Abstract
Blood biomarkers provide critical information about the health of older populations, especially in large developing countries where self-reports of health are often inaccurate due to lack of access to health care. However, it is very difficult to collect blood samples in representative population surveys in such countries. The China Health and Retirement Longitudinal Study (CHARLS), a nationally representative study of middle-aged and older Chinese, represents one of the first efforts to include blood biomarkers in a nationally representative survey of China. In the 2015 wave of CHARLS, 13,013 respondents located in 150 counties around China donated whole blood, which was assayed on a range of indicators. Here we describe the process of the sample collection, transportation, storage, and analysis and present basic statistics.
Keywords: aging, biomarkers, CHARLS, study design
By 2050, a little more than 1 in 4 Chinese will be 65 years or older, a population totaling more than 366 million (1). To address population aging in China, the China Health and Retirement Longitudinal Study (CHARLS) has been developed to provide high-quality public-use data to enable research on issues related to aging and provide a basis for interventions to promote the health of older adults in China. CHARLS is part of a group of aging studies around the world that are increasingly harmonized to the Health and Retirement Study (HRS) in the United States.
CHARLS is a multipurpose population-based survey that evaluates numerous health indicators. Blood-based biomarkers provide valuable indicators of health for 2 reasons. First, these biomarkers offer objective health information, which may not be known to respondents. As a developing country, a significant proportion of older Chinese do not have access to regular, high-quality health care, resulting in limited awareness of diseases, which varies by socioeconomic status and urban-rural residency (2, 3). Second, biological measures offer information on pathophysiological states prior to clinically diagnosed diseases, disability, and mortality. Examining biomarkers within a large representative sample with a wealth of individual social, economic, behavioral, psychological, and other self-reported health and physical performance data will provide a resource to uncover mechanisms or pathways through which social and environmental factors “get under the skin” to influence downstream health outcomes (4, 5). Therefore, collection of venous blood has been included in a growing number of population-based aging surveys, including the HRS, the English Longitudinal Study of Ageing, the Mexican Health and Aging Study, the Irish Longitudinal Study on Ageing, the Northern Ireland Cohort for the Longitudinal Study of Ageing, and the Brazilian Longitudinal Study of Aging. However, in a large and diverse country such as China, collecting venous blood in a randomly selected representative population survey can be highly challenging because venous blood samples have to be processed within a relatively short period of time and transported in a controlled temperature environment. Nevertheless, CHARLS successfully collected and assayed venous blood samples in both the national baseline wave in 2011 and the 2015 follow-up wave. In this paper, we describe the field procedures for the 2015 wave. In doing this, we provide details on how the samples were collected and analyzed that should give the users of our data confidence in its quality. In addition, our experience can perhaps be useful for population surveys in other countries.
METHODS
The study sample
CHARLS is a nationally representative survey of the middle-aged and elderly in China, run by the National School for Development (China Center for Economic Research) together with the Institute for Social Science Survey at Peking University. The study is directed by Yaohui Zhao, John Strauss, and Gonghuan Yang, with a multidisciplinary research team composed of economists, epidemiologists, and public health/medical professionals. Decisions on the use of stored specimens are made by the principal investigator team following advice from our public health/medical professionals. CHARLS has a scientific advisory committee led by James Smith, consisting mainly of principal investigators of major international HRS-family studies.
The CHARLS sample consists of approximately 10,000 households with close to 20,000 individual respondents aged 45 years or older. CHARLS began with a pilot study in 2 provinces in 2008 (6). The national baseline survey was conducted in 2011–2012, with follow-up waves in 2013, 2015, and 2018. The CHARLS national sample covered 150 counties/districts and 450 villages/urban communities within these counties. The multistage sample was drawn at each stage based on probability-proportional-to-size random-sampling procedures. Respondents were randomly chosen from a sampling frame reflecting a census of all dwelling units within a village/community, derived from an electronic mapping software using a Google Earth map designed by the CHARLS team. Within a dwelling unit containing at least 1 individual 45 years or older, a main respondent was randomly chosen. The final sample also includes the respondent’s spouse regardless of age. At the baseline wave in 2011–2012, 17,708 individuals in 10,257 households were surveyed, all face to face. Key design elements of the CHARLS project are discussed elsewhere (7, 8) and on the study’s website (http://charls.pku.edu.cn/en). Data, including the blood data, are also available publicly online at that website.
