ABSTRACT
Introduction
Beverage consumption is a modifiable risk factor for type 2 diabetes (T2D), but there is insufficient evidence to inform the suitability of substituting 1 type of beverage for another.
Objective
The aim of this study was to estimate the risk of T2D when consumption of sugar-sweetened beverages (SSBs) was replaced with consumption of fruit juice, milk, coffee, or tea.
Methods
In the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct case–cohort study of 8 European countries (n = 27,662, with 12,333 cases of incident T2D, 1992–2007), beverage consumption was estimated at baseline by dietary questionnaires. Using Prentice-weighted Cox regression adjusting for other beverages and potential confounders, we estimated associations of substituting 1 type of beverage for another on incident T2D.
Results
Mean ± SD of estimated consumption of SSB was 55 ± 105 g/d. Means ± SDs for the other beverages were as follows: fruit juice, 59 ± 101 g/d; milk, 209 ± 203 g/d; coffee, 381 ± 372 g/d; and tea, 152 ± 282 g/d. Substituting coffee for SSBs by 250 g/d was associated with a 21% lower incidence of T2D (95% CI: 12%, 29%). The rate difference was −12.0 (95% CI: −20.0, −5.0) per 10,000 person-years among adults consuming SSBs ≥250 g/d (absolute rate = 48.3/10,000). Substituting tea for SSBs was estimated to lower T2D incidence by 22% (95% CI: 15%, 28%) or −11.0 (95% CI: −20.0, −2.6) per 10,000 person-years, whereas substituting fruit juice or milk was estimated not to alter T2D risk significantly.
Conclusions
These findings indicate a potential benefit of substituting coffee or tea for SSBs for the primary prevention of T2D and may help formulate public health recommendations on beverage consumption in different populations.
Keywords: diabetes, epidemiology, dietary guidelines, beverages, sugar-sweetened beverages
Introduction
Beverage consumption is one of the modifiable risk factors for noncommunicable diseases, including type 2 diabetes (T2D) (1–4). Observational studies have indicated a benefit or harm of different types of beverages for the primary prevention of noncommunicable diseases. For instance, adults consuming ≥1 daily serving of sugar-sweetened beverages (SSBs) are likely to experience a 13% greater incidence of T2D (5), whereas adults consuming 2–3 daily servings of coffee or tea are likely to experience a 15–25% lower incidence of T2D (6–8). This evidence has been supported by analyses that evaluated associations of different types of beverages with incident T2D separately. Evidence remains limited, however, on the potential benefit of substituting 1 type of beverage for another by examining different types of beverages simultaneously (9).
Effects of substituting alternative beverages for SSBs are of particular interest to inform clinical and public health recommendations. This is because consumption of SSBs has been related to cardiometabolic burden worldwide (2–4) and because multiple alternatives of beverages to SSBs are available, with diversity in sales and consumption across countries (10, 11). The effects on cardiometabolic risks have been tested in trials that, for example, examined the effect of replacing SSBs with milk, fruit juice, water, or noncaloric artificially-sweetened beverages (ASBs) on cardiometabolic risk factors (12–14). Clinical endpoints have been studied only in a few cohorts for stroke (15) or T2D (9, 16–18) in the United States and the United Kingdom (15–17). We aimed to provide further evidence by evaluating participants in the European Prospective Investigation into Cancer and Nutrition (EPIC)–InterAct consortium (19), a case–cohort study of T2D that reported associations of selected beverages with T2D, including tea (20), milk (21), fruit juice (22), ASBs (22), and SSBs (22), but had not yet evaluated coffee, milk beverages, or water. Accounting for the available evidence (4–8, 19–24), we hypothesized that substituting tea or coffee for SSBs could lower risk of T2D, whereas substituting the other beverages for SSBs would not affect the risk.
