, as a comparison. Thus, the total number of participants with sequential hair analyses was 18. Some participants elected to participate in follow-up data collection, which took place from May to June 2010 in community A and November to December 2009 in community B. If participants included in the segmental hair analysis had a hair sample collected during the follow-up study, the follow-up sample was used (11 out of 18 participants, 61%). This was done to provide an opportunity to match the NIR of the earlier-collected, proximal hair sample with its imputed location on the later-collected, segmentally analyzed hair sample, assuming a hair growth rate of 1 cm/mo.
Diet assessment
Repeated 24-h recalls are commonly used methods of total diet assessment (39) that have been shown to accurately represent “usual” intake. Four unannounced 24-h recalls were collected from each participant by certified interviewers using computer-assisted software [Nutrition Data System for Research (NDSR) software 2018; University of Minnesota], as described in detail elsewhere (36). Dietary interviews were 9 ± 5 d apart, with a minimum of 2 d between recalls. Intake of dietary components was calculated from the NDSR food and nutrient database by assigning NDSR food codes to categories of traditional foods (40). All traditional food items were assigned to 1 of the following categories of traditional foods: fish, marine mammals, terrestrial mammals, birds, greens, and berries (36).
Sample preparation
Blood samples were collected as described elsewhere (18). The RBC portion was frozen at −12°C, transported to the University of Alaska Fairbanks and placed in an ultralow freezer at −80°C.
Hair samples were collected by cutting a small pinch of hair (ca. 30–50 hairs) from the back of the head, just below the postoccipital protuberance. Samples were taped with the follicle end labeled, folded into aluminum foil strips, and stored in plastic bags for analysis. The preparation of RBC and hair samples from the first phase of data collection is described elsewhere (36, 37). Hair samples selected for sectional analysis were aligned at the cut end, stretched out on glass, and taped down with a single piece of laboratory tape parallel to and covering the strand. Hair length was marked on the tape at 0.33-cm intervals from the cut end. At each cm a 0.33 cm section of tape and underlying hair was removed using a razor blade. Hair pieces were then removed from the tape sections with forceps and cleaned with triplicate 30-min washes with sonication in 2:1 methanol chloroform to remove adhesive, natural oils, and shampoo residues, and then the samples were washed and sonicated 3 times in distilled water and oven-dried at 50°C for 24 h (41–43). The dried hair sections were placed into 11 × 8 mm tin capsules, and the capsules were crushed into balls and loaded into an auto-sampler for stable isotope analysis. The resulting sample masses ranged from 0.3 to 0.6 mg.
Stable isotope analysis
Hair and RBC samples were analyzed for their NIR at the Alaska Stable Isotope Facility at the University of Alaska Fairbanks by elemental analysis-isotope ratio mass spectrometry, as described previously (4, 19). Although this study was focused on the NIR, the carbon isotope ratio is measured concurrently during elemental analysis-isotope ratio mass spectrometry and the carbon isotope ratio of segmental hair analyses are reported in the supplemental materials (Supplemental Tables 1 and 2). Samples were analyzed as described elsewhere (25), and nitrogen and carbon isotope ratios were presented as delta (δ) values relative to atmospheric N2 and the Vienna Pee Dee Belemnite, respectively: δX = (R sample/R standard -1) × 1000‰, where X is 15N or 13C and R is the ratio of heavy to light isotope. Precision as assessed by the standard deviation of concurrently analyzed laboratory materials (peptone) was within 0.3‰ for δ15N and within 0.2‰ for δ13C values. The integrity of the stable isotope values from hair was assessed using the carbon to nitrogen (C/N) ratio. The accepted atomic C/N ratio for modern hair is 2.9–3.8 (41) and the mean atomic C/N ratio of hair analyzed for this study was 3.0 ± 0.2. For all individuals, the C/N ratio fell within the acceptable range (Supplemental Tables 1 and 2).
Statistical analyses
All statistical analyses were performed using JMP version 8 (SAS Institute). Differences in age, sex, and BMI between the study sample with RBC measurements (n = 68), the study sample with hair measurements (n = 58), and the study sample with segmental hair analyses (n = 18) were assessed using t tests. Associations among intakes of traditional food groups were assessed using Pearson product moment correlation (r), as were intakes of each traditional food group and total traditional foods with the NIR of RBC and hair. The relation between total traditional food intake and NIR of RBCs and hair was described using linear regression, both unadjusted and adjusted for age, sex, and BMI. We also evaluated the associations of the NIR and total traditional food intake with age, sex, BMI, and community using multiple linear regression models. For the participants with segmental analyses of hair NIR we used the intra-individual range of NIR among all hair sections as a proxy for the seasonality of a participant's traditional food intake. Means are presented ± SDs, β coefficients are given with upper and lower 95% CI and a significance level of α = 0.05 was used throughout the analyses.