CHARLS is harmonized to the HRS and sister surveys in the HRS family, such as the English Longitudinal Study of Ageing; the Survey of Health, Ageing and Retirement in Europe (SHARE); and the Longitudinal Aging Study in India. Similar to these studies, core CHARLS questionnaires include sections on demographics, family structure and transfers, health status and functioning, lifestyle and health-related behaviors (smoking, drinking, physical activities), health care and insurance, work, retirement and pension, income and consumption, and assets (individual and household). Four of the blood-based markers—total cholesterol, high-density lipoprotein (HDL) cholesterol, high-sensitivity C-reactive protein (hsCRP), and glycated hemoglobin (HbA1C)—are being harmonized with the other HRS studies by comparing assays done across labs and adjusting for the effects of collection, handling, and freezing protocols.
Blood assay data
CHARLS collected dried blood spots in the pilot survey of 2 provinces in 2008 but switched to venous blood in the national baseline survey in 2011, when we collected blood samples for 11,847 individuals (7). Due to fieldwork difficulties, blood collection lagged behind the survey by half a year on average, and the temperature for some batches of blood rose above freezing during shipment from study sites to the central storage location.
Based on lessons learned during the first wave, the CHARLS team redesigned procedures for collecting, transporting, and assaying the blood samples in the third wave of CHARLS in 2015, so that blood collection occurred simultaneously with the interviews, and the shipping temperature was strictly controlled and monitored. As a result, the quality of the samples and results were significantly improved. In wave 3, 20,284 respondents were interviewed and asked to consent to a venous blood draw; 13,013 provided venous blood. The number of interviews was higher than the baseline wave due to 2 factors: first, CHARLS recontacted those who did not respond in the baseline wave; second, younger cohorts could “age” into the sample, making the total sample representative of those aged 45 or older.
Analysis of blood samples took place in 2 stages. A complete blood count (CBC) analysis was done at local county health centers right after collection (this included hemoglobin, hematocrit, white blood cell count, platelet count, and mean corpuscular volume). The samples were then shipped to the study headquarters where they were assayed for hsCRP, HbA1C, total cholesterol, HDL cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, glucose, blood urea nitrogen, creatinine, uric acid, and cystatin C.
Protocol for blood collection
CHARLS organized fieldwork by teams of students who were recruited and trained in Beijing. Each team had 8–10 students and was responsible for 6 villages/communities in 2 counties. Each student had a role to play in the team. Two students went to a village/community ahead of the rest of the team and were responsible for finding a central location for physical examinations/blood collection. They also contacted local community hospitals or clinics to hire nurses to draw blood, negotiated the use of a centrifuge for the separation of blood into plasma and buffy coat, and located a freezer to store the specimens before they were shipped to Beijing.
Each team had at least 2 medical students. One was responsible for organizing physical measurements. The other was responsible for blood collection, working with local nurses; this student was trained on how to put respondent identification labels on the blood tubes to prevent mix-up of specimens, both at the blood collection stage and at the centrifuging stage. This student was also responsible for storing the specimens and preparing the specimens for shipment. Most team members were interviewers. They were responsible for locating the respondents, doing the interviews, and informing respondents of the time and location of physical examinations and blood collection.
Respondents were asked to fast overnight and come to the blood collection location in the morning. If the respondents were too sick to travel, blood collection took place in their homes. When respondents arrived at the blood collection site, the first step was to verify their identity. Respondents were asked to bring their national identity cards, which indicate name, sex, and birth date. In addition, respondents’ photos, previously taken by CHARLS interviewers during waves 1 and 2, were used for verifying identity. After the verification, blood was drawn and the respondent was given food before proceeding to other measurements. Most respondents fasted (85%), but blood was collected even if they had not fasted, and we noted their status.
A team stayed in a village or community for about 5 days, so respondents had multiple opportunities for blood collection. Blood samples were sent to the local laboratory within 2 hours of collection, using protocols that varied by tube.