Methods
Study population
EPIC-InterAct is a prospective case–cohort study nested within the EPIC study cohort from 8 European countries (France, Italy, Spain, the United Kingdom, the Netherlands, Germany, Sweden, and Denmark) (19). From a total of 340,234 cohort participants (3.99 million person-years of follow-up) with stored blood and reported diabetes status, EPIC-InterAct identified 16,835 adults randomly selected (subcohort) and ascertained 12,403 incident cases of T2D occurring by 31 December, 2007; the identified cases included 778 cases in the subcohort (19, 25). In the current study, we excluded adults with prevalent diabetes (n = 548) or without information on T2D (n = 129) or baseline diet (n = 117). After these exclusions, 27,662 adults were evaluated in our analysis (n cases = 12,333) (Supplemental Figure 1). All participants gave written informed consent. The study was approved by local ethics committees and the Internal Review Board of the International Agency of Research on Cancer.
Ascertainment of T2D
A diagnosis of incident T2D was defined as self-report of physician's diagnosis verified by at least 1 independent source, including multiple information sources reviewed by each participating center (19): self-report, linkage to primary-care registers, secondary-care registers, medication use (drug registers), hospital admissions, and mortality data (Supplemental Table 1). Information from any follow-up visit or external evidence with a date later than the baseline visit was used. In Denmark and Sweden, incident cases were identified via local and national diabetes and pharmaceutical registers and were considered to be verified. Follow-up was to the date of diagnosis, 31 December, 2007, or the date of death, whichever occurred first.
Beverage consumption
In EPIC, dietary consumption was assessed by a dietary FFQ or diet history harmonized across EPIC cohorts (26–29). Five beverages were used as the primary exposures: SSBs (carbonated/soft drinks or diluted syrups, and sweetened milk beverages), fruit juice (fruit or vegetable juices, and fruit concentrates), milk, coffee (caffeinated or decaffeinated), and tea (Supplemental Table 2 for classification). We included sweetened milk beverages (e.g., milkshakes) as SSBs, not as milk, because of their presumably high sugar contents and their significant positive association with T2D incidence, similar to SSB consumption, in the EPIC-Norfolk study using 7-d food records (18). Based on published reviews and previous EPIC-InterAct analyses, we combined decaffeinated coffee with coffee (8) and vegetable juice with fruit juice (22). Reported consumption of each beverage had moderate correlations—adjusted for age, sex, and energy intake—with corresponding measures of 24-h recalls on average across countries (n = 2347): r = 0.33 for SSB, r = 0.38 for fruit juice, r = 0.52 for milk, r = 0.60 for coffee, and r = 0.52 for tea.
We separately evaluated ASBs (available among 82.8% of participants), decaffeinated coffee (72.1%), vegetable juice (57.0%), and water (58.4%) (Supplemental Table 1). Possible alternative groupings (e.g., classifying sweetened milk beverages as milk rather than SSBs) were evaluated additionally. These beverages were evaluated in the secondary analyses because the missing information (not at random) could limit validity of estimating absolute incidence and because these beverages were assumed to be irregularly consumed and thus the validity of self-reported consumption of these beverages was considered unclear. Alcohol, evaluated previously in EPIC-InterAct (24), was used as a covariate because alcoholic beverages cannot be considered as an alternative to the other beverages in practice.
Other study variables
At baseline, weight, height, and waist circumference were measured directly in every center, except waist circumference was not assessed in Oxford, UK, and Umea, Sweden (19). Sociodemographic factors, smoking status, physical activity, menopausal status and use of hormone replacement therapy (women only), use of medication, and prevalent diseases were self-reported (19, 30). Food groups were derived from dietary questionnaires or history (28) and considered as dietary confounders. Total energy intake was estimated from European food composition tables and centrally analyzed for EPIC (28). As an indicator of under-, normal, or overreporting of individuals’ diet, we calculated a ratio of total energy intake to total energy requirement as previously performed in EPIC-Spain (31) and categorized the ratio into the 3 levels by 1.0 ± 2 × within-individual SD of the ratio (31).