Results
Study sample characteristics
The demographic characteristics and stable isotope ratios for the study samples with RBC analyses (n = 68), hair analyses (n = 58) and hair segmental analyses (n = 18), are shown in Table 1. Participant characteristics and the RBC NIR did not differ between the overall study sample and the subset with hair analyses; however, participants selected for hair segmental analysis were predominantly female, older, had higher BMI, and higher RBC and hair NIR (Table 1).
TABLE 1.
Demographic characteristics and biomarker measurements by study sample1
| Complete sample | Hair subset | Segmental hair subset | |
|---|---|---|---|
| n | 68 | 58 | 18 |
| Sex, % female | 49 | 52 | 892 |
| Age, y | 41 ± 18 | 41 ± 17 | 49 ± 122 |
| 14–19, % | 16 | 12 | 0 |
| 20–39, % | 32 | 34 | 28 |
| 40–59, % | 40 | 41 | 61 |
| ≥60, % | 12 | 12 | 11 |
| BMI, kg/m2 | 27.2 ± 6.3 | 27.4 ± 6.3 | 30.8 ± 6.92 |
| <18.5, % | 1 | 0 | 0 |
| ≥18.5 to ≤25, % | 44 | 41 | 22 |
| >25 to ≤30, % | 24 | 29 | 11 |
| >30 kg/m2, % | 31 | 29 | 67 |
| Stable isotope ratios | |||
| RBC NIR, ‰ | 9.3 ± 1.8 (6.3, 13.5) | 9.3 ± 1.8 (6.3, 13.5) | 10.4 ± 1.62 (7.8, 13.5) |
| Hair NIR, ‰ | — | 10.8 ± 2.0 (6.9, 15.2) | 12.0 ± 1.92 (8.2, 15.2) |
Values are percentages, mean ± SD, or ranges (min, max). NIR, nitrogen isotope ratio, expressed as δ15N values.
Differs from complete sample and hair subset, P < 0.05 (t test or chi-square test).
Characteristics of traditional food intake
Total intake of traditional foods varied from 0 to 65% of energy (20 ± 18%) and fish and marine mammals were the largest contributors at 50% and 30% of traditional food energy, respectively (Table 2). Intakes of fish and marine mammals were strongly associated with each other (r = 0.70, P < 0.0001), and with intake of berries (r = 0.51 and 0.47, respectively, both P < 0.0001). Intakes of fish and marine mammals were also associated with intake of greens (r = 0.31, P = 0.0089 and r = 0.29, P = 0.015, respectively).
TABLE 2.
Associations of Yup'ik traditional food intakes with the NIR of RBCs (n = 68) and hair (n = 58)1
| RBC NIR | Hair NIR | ||||
|---|---|---|---|---|---|
| Dietary variable | % of energy | r 2 (95% CI) | P | r 2 (95% CI) | P |
| Fish | 10 ± 10 | 0.65 (0.48, 0.77) | <0.0001 | 0.67 (0.50, 0.79) | <0.0001 |
| Marine mammals | 6 ± 8 | 0.74 (0.60, 0.83) | <0.0001 | 0.65 (0.47, 0.78) | <0.0001 |
| Land mammals | 2 ± 3 | 0.21 (−0.03, 0.43) | 0.08 | 0.23 (−0.03, 0.46) | 0.08 |
| Birds | 1 ± 3 | 0.21 (−0.03, 0.43) | 0.08 | 0.13 (−0.13, 0.38) | 0.32 |
| Greens | 0.1 ± 0.3 | 0.36 (0.14, 0.55) | 0.002 | 0.30 (0.05, 0.52) | 0.02 |
| Berries | 0.4 ± 0.8 | 0.57 (0.39, 0.71) | <0.0001 | 0.57 (0.36, 0.72) | <0.0001 |
| Total traditional foods | 20 ± 18 | 0.78 (0.66, 0.86) | <0.0001 | 0.75 (0.61, 0.85) | <0.0001 |
NIR, nitrogen isotope ratio, expressed as δ15N values.
Pearson's product moment correlation.