Three tubes of venous blood were collected from each respondent using a standard protocol and ethylenediaminetetraacetic acid–K2 anticoagulant vacuum tubes. The first tube of blood, a 2-mL tube, was used for CBC tests measured on automated analyzers available at local county hospitals or town health centers. This tube was transported at ambient temperature to the lab. Second, a 6-mL tube of whole blood was collected to obtain plasma and buffy coat, which contains predominantly white blood cells. The venous blood was kept under 4°C until the specimens were centrifuged at local hospitals or health centers. After the venous blood was separated, plasma from each participant was stored in two 1-mL cryovials, with the buffy coat in a separate cryovial. These cryovials were immediately stored frozen at −20°C in local hospitals until they were picked up by a cold-chain shipping company. Finally, one 2-mL tube was collected for the HbA1C assay. This 2-mL tube of whole blood was stored at 4°C in local hospitals until the tubes were picked by the cold-chain shipping company.
Transportation, storage, and assays
Transportation was provided by KingMed Diagnostics, located in Tianjin, a city neighboring Beijing. The company’s main business is to provide blood testing services to hospitals around China, and thus it maintains a large network for cold-chain shipping of biological specimens.
After finishing fieldwork in a county (containing 3 villages/communities), plasma, buffy coat, and whole blood for HbA1C, which had been stored in local hospitals, were collected by the cold-chain company within 14 days after the first collection day and shipped with dry ice. A temperature monitor, which measured temperature every 5 minutes, was included with each shipment, except one (which had no temperature monitor, but there was plenty of dry ice left in the plastic foam container when the samples arrived at the destination, so we assume temperature control was adequate during the shipment).
Samples went directly to Peking University, which is responsible for tracking all the samples and for long-term storage. Staff at Peking University logged whole blood tubes and cryovials as they were delivered by the cold-chain company. Temperature data were downloaded from monitors that were shipped with samples. All tubes and cryovials had study barcodes attached to them. These specimens were placed in −80°C freezers with a tracking system for sample locations. These freezers have battery-power backup and are equipped with a temperature monitoring system that alerts staff via text messages in the event the temperature goes above a predefined level. New bar codes from KingMed Diagnostics, the testing laboratory, were added to the whole blood tubes and cryovials that were used for assays at KingMed. These specimens remained in the −80°C deep freezer, until they were shipped to KingMed via cold chain and assayed the following day.
KingMed Diagnostics is the leading provider of diagnostic services and the largest third-party clinical test laboratory system in China. It has testing laboratories in 27 provincial-level cities. In 2011, it established an alliance partnership with Quest Diagnostics. KingMed central laboratories have both College of American Pathologists and International Organization for Standardization ISO 15189 certifications. KingMed laboratory runs quality-control specimens daily. The CHARLS team monitored quality-control results on a weekly basis. Data supported assay stability during the period when CHARLS samples were tested. Cumulative interassay coefficients of variation were 2%–3% for HbA1C, lipid panel, blood urea nitrogen, creatinine, and uric acid. Interassay coefficients of variation were approximately 5% for cystatin C, hsCRP, and glucose.
KingMed sent results electronically to Peking University weekly. The CHARLS team monitored the distributions of bioassay data on a regular basis. Shortly after tests were finished, reports of biochemical laboratory tests were sent to respondents through express mail. (The CBC reports were sent back to the respondents by our field team right after the tests.)
Methods for blood-based bioassays
In addition to the CBC, which was performed at the laboratories near study sites, we measured HbA1C, hsCRP, lipid panel (total, HDL, and LDL cholesterol, as well as triglyceride), glucose, blood urea nitrogen, creatinine, uric acid, and cystatin C in whole blood or plasma specimens at KingMed laboratory. The assay methods used in this laboratory, laboratory coefficients of variation, and detection limits are summarized in Table 1.
Table 1.