Serum triglycerides, HDL cholesterol, high-sensitivity C-reactive protein (hsCRP), and hemoglobin A1c (HbA1c) were measured at Stichting Ingenhousz Laboratory using serum samples stored at −196°C (or −150°C in Denmark), with exception of Umeå, where plasma samples were used (details in Supplemental Methods). These markers were additionally examined as potential mediators for associations of beverage consumption with incident T2D.
Statistical analysis
All analyses were performed using Stata version 14.0 software (Stata). Outlying values of beverage consumption were Winsorized (replaced with each mean +3 × SD, country-specific) to reduce their influence. The prospective association between consumption of each type of beverage (SSBs, fruit juice, milk, coffee, and tea) and incident T2D was evaluated using Prentice-weighted Cox regression for every country separately, with age as the underlying timescale, providing estimates of HRs and 95% CIs (32). HR per 250 g/d of each beverage was estimated to evaluate the same quantity of each beverage; sensitivity analyses assigned different amounts to different beverages [e.g., 150 g/d for tea (33)] accounting for varying portion sizes per serving. Country-specific estimates were then pooled across countries by multivariable random-effects meta-analysis (34). Models were adjusted for different types of beverages mutually and for potential confounders: research center, sex, education level, occupation, marital status, menopausal status and use of hormone replacement therapy (only for women), family history of diabetes, prevalent clinical conditions (hypertension, dyslipidemia, cardiovascular diseases, and cancer), and the aforementioned quality indicator of dietary reporting (31). We also adjusted for lifestyle factors: smoking status, physical activity level, dietary supplement use, alcohol consumption, and dietary consumption (total energy intake, vegetables, fruits, nuts, cheese, yogurt, red meats, processed meats, fish, confectionary, and cereals). We further adjusted for BMI (in kg/m2) and waist circumference because obesity status may have altered beverage consumption (22). We additionally tested nonlinearity of an association between each of the beverages and incident T2D by meta-regression with restricted cubic spline.
We estimated rate differences (RDs) in T2D incidence comparing different amounts of beverage servings (e.g., between 250 and 0 g/d), using parameter estimates from Cox regression models. RD for each beverage was estimated based on the absolute incidence rate of T2D of adults consuming ≥250 g/d of each beverage. In the main analysis, difference in 10-y rates was modeled using a weighted average of the cumulative incidence of T2D in each country-specific subcohort, weighted for sampling probability (35). Differences during 5 and 20 y of follow-up were also estimated. CIs were estimated using bootstrapping (1000 iterations).
We estimated the potential effects of substituting 250 g/d of 1 type of beverage for the same quantity of another type of beverage (16). The substitution effect was estimated as the difference in effect measures (e.g., βcoffee – βSSB for the association of substituting coffee for SSB), as previously conducted (15–17). This modeling produces, for example, an estimated change in T2D incidence if an individual decreased SSB and then increased coffee consumption, whereby the interpretation makes an assumption of causality for 2 exposure variables. As the secondary analysis, we also modeled substitution between 2 beverages by an average amount of each beverage in each country, not assuming serving sizes of beverages.
Sensitivity analyses were performed to examine the impact of various assumptions on the results. We estimated HRs for substitution between 250 g/d of SSBs and 150 g/d of 1 of the other beverages, considering possibly different amounts/serving across beverages; for beverage variables without Winsorization; and for beverages with log-transformation. Multiple imputation (20 data sets) was performed to investigate the impact of missing covariate data (36); the main results were from a single imputation in which between-imputation variability was considered small (0.3% of total variability). We assessed influences of reverse causation by censoring T2D cases diagnosed within the first 6 y (approximately the midpoint of follow-up time in EPIC-InterAct). We also examined the influence of errors in dietary measurements (21, 37). Using dietary estimates from single 24-h recalls in a subset (n = 2347) as a reference for dietary questionnaire or history data, we conducted multivariable calibration for HRs for different beverages by computing
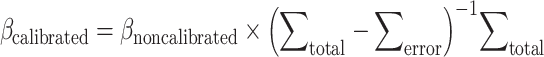 |
(1) |
where β is a vector of regression coefficients, log(HRs), and ∑ is the total and error variance–covariance matrices of beverage consumption estimates (37).