Biomarker associations with traditional food intake
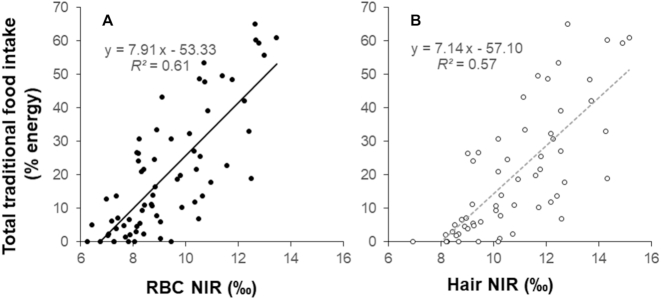
Dietary intakes of fish, marine mammals, greens, and berries were significantly associated with the NIR of RBCs and hair (Table 2). The associations of land mammal and bird intakes with the NIR of RBCs were marginally nonsignificant (both P = 0.08, Table 2) and there was a marginally nonsignificant association of land mammal intake with the hair NIR (P = 0.08, Table 2). Total traditional food intake was strongly and linearly associated with the NIR in RBCs and hair (R2 = 0.61 and 0.57, respectively, both P < 0.0001), and each 1‰ increase in the NIR corresponded to an increase of 7–8% of energy from total traditional foods [βRBC-NIR = 7.9 (6.3, 9.5), βhair-NIR = 7.1 (5.5, 8.8), Figure 1]. Sex and BMI were not significant when included as covariates, whereas age was marginally nonsignificant (P = 0.052 for RBC NIR, P = 0.09 for hair NIR).
FIGURE 1.
Associations of total Yup'ik traditional food intake (% energy) with (A) RBC NIR, n = 68, and (B) hair NIR, n = 58. P < 0.0001 for both. NIR, nitrogen isotope ratio, expressed as δ15N values
The RBC NIR, hair NIR, and total traditional food intake were all strongly associated with age [βRBC-NIR = 0.08‰/y (0.06, 0.09), βhair-NIR = 0.09‰/y (0.07, 0.11), and βtotal-traditional = 0.75%/y (0.6, 0.9), respectively, all P < 0.0001], but not with sex, BMI, or community (A or B) (all P > 0.1).
Seasonality of traditional food intake
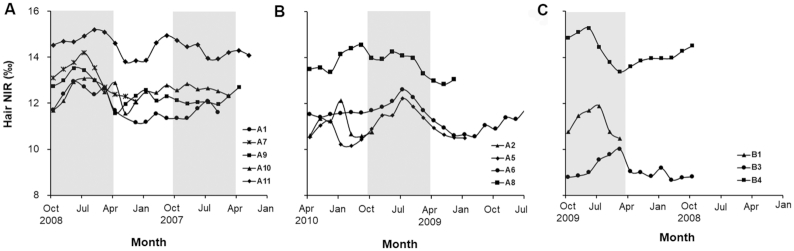
The intraindividual mean and range of NIR from sequential hair segments from 18 individuals with hair >6 cm in length are summarized in Table 3. All original data are presented in Supplemental Tables 1 and 2. Within individuals, NIR ranges varied from 0.26‰ to 2.20‰ (mean = 1.45 ± 0.61‰). NIR tended to peak during the summer months among participants whose NIR was variable (range ≥1.4‰; Figure 2). This resulted in some, although not perfect, synchronicity among individuals from a given sampling location and date (Figure 2). There were 4 individuals who participated in both first and second hair collections and who had long enough hair that the first hair collection time was reflected in the second, sequentially analyzed hair sample (>18 cm for community A and >8 cm for community B, based on sampling dates). The mean difference in hair NIR between those time points for those 4 individuals was 0.5 ± 0.3‰.
TABLE 3.