Methods and Technical Parameters of Bioassays at KingMed Diagnostics Laboratory, Tianjin, China
| Biomarker | Method | Coefficient of Variation, % | Detection Limit | |
|---|---|---|---|---|
| Within-Assay | Between-Assay | |||
| HbA1C, % | High performance liquid chromatography | <1.16 | <1.27 | 3.85–17.80 |
| hsCRP, mg/L | Immunoturbidimetric assay | <2.94 | <2.09 | 0.10–140.00 |
| Total cholesterol, mmol/L | Oxidase method | <0.70 | <3.18 | 0.13–18.18 |
| HDL cholesterol, mmol/L | Direct method | <0.67 | <3.14 | 0.09–3.08 |
| LDL-cholesterol, mmol/L | Direct method | <1.38 | <2.90 | 0.22–14.14 |
| Triglycerides, mmol/L | Oxidase method | <0.41 | <3.64 | 0.12–9.78 |
| Glucose, mmol/L | Hexokinase | <0.40 | <2.50 | 0.13–39.58 |
| Uric acid, μmol/L | Uricase, catalase | <0.25 | <2.32 | 38.50–1044.00 |
| Cystatin C, mg/L | Immunoturbidimetric assay | <1.84 | <1.87 | 0.10–6.00 |
| Blood urea nitrogen, mmol/L | Enzymatic | <0.71 | <2.82 | 0.65–39.05 |
| Creatinine, μmol/L | Picric acid method | <1.87 | <4.34 | 22.85–2151.70 |
Abbreviations: HbA1C, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Quality assurance and control
Prior to the fieldwork, a small pilot study was performed in a community in Beijing to identify and correct potential problems in the blood collection procedures. Central training of the medical students responsible for blood collection was performed according to the protocol for venous blood sample collection. Best practices were followed to ensure quality of the samples’ processing, transportation, storage, and testing.
In the fieldwork, although we used nurses or phlebotomists from local hospitals to collect venous blood, all blood collection tubes and needles were provided by the CHARLS team, and nurses from local hospitals brought tourniquets, antiseptic agents such as alcohol or iodophor, and other medical products needed for blood collection. For quality-control purposes, we recorded the time of each stage of blood collection in the field: time when blood was collected, was centrifuged, was shipped, and arrived at destination.
Weighting
A sample weight for the wave 3 blood data was generated, to correct for nonparticipation, by multiplying the wave 3 individual sample weight by the propensity score (predicted probability) that someone who was in wave 3, and was eligible to give blood, did provide a blood sample. The propensity score was calculated from a logit regression.
Ethical approval
The biomarker sample collection study protocol was approved by the ethical review committee (institutional review board) of Peking University (IRB 00001052–11014). Written informed consent was obtained from all study participants.
RESULTS
Characteristics of study participants and the processing of blood samples