Range of NIR of sequential hair segments from participants in the upper 2 quartiles of traditional food intake with hair >6 cm long (n = 18)1
| Individual | Community | Visit | Sex | Age, y | n 2 | NIR, ‰3 | NIR range, ‰ |
|---|---|---|---|---|---|---|---|
| A1 | A | 1 | F | 39 | 16 | 11.9 ± 0.6 | 1.8 (11.2–13.0) |
| A2 | A | 2 | F | 39 | 7 | 11.1 ± 0.6 | 1.5 (10.6–12.1) |
| A3 | A | 2 | M | 58 | 8 | 11.5 ± 0.2 | 0.6 (11.1–11.7) |
| A44 | A | 2 | M | 37 | 7 | 10.5 ± 0.3 | 0.8 (10.1–10.9) |
| A5 | A | 2 | F | 43 | 16 | 11.0 ± 0.6 | 2.1 (12.1–14.2) |
| A6 | A | 2 | F | 45 | 56 | 11.6 ± 0.5 | 2 (10.6–12.6) |
| A7 | A | 1 | F | 56 | 9 | 13.1 ± 0.7 | 2.1 (12.1–14.2) |
| A8 | A | 2 | F | 55 | 22 | 13.4 ± 0.6 | 2.2 (12.3–14.5) |
| A9 | A | 1 | F | 39 | 19 | 12.4 ± 0.5 | 2 (11.5–13.5) |
| A10 | A | 1 | F | 50 | 17 | 12.5 ± 0.4 | 1.5 (11.6–13.1) |
| A11 | A | 1 | F | 57 | 21 | 14.4 ± 0.4 | 1.4 (13.8–15.2) |
| A12 | A | 2 | F | 72 | 8 | 15.0 ± 0.3 | 0.8 (14.6–15.4) |
| B1 | B | 2 | F | 40 | 6 | 11.2 ± 0.6 | 1.4 (10.5–11.9) |
| B2 | B | 1 | F | 55 | 13 | 10.9 ± 0.3 | 0.8 (10.5–11.3) |
| B34 | B | 2 | F | 22 | 23 | 9.0 ± 0.4 | 2 (8.0–10.0) |
| B4 | B | 2 | F | 71 | 13 | 14.2 ± 0.6 | 1.9 (13.4–15.5) |
| B5 | B | 1 | F | 56 | 5 | 14.0 ± 0.1 | 0.3 (14.4–14.7) |
| B6 | B | 1 | F | 50 | 11 | 11.4 ± 0.3 | 1 (10.9–11.9) |
NIR, nitrogen isotope ratio, expressed as δ15N values.
n = number of hair segments analyzed.
Values are means ± SDs.
Participants from the lower 2 quartiles of traditional food intake, included for comparison.
FIGURE 2.
NIR of sequential hair segments collected at 2 locations: community A visit 1 (A) and visit 2 (B) and community B visit 2 (C). Only participants with ranges in sequential hair NIR ≥1.4‰ are shown. Community B visit 1 is omitted because no participants had ranges ≥1.4‰. The “summer” months (Apr–Oct) are marked with a gray vertical bar. NIR, nitrogen isotope ratio, expressed as δ15N values.
Discussion
This study explores how groups of Yup'ik traditional foods are associated with the NIR of RBCs and hair and calibrates these biomarkers to total traditional food intake, using intake data from four 24-h recalls collected over the period of RBC and hair synthesis. Although the NIRs of RBCs and hair have been evaluated previously as biomarkers of marine food intake for this population using RBC EPA and DHA as reference measures (18, 19), their relations to specific groups of traditional foods or to total traditional foods had not been very precisely characterized. Most groups of Yup'ik traditional foods were associated with the NIR of RBCs and hair, and each 1‰ increase in the NIR corresponded to an increase of ∼8% of energy from total traditional foods. In an exploratory analysis, we showed that NIR analyzed along sequential segments of hair reflected seasonality of traditional food intake in some individuals. The maximum observed fluctuation in NIR was 2.2‰, with peaks occurring during the summer months. Seasonal patterns of traditional food intake are important to consider when designing health studies in the region.
A previous analysis of Yup'ik traditional foods showed that NIR values were high in marine mammals, moderately high to high in both marine and freshwater fish, moderately high in birds (cranes, ducks, geese, swans), and low in terrestrial herbivores (moose, caribou, muskoxen) and plants (berries and greens) (4). In the present study, intakes of fish and marine mammals were strongly associated with the NIR of RBCs and hair, whereas intakes of land mammals and birds had more modest associations that did not reach statistical significance. Surprisingly, intakes of greens and berries were also associated with the NIR of RBCs and hair, despite those foods having low NIR. These associations are likely to be the result of a dietary pattern in our study, in which intakes of berries and greens were associated with intakes of fish and marine mammals. Previous studies in a Yup'ik population have differentiated 3 dietary patterns, termed “subsistence foods” (here termed “traditional foods”), “fruits and vegetables,” and “processed foods” based on data from FFQ (12, 44). In those studies, intakes of seal/walrus soup, non-oily fish, greens, and bird soup were the factors that best predicted the “subsistence foods” dietary pattern, supporting our biomarker associations with “greens.” Further studies of the Yup'ik traditional dietary pattern are warranted.
The cohort recruited for this study exhibited a wide range of traditional food intake, ranging from 0% to 65% of energy, which was ideal for evaluating linear models of traditional food intake based on the NIR. As has been previously described for this population, both reported % energy from traditional foods and the NIR were strongly associated with age, as elders tend to consume a much more traditional diet than youth (2, 4, 45, 46). However, there was no association of the NIR with either sex or community, in contrast to previous findings (4). This discrepancy may be attributable to the smaller number of participants in the present study. Coefficients of biomarker–diet relations were very similar for RBCs and hair, supporting the use of either sample type as determined by study suitability. This finding is consistent with previous studies showing a very strong association between the NIR measured in RBCs and hair (19, 25), suggesting that the measurements are essentially interchangeable after a small mathematical correction.
Sequential measurement of NIR in segments of long hair samples showed modest temporal variation of traditional food intake in most individuals, with 61% of the selected individuals having ranges equal to or exceeding 1.4‰, which is more than double that expected from analytical error. The maximum variation of 2.2‰ would correspond to changes in % energy intake from traditional foods of up to ∼18%. These variations appeared to peak roughly during the summer months, indicating the potential for the NIR to indicate seasonality in dietary intake among Yup'ik people. The seasonal nature of subsistence activities by Yup'ik people has been well described (24, 47, 48), including seasonal harvest of marine mammals, land mammals, birds, berries, greens, and many fish species. Fish runs occur throughout the spring and summer, starting with herring and followed by salmon (king, red, chum, pink, and silver). The seasonal availability of salmon is likely a major contributor to the fluctuations observed in hair NIR. Overall, however, seasonal shifts in the NIR within individuals were relatively modest compared to differences in NIR among individuals, suggesting that seasonality of diet does not preclude using the NIR as an index of traditional food intake in studies that collect measurements at varying times of year.
Some individuals consuming large amounts of traditional food showed little to no seasonal fluctuation in NIR. During salmon harvest, large numbers of fish are dried and smoked for long-term storage, which may dampen seasonal variation in intake in very traditional-living individuals. There are also fish that are harvested through the ice during the winter months, including Dolly Varden, smelt, pike, lushfish, whitefish, and blackfish. Conversely, 1 of the individuals with low traditional food intake showed surprisingly pronounced variability in hair NIR. This individual may be taking advantage of traditional foods only when seasonally abundant, or may be having subsistence foods shared with them. Fall (1990) described food sharing in a Yup'ik community, noting that a relatively small percentage of the households harvested most of the subsistence resources during summer and shared a large amount of these harvests with other households (24). This observation underscores the cultural and health importance of traditional foods to these communities, even for individuals with relatively low traditional food consumption overall. Further study of dietary seasonality in low consumers of traditional foods is warranted.
A limitation of this study is that intakes of traditional food groups and total traditional foods were evaluated against self-reported measures, which are known to have substantial error and bias. Although little is known about the factors contributing to reporting bias in this population, bias is commonly observed by sex, BMI, or ethnicity (49, 50), which were not associated with NIR in this study. Thus, bias cannot explain the strong diet-biomarker associations reported here. Furthermore, repeated 24-h recalls are among the least biased and underreported of the self-reported measures of “usual” intake (32, 34). Relatively few males had hair >6 cm in length; thus, our characterization of seasonality in traditional food consumers was limited to a small number of individuals (n = 18), and females were greatly overrepresented (89%). The study also had some unique strengths. Although the study population was not large it captured a wide range of dietary behaviors, representing diets with almost no traditional food intake and diets with extremely high traditional food intake. This was ideal for evaluating a biomarker of traditional food intake and its pattern of seasonality.
In summary, we found that the NIR of RBCs and hair was strongly associated with several groups of Yup'ik traditional foods, as well as total traditional food intake, with a 1‰ increase in NIR corresponding to an increase of 7–8% of calories from Yup'ik traditional foods. In an exploratory analysis, we found that the NIR of sequentially measured segments of hair reflected seasonal variation in traditional food intake, peaking roughly during the summer months. However, the magnitude of seasonal variations in traditional food intake demonstrated in our sample was relatively modest compared to differences in traditional food intake between individuals. Our study shows that the NIR is a useful and potentially noninvasive biomarker for assessing traditional food intake, with the potential for assessing seasonal and annual patterns of traditional food intake.