The mean age of CHARLS participants who gave blood was 59.4 (standard deviation, 10.4) years. Approximately half were female and 70.8% had agricultural hukou residence permits (Table 2). About 25% were illiterate and 86.9% of them were married. Ninety-four percent of respondents came to centralized locations for venous blood collection, and survey teams collected blood at the homes of the remaining 6%. Approximately 85% of respondents who gave blood reported that they were fasting. The median time from blood collection to CBC assay was 107 minutes (mean = 116 minutes; 95th percentile: 236 minutes). The median time from collection to centrifuge was 78 minutes (mean = 79 minutes; 95th percentile: 140 minutes; 99th percentile: 174 minutes).
Table 2.
Sociodemographic Characteristics of the Respondents (n = 13,013), China Health and Retirement Longitudinal Study, China, 2015
| Variable | % |
|---|---|
| Age, years | |
| <45 | 3.8 |
| 45–49 | 16.0 |
| 50–54 | 16.8 |
| 55–59 | 16.2 |
| 60–64 | 17.2 |
| 65–69 | 12.2 |
| 70–74 | 8.6 |
| 75–79 | 5.6 |
| ≥80 | 3.6 |
| Female sex | 52.0 |
| Hukou | |
| Agricultural hukou | 70.8 |
| Nonagricultural hukou | 28.8 |
| Unified hukou | 0.3 |
| No hukou | <0.1 |
| Education | |
| Illiterate | 25.5 |
| Literate | 7.8 |
| Primary school | 24.0 |
| Middle school | 25.6 |
| High school or above | 17.1 |
| Marital status | |
| Married | 86.9 |
| Widowed | 11.5 |
| Separated, divorced, or never married | 1.7 |
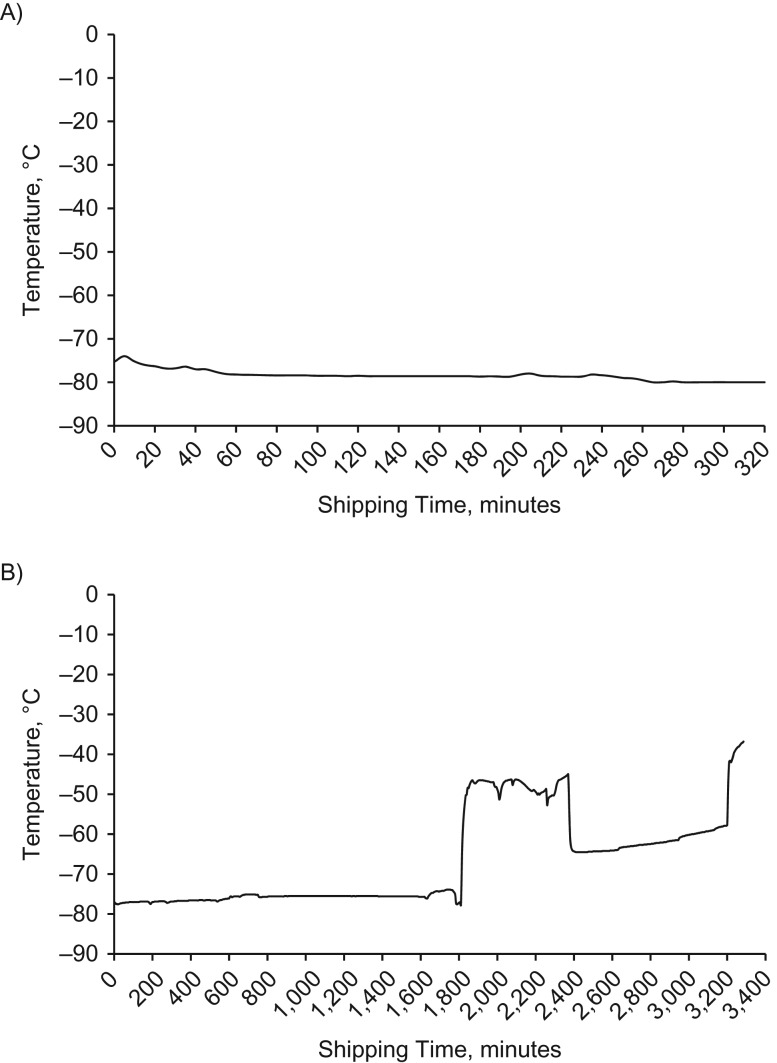
Plasma, buffy coat, and whole blood for HbA1C were collected by the cold-chain company within a median of 5 days (mean = 5.7 days; 90th percentile: 11 days; maximum: 16 days). More than two-thirds of the specimens were collected within a week. The median shipping duration from counties to Peking University was 48.6 hours (mean = 50.8 hours; 90th percentile: 83.7 hours; 95th percentile: 107.7 hours; maximum: 189.2 hours). For the 149 shipments with temperature monitors, 92.0% had a highest temperature below −40°C, 5.4% had a highest temperature between −40°C and −20°C, and 1.3% between −20°C and −10°C. Only 2 shipments (1.3%) had a highest temperature above 0°C. One was above 0°C for 3.2 hours, the other for more than 33.9 hours. A total of 181 specimens from these 2 shipments were sent to the laboratory and assayed immediately. The analyte distributions from these 2 shipments look similar to the data from other shipments. Figure 1 shows temperature graphs from 2 representative primary sampling units that never went over 0°C.
Figure 1.
Temperature graphs from 2 representative primary sampling units that never went over 0°C, China Health and Retirement Longitudinal Study, China, 2015. A) Example 1; B) example 2.
The sample storage time at Peking University differed according to the type of assay. HbA1C levels were measured within a median of 34 days (mean = 26 days; 95th percentile: 53 days; maximum: 69 days). Other plasma-based assays were done within a median of 53 days (mean = 58 days; 95th percentile: 83 days; maximum: 89 days).
Blood assay results
The data distributions in Tables 3–5 use sample weights to correct for nonresponse. The CBC assay results are shown in Table 3. The median white blood cell count and hemoglobin were 5,700 per cubic millimeter and 13.7 g per deciliter, respectively.
Table 3.