Supplementary Material
Acknowledgments
We thank Eliza Orr for sharing her knowledge of Yup'ik traditional foods and culture and Marjorie Richards for laboratory assistance. We thank Tim Howe at the Alaska Stable Isotope Facility for assistance with the stable isotope analyses. The authors’ responsibilities were as follows—DMO, SHN, and AB: designed research; KC, SHN, SEH, BBB, and DMO: conducted research; KC, SHN, CH, and DMO: analyzed data; KC and DMO: wrote the paper; DMO: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported by the National Center for Research Resources and the National Institute of General Medical Sciences of the NIH through grant nos. P20RR016430 and P30GM103325.
Author disclosures: KC, SHN, CH, AB, SEH, BBB, and DMO, no conflicts of interest.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Research Resources or the NIH.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: C/N ratio, carbon to nitrogen ratio; NDSR, Nutrition Data System for Research; NIR, nitrogen isotope ratio.
References
- 1. Kuhnlein HV, Soueida R, Receveur O. Dietary nutrient profiles of Canadian Baffin Island Inuit differ by food source, season, and age. J Am Diet Assoc. 1996;96:155–62. [DOI] [PubMed] [Google Scholar]
- 2. Bersamin A, Zidenberg-Cherr S, Stern JS, Luick BR. Nutrient intakes are associated with adherence to a traditional diet among Yup'ik Eskimos living in remote Alaska Native communities: the CANHR study. Int J Circumpolar Health. 2007;66:62–70. [DOI] [PubMed] [Google Scholar]
- 3. Wilkinson MJ, Yai Y, O'Brien DM. Age-related variation in red blood cell stable isotope ratios (δ13C and δ15N) from two Yup'ik villages in Southwest Alaska: a pilot study. Int J Circumpolar Health. 2007;66:31–41. [DOI] [PubMed] [Google Scholar]
- 4. Nash SH, Bersamin A, Kristal AR, Hopkins SE, Church RS, Pasker RL, Luick BR, Mohatt GV, Boyer BB, O'Brien DM. Stable nitrogen and carbon isotope ratios indicate traditional and market food intake in an indigenous circumpolar population. J Nutr. 2012;142:84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luick B, Bersamin A, Stern JS. Locally harvested foods support serum 25-hydroxyvitamin D sufficiency in an indigenous population of Western Alaska. Int J Circumpolar Health. 2014;73 doi:10.3402/ijch.v73.22732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson JS, Nobmann ED, Asay E, Lanier AP. Dietary intake of Alaska Native people in two regions and implications for health: the Alaska Native Dietary and Subsistence Food Assessment Project. Int J Circumpolar Health. 2009;68:109–22. [DOI] [PubMed] [Google Scholar]
- 7. Fall JA. Regional patterns of fish and wildlife harvests in contemporary Alaska. Arctic. 2016;69(1):47–64. [Google Scholar]
- 8. Fall JA, Braem NS, Brown CL, Hutchinson-Scarbrough LB, Koster DS, Krieg TM. Continuity and change in subsistence harvests in five Bering Sea communities: Akutan, Emmonak, Savoonga, St. Paul, and Togiak. Deep Sea Res Part II. 2013;94:274–91. [Google Scholar]
- 9. Maurice AC, Philip J, Bersamin A. Yup'ik identity and socioeconomic status are associated with child consumption of traditional food and weight in rural Yup'ik communities. Ethn Health. 2019;24:312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walch A, Loring P, Johnson R, Tholl M, Bersamin A. Traditional food practices, attitudes, and beliefs in urban Alaska Native women receiving WIC assistance. J Nutr Educ Behav. 2019;51:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Brien DM, Thummel KE, Bulkow LR, Wang Z, Corbin B, Klejka J, Hopkins SE, Boyer BB, Hennessy TW, Singleton R. Declines in traditional marine food intake and vitamin D levels from the 1960s to present in young Alaska Native women. Public Health Nutr. 2017;20:1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ryman TK, Boyer BB, Hopkins S, Philip J, O'Brien D, Thummel K, Austin MA. Characterising the reproducibility and reliability of dietary patterns among Yup'ik Alaska Native people. Br J Nutr. 2015;113:634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nobmann ED, Byers T, Lanier AP, Hankin JH, Jackson MY. The diet of Alaska Native adults: 1987–1988. Am J Clin Nutr. 1992;55:1024–32. [DOI] [PubMed] [Google Scholar]