Descriptive Statistics of Complete Blood Count (Weighted), China Health and Retirement Longitudinal Study, China, 2015
| Biomarker | No. (Unweighted) | Mean (SD) | Median | 5th Percentile | 25th Percentile | 75th Percentile | 95th Percentile | Range |
|---|---|---|---|---|---|---|---|---|
| White blood cells, thousands per mm3 | 12,845 | 5.97 (2.13) | 5.70 | 3.68 | 4.74 | 6.90 | 9.05 | 1.39–116.00 |
| Hemoglobin, g/dL | 12,845 | 13.77 (1.91) | 13.70 | 10.90 | 12.60 | 14.90 | 16.70 | 4.30–28.60 |
| Hematocrit, % | 12,844 | 41.63 (5.54) | 41.40 | 33.30 | 38.20 | 44.80 | 50.10 | 12.80–84.50 |
| Mean corpuscular volume, fL | 12,845 | 91.27 (7.62) | 91.80 | 79.00 | 88.00 | 95.60 | 101.90 | 24.40–137.60 |
| Platelets, 109/L | 12,840 | 203.42 (73.60) | 200.00 | 97.00 | 161.00 | 242.00 | 314.00 | 8.00–1313.00 |
Abbreviation: SD, standard deviation.
Table 5.
Percentages With High Risk Levels According to Sex (Weighted), China Health and Retirement Longitudinal Study, 2015, China
| Biomarkers at High Risk | Total | Men | Women |
|---|---|---|---|
| % low hemoglobin (<13 g/dL for men; <12 g/dL for women) | 18.13 | 13.29 | 22.59 |
| % C-reactive protein >3 mg/L | 20.34 | 21.32 | 19.44 |
| % HbA1C ≥6.5% | 13.99 | 14.05 | 13.94 |
| % glucose ≥126 mg/dL | 11.96 | 12.56 | 11.40 |
| % total cholesterol ≥240 mg/dL | 6.75 | 5.38 | 8.02 |
| % HDL cholesterol <40 mg/dL | 15.96 | 21.31 | 11.01 |
| % LDL cholesterol >160 mg/dL | 3.63 | 3.49 | 3.77 |
| % triglycerides ≥200 mg/dL | 18.15 | 17.55 | 18.71 |
| % blood urea nitrogen >20 mg/dL | 13.00 | 15.57 | 10.62 |
| % creatinine >1.4 mg/dL | 2.36 | 3.00 | 1.78 |
| % high cystatin C (>1.03 mg/L) | 11.48 | 14.21 | 8.96 |
Abbreviations: HbA1C, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 4 shows summary statistics for the assays done on plasma or whole blood at the KingMed laboratory. Essentially, all analytes had right-skewed distributions, although the skew was less for cholesterols and uric acid. For triglycerides, the values are top-coded at 500 mg/dL. Because the usual cutoff for hypertriglyceridemia is 200 mg/dL, observations with raw readings over 500 are very high indeed. Very few observations needed top coding. Because cholesterol and glucose are usually measured on fasting specimens, we also examined the lipid panel and plasma glucose levels among the 10,983 respondents who reported fasting overnight. The distributions for these fasting individuals were close to those for the entire sample. The medians for fasting specimens were 183 mg/dL for total cholesterol, 49 mg/dL for HDL cholesterol, 102 mg/dL for LDL cholesterol, 115 mg/dL for triglyceride, and 96 mg/dL for glucose.
Table 4.
Descriptive Statistics of Blood-Based Biomarkers in Entire Wave 3 (Weighted), China Health and Retirement Longitudinal Study, China, 2015
| Biomarker | No. (Unweighted) | Mean (SD) | Median | 5th Percentile | 25th Percentile | 75th Percentile | 95th Percentile | Range |
|---|---|---|---|---|---|---|---|---|
| hsCRP, mg/L | 12,947 | 2.73 (5.94) | 1.40 | 0.30 | 0.80 | 2.60 | 8.80 | 0.10–150.20 |
| HbA1C, % | 13,002 | 5.99 (0.99) | 5.80 | 5.10 | 5.50 | 6.10 | 7.60 | 3.80–18.20 |
| Glucose, mg/dL | 12,981 | 104.91 (36.64) | 95.50 | 77.48 | 88.29 | 108.11 | 156.76 | 46.85–634.23 |
| Total cholesterol, mg/dL | 12,949 | 185.63 (37.77) | 182.63 | 131.27 | 161.00 | 206.95 | 245.17 | 69.88–616.60 |
| HDL cholesterol, mg/dL | 12,949 | 50.70 (11.37) | 49.42 | 34.75 | 42.86 | 57.14 | 70.66 | 6.18–159.85 |
| LDL cholesterol, mg/dL | 12,948 | 103.77 (30.34) | 101.93 | 60.23 | 83.40 | 121.24 | 153.67 | 16.99–367.18 |
| Triglycerides, mg/dL | 12,949 | 146.81 (92.83) | 118.58 | 57.52 | 85.84 | 174.34 | 347.79 | 26.55–500.00 |
| Uric acid, mg/dL | 12,981 | 5.09 (1.45) | 4.90 | 3.10 | 4.10 | 55.90 | 7.70 | 1.00–14.40 |
| Blood urea nitrogen, mg/dL | 12,947 | 15.27 (4.53) | 14.57 | 9.24 | 12.32 | 17.65 | 23.25 | 2.80–93.84 |
| Creatinine, mg/dL | 12,981 | 0.82 (0.29) | 0.78 | 0.55 | 0.67 | 0.91 | 1.19 | 0.28–11.88 |
| Cystatin C, mg/L | 12,943 | 0.85 (0.24) | 0.83 | 0.58 | 0.72 | 0.95 | 1.21 | 0.36–7.27 |
Abbreviations: HbA1C, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SD, standard deviation.
Table 5 summarizes the prevalence of high risk levels for select assays according to sex, using commonly used clinical cutoff points, except for cystatin C, for which the cutoff is that suggested by the manufacturer of the assay kit. Eighteen percent of the respondents had anemia. Approximately 20% had at-risk hsCRP levels, and 14% had HbA1C values equal to or greater than 6.5%, the criteria used to diagnose diabetes. More respondents had elevated cystatin C levels than elevated creatinine levels (11.48% versus 2.36%). Substantial sex differences in prevalence were found for anemia, low HDL cholesterol, high creatinine, and high cystatin C.
DISCUSSION
CHARLS provides longitudinal data on a wide range of measures, from socioeconomic status to health, in a nationally representative sample of middle-aged and older Chinese. It has adopted a conceptual approach and measures that are harmonized to the HRS and other sister surveys. Field experience from CHARLS wave 3 in 2015 suggests that it is feasible to collect and assay venous blood samples in a nationally representative sample in China. Timestamp and temperature monitoring data have shown that venous blood can be processed within a reasonable period of time, stored and shipped to a central location under appropriate temperature, and assayed for select biomarkers relevant for health and aging.
Quality of venous blood collection is further supported by the laboratory test results from the collected venous blood sample. The distributions of bioassay data not only look reasonable biologically but the distributions look similar to those from the National Health and Nutrition Examination Survey (NHANES) in the United States. For example, 14% of CHARLS participants had HbA1C values equal to or greater than 6.5%. In comparison, diabetes prevalence ranged from 18.4% to 25.7% between 1999 and 2014 among NHANES participants who were 65 years or older (9). It is important to note that NHANES defined diabetes as self-reported diagnosis of diabetes as well as HbA1C ≥6.5%, which could explain the higher prevalence in NHANES than ours, based on HbA1C alone. Similarly, 37% of NHANES participants had hsCRP concentrations above 3 mg/L, compared with 20% in CHARLS (10). NHANES data have also shown a stable prevalence of chronic kidney disease at 6.9% between 2003–2004 and 2011–2012 (11), a number in the similar range of our estimate based on creatinine and cystatin C levels.
The ability to include venous blood-based biomarkers in a population-representative survey of socioeconomic status and health offers several important advantages. In addition to more accurate diagnosis among those respondents who do not have regular access to high-quality health care, biomarkers provide an opportunity to evaluate how well chronic medical conditions are being managed among those respondents with known diseases, such as diabetes (3). Biomarkers also allow richer modeling of pathways linking socioeconomic status to physical health by measuring pathophysiological states or preclinical dysregulation that are unknown to survey participants or cannot be reliably assessed by self-reported information, such as inflammatory burden (12). Compared with dried blood spot specimens, which were collected during the CHARLS pilot study, venous blood offers more straightforward testing and result interpretation, as well as more flexibility in assay selection both during fieldwork and in the future.