- 14. Ballew C, Tzilkowski AR, Hamrick K, Nobmann ED. The contribution of subsistence foods to the total diet of Alaska Natives in 13 rural communities. Ecol Food Nutr. 2006;45:1–26. [Google Scholar]
- 15. Prentice RL. Use of intake biomarkers in nutritional epidemiology. In: Schoeller D, Westerterp-Plantenga Meditors. Advances in the Assessment of Dietary Intake. Boca Raton (FL): CRC Press, Taylor & Francis, 2017;221–34. [Google Scholar]
- 16. Prentice RL, Mossavar-Rahmani Y, Huang Y, Van Horn L, Beresford SA, Caan B, Tinker L, Schoeller D, Bingham S, Eaton CB et al.. Evaluation and comparison of food records, recalls, and frequencies for energy and protein assessment by using recovery biomarkers. Am J Epidemiol. 2011;174:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Brien DM, Kristal AR, Nash SH, Hopkins SE, Luick BR, Stanhope KL, Havel PJ, Boyer BB. A stable isotope biomarker of marine food intake captures associations between n-3 fatty acid intake and chronic disease risk in a Yup'ik study population, and detects new associations with blood pressure and adiponectin. J Nutr. 2014;144:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Brien DM, Kristal AR, Jeannet MA, Wilkinson MJ, Bersamin A, Luick B. Red blood cell d15N: a novel biomarker of dietary eicosapentaenoic acid and docosahexaenoic acid intake. Am J Clin Nutr. 2009;89:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nash SH, Kristal AR, Boyer BB, King IB, Metzgar JS, O'Brien DM. Relation between stable isotope ratios in human red blood cells and hair: implications for using the nitrogen isotope ratio of hair as a biomarker of eicosapentaenoic acid and docosahexaenoic acid. Am J Clin Nutr. 2009;90:1642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fohner AE, Wang Z, Yracheta J, O'Brien DM, Hopkins SE, Black J, Philip J, Wiener HW, Tiwari HK, Stapleton PL et al.. Genetics, diet, and season are associated with serum 25-hydroxycholecalciferol concentration in a Yup'ik study population from Southwestern Alaska. J Nutr. 2015;146(2):318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Eilat-Adar S, Mete M, Nobmann ED, Xu J, Fabsitz RR, Ebbesson SO, Howard BV. Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr. 2009;139:2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beaulieu-Jones BR, O'Brien DM, Hopkins SE, Moore JH, Boyer BB, Gilbert-Diamond D. Sex, adiposity, and hypertension status modify the inverse effect of marine food intake on blood pressure in Alaska native (Yup'ik) people. J Nutr. 2015;145:931–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, Boyer B, Mohatt GV. Associations of very high intakes of eicosapentaenoic and docosahexaenoic acids with biomarkers of chronic disease risk among Yup'ik Eskimos. Am J Clin Nutr. 2010;91:777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fall JA. The Division of Subsistence of the Alaska Department of Fish and Game: an overview of its research program and findings:1980–1990. Arctic Anthropol. 1990;27:68–92. [Google Scholar]
- 25. Nash SH, Kristal AR, Hopkins SE, Boyer BB, O'Brien DM. Stable isotope models of sugar intake using hair, red blood cells, and plasma, but not fasting plasma glucose, predict sugar intake in a Yup'ik study population. J Nutr. 2014;144:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thompson AH, Chesson LA, Podlesak DW, Bowen GJ, Cerling TE, Ehleringer JR. Stable isotope analysis of modern human hair collected from Asia (China, India, Mongolia, and Pakistan). Am J Phys Anthropol. 2010;141:440–51. [DOI] [PubMed] [Google Scholar]
- 27. Valenzuela LO, Chesson LA, O'Grady SP, Cerling TE, Ehleringer JR. Spatial distributions of carbon, nitrogen and sulfur isotope ratios in human hair across the central United States. Rapid Commun Mass Spectrom. 2011;25:861–8. [DOI] [PubMed] [Google Scholar]
- 28. Valenzuela LO, Chesson LA, Bowen GJ, Cerling TE, Ehleringer JR. Dietary heterogeneity among Western Industrialized countries reflected in the stable isotope ratios of human hair. PLoS One. 2012;7:e34234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Britton K, Knecht R, Nehlich O, Hillerdal C, Davis RS, Richards MP. Maritime adaptations and dietary variation in prehistoric Western Alaska: stable isotope analysis of permafrost-preserved human hair. Am J Phys Anthropol. 2013;151:448–61. [DOI] [PubMed] [Google Scholar]