In the last several decades, China has been undergoing rapid economic development, major societal changes, and an epidemiologic transition from predominantly infectious diseases to chronic noncommunicable diseases (13). This emergence of cardiometabolic diseases is supported by biomarker data from CHARLS wave 3, with high prevalence of diabetes mellitus, hypercholesterolemia, and elevated inflammatory burden. As China continues to pursue its development, the longitudinal nature of CHARLS data will offer a rare opportunity to allow the international research community to continue to study the ways that socioeconomic changes at both individual and policy levels could affect the health and aging of older Chinese.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Institute of Social Survey Research, Peking University, Beijing, China (Xinxin Chen, Qinqin Meng, Yafeng Wang); Leonard Davis School of Gerontology, University of Southern California, Los Angeles, California (Eileen Crimmins, Jung Ki Kim); David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Peifeng (Perry) Hu); Department of Economics, University of Southern California, Los Angeles, California (John Strauss); Rural Development Institute, Chinese Academy of Social Science, Beijing, China (Junxia Zeng); Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Yuan Zhang); and National School of Development, Peking University, Beijing, China (Yaohui Zhao).
This work was supported by the National Institute on Aging, National Institute of Health (grants R01AG037031, P30AG017265, and T32AG000037), the Natural Science Foundation of China (grants 71450001, 71603013, and 71873010), the China Medical Board (grant 16-249), the Knowledge for Change Program at the World Bank (contract 7172961), and Peking University.
All authors contributed equally.
Conflict of interest: none declared.
Abbreviations
- CBC
complete blood count
- CHARLS
China Health and Retirement Longitudinal Study
- HbA1C
glycated hemoglobin
- HDL
high-density lipoprotein
- HRS
Health and Retirement Study
- hsCRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
- NHANES
National Health and Nutrition Examination Survey
REFERENCES
- 1. United Nations, Population Division World Population Prospects 2019 https://population.un.org/wpp/. Accessed on July 2, 2019.
- 2. Pan L, Yang Z, Wu Y, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. 2016;248:2–9. [DOI] [PubMed] [Google Scholar]
- 3. Zhao Y, Crimmins EM, Hu P, et al. Prevalence, diagnosis, and management of diabetes mellitus among older Chinese: results from the China Health and Retirement Longitudinal Study. Int J Public Health. 2016;61(3):347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Crimmins EM, Vasunilashorn S. Links between biomarkers and mortality In: Rogers RG, Crimmins EM, eds. International Handbook of Adult Mortality. Dordrecht, the Netherlands: Springer Netherlands; 2011:381–398. [Google Scholar]
- 5. Turra CM, Goldman N, Seplaki CL, et al. Determinants of mortality at older ages: the role of biological markers of chronic disease. Popul Dev Rev. 2005;31(4):675–698. [Google Scholar]
- 6. Crimmins E, Hu J, Hu P, et al. 2008 CHARLS Pilot: User’s Guide Beijing, China: China Center for Economic Research, Peking University. http://charls.pku.edu.cn/en/page/documentation/2008_pilot. Updated February 16, 2013. Accessed August 13, 2019.
- 7. Zhao Y, Hu Y, Smith JP, et al. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao Y, Strauss J, Yang G, et al. China Health and Retirement Longitudinal Study: 2011–2012 National Baseline Users’ Guide Beijing, China: National School of Development, Peking University; 2013. http://charls.pku.edu.cn/uploads/document/2011-charls-wave1/application/CHARLS_nationalbaseline_users_guide.pdf. Accessed on July 2, 2019.
- 9. Caspard H, Jabbour S, Hammar N, et al. Recent trends in the prevalence of type 2 diabetes and the association with abdominal obesity lead to growing health disparities in the USA: an analysis of the NHANES surveys from 1999 to 2014. Diabetes Obes Metab. 2018;20(3):667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alley DE, Seeman TE, Ki Kim J, et al. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain Behav Immun. 2006;20(5):498–504. [DOI] [PubMed] [Google Scholar]
- 11. Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weir D. Elastic powers: the integration of biomarkers into the Health and Retirement Study In: Weinstein M, Vaupel J, Wachter K, eds. National Research Council (US) Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys. Washington, DC: National Academies Press (US); 2008. [PubMed] [Google Scholar]
- 13. Cook IG, Dummer TJ. Changing health in China: re-evaluating the epidemiological transition model. Health Policy. 2004;67(3):329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.