- 30. Williams JS, Katzenberg MA. Seasonal fluctuations in diet and death during the late horizon: a stable isotopic analysis of hair and nail from the central coast of Peru. J Archaeolog Sci. 2012;39:41–57. [Google Scholar]
- 31. Webb EC, White CD, Uum SV, Longstaffe FJ. Integrating cortisol and isotopic analyses of archaeological hair: reconstructing individual experiences of health and stress. Am J Phys Anthropol. 2015;156:577–94. [DOI] [PubMed] [Google Scholar]
- 32. Thompson FE, Kirkpatrick SI, Krebs-Smith SM, Reedy J, Schap TE, Subar AF, Wilson MM. The National Cancer Institute's dietary assessment primer: a resource for diet research. J Acad Nutr Diet. 2015;115:1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, Schneider KL, Merriam PA, Hebert JR. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19:553–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R et al.. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN Study. Am J Epidemiol. 2003;158:1–13. [DOI] [PubMed] [Google Scholar]
- 35. Harding H, Rogers G. Physiology and growth of hair. In: Robertson J.eds. Forensic examination of hair. London: Taylor & Francis; 1999;1–77. [Google Scholar]
- 36. Nash SH, Kristal AR, Bersamin A, Hopkins SE, Boyer BB, O'Brien DM. Carbon and nitrogen stable isotope ratios predict intake of sweeteners in a Yup'ik study population. J Nutr. 2013;143:161–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choy K, Nash SH, Kristal AR, Hopkins SE, Boyer BB, O'Brien DM. The carbon isotope ratio of alanine in red blood cells is a new candidate biomarker of sugar-sweetened beverage intake. J Nutr. 2013;143:878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cohen RM, Franco RS, Khera PK, Smith EP, Lindsell CJ, Ciraolo PJ, Palascak MB, Joiner CH. Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c. Blood. 2008;112:4284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Subar AF, Freedman LS, Tooze JA, Kirkpatrick SI, Boushey C, Neuhouser ML, Thompson FE, Potischman N, Guenther PM, Tarasuk V et al.. Addressing current criticism regarding the value of self-report dietary data. J Nutr. 2015;145:2639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schakel SF. Maintaining a nutrient database in a changing marketplace: keeping pace with changing food products: a research perspective. J Food Compos Anal. 2001;14:315–22. [Google Scholar]
- 41. O'Connell TC, Hedges REM. Isotopic comparison of hair and bone: archaeological analyses. J Archaeolog Sci. 1999;26:661–5. [Google Scholar]
- 42. O'Connell TC, Hedges REM, Healey MA, Simpson AHRW. Isotopic comparison of hair, nail and bone: modern analyses. J Archaeolog Sci. 2001;28:1247–55. [Google Scholar]
- 43. Hedges REM, Thompson JMA, Hull BD. Stable isotope variation in wool as a means to establish Turkish carpet provenance. Rapid Commun Mass Spectrom. 2005;19:3187–91. [DOI] [PubMed] [Google Scholar]
- 44. Ryman TK, Austin MA, Hopkins S, Philip J, O'Brien D, Thummel K, Boyer BB. Using exploratory factor analysis of FFQ data to identify dietary patterns among Yup'ik people. Public Health Nutr. 2014;17:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bersamin A, Luick BR, Ruppert E, Stern JS, Zidenberg-Cherr S. Diet quality among Yup'ik Eskimos living in rural communities is low: the Center for Alaska Native Health Research Pilot Study. J Am Diet Assoc. 2006;106:1055–63. [DOI] [PubMed] [Google Scholar]
- 46. Bersamin A, Luick BR, King IB, Stern JS, Zidenberg-Cherr S. Westernizing diets influence fat intake, red blood cell fatty acid composition, and health in remote Alaskan Native Communities in the Center for Alaska Native Health. J Am Diet Assoc. 2008;108:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barker JH, Barker R. Always getting ready, upterrlainarluta: Yup'ik Eskimo subsistence in southwest Alaska. Seattle: University of Washington Press. [Google Scholar]
- 48. Burch ES. The Inupiaq Eskimo nations of northwest Alaska. Fairbanks: University of Alaska Press. [Google Scholar]
- 49. Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. 2003;133:895–920. [DOI] [PubMed] [Google Scholar]
- 50. Macdiarmid J, Blundell J. Assessing dietary intake: who, what and why of under-reporting. Nutr Res Rev. 1998;11:231–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.