We tested if the estimated substitution effects were consistent across country, baseline age, sex, smoking, BMI, and the measure of under- or overreporting of dietary data. Heterogeneity by country was assessed by I2. For the other variables, we performed stratified analyses followed by meta-regression testing between-strata heterogeneity. We also examined if associations of interest were mediated by triglycerides, HDL cholesterol, hsCRP, and HbA1c by including these variables in the model adjusting for potential confounders.
Results
Participants’ characteristics at baseline are presented in Table 1. Mean ± SD consumption of SSBs was 55 ± 105 g/d in the subcohort. Means ± SDs for the other beverages were as follows: fruit juice, 59 ± 101 g/d; milk, 209 ± 203 g/d; coffee, 381 ± 372 g/d; and tea, 152 ± 282 g/d. Different types of beverage consumption were not correlated or weakly correlated mutually (r = −0.02 to −0.23) (data not shown). Socioeconomic and lifestyle factors and prevalent comorbidity showed complex associations with beverage consumption (Supplemental Table 3). For example, longer education history was significantly related to higher consumption of fruit juice, coffee, or tea, but lower consumption of SSBs and milk (P < 0.007 for each). Higher consumption of fruit juice was related to lower use of dietary supplements and greater prevalence of hypertension, dyslipidemia, and cardiovascular diseases (P < 0.001 for each). Underreporting of dietary intakes was more prevalent among consumers of tea (12%) or coffee (10%) than among consumers of other beverages (<10%).
TABLE 1.
Baseline characteristics of study participants in the EPIC-InterAct case–cohort study1
| Subcohort2 (n = 16,103) | Cases of diabetes (n = 12,333) | |
|---|---|---|
| Age, y | 52 ± 9.0 | 56 ± 7.7 |
| Women, % | 62 | 50 |
| Education ≥high school, % | 21 | 13 |
| Currently employed, % | 51 | 45 |
| Current smokers, % | 26 | 28 |
| Physical activity, ≥moderately active, % | 43 | 37 |
| Postmenopausal,3 % | 51 | 34 |
| Hormone therapy,3 % | 15 | 14 |
| Family history of diabetes,4 % | 23 | 38 |
| Prevalent conditions, % | ||
| Hypertension | 19 | 38 |
| Dyslipidemia | 18 | 36 |
| Cardiovascular disease | 3.0 | 7.0 |
| Dietary supplement use, % | 39 | 39 |
| Dietary consumption | ||
| Energy, MJ/d | 9.0 ± 2.8 | 9.1 ± 3.1 |
| Alcohol,5 servings/d | 1.3 ± 2.0 | 1.5 ± 2.5 |
| Fiber, g/d | 23 ± 8.0 | 23 ± 8.4 |
| Vegetables, g/d | 184 ± 120 | 180 ± 126 |
| Fruits, g/d | 235 ± 191 | 229 ± 201 |
| Processed meat, g/d | 37 ± 33 | 42 ± 39 |
| Yogurt, g/d | 63 ± 88 | 59 ± 91 |
| Confectionery, g/d | 25 ± 51 | 29 ± 69 |
| Ratio of energy intake to energy requirement | 0.92 ± 0.30 | 0.90 ± 0.30 |
| Underreporters of dietary consumption, % | 11.0 | 14.0 |
| BMI, kg/m2 | 26 ± 4 | 30 ± 5 |
| Waist circumference, cm | 86 ± 13 | 97 ± 13 |
| Hemoglobin A1c, mmol/mol | 36 ± 4.8 | 43 ± 10 |
| Triglycerides, mmol/L | 1.4 ± 0.9 | 2.0 ± 1.3 |
| HDL cholesterol, mmol/L | 1.5 ± 0.4 | 1.2 ± 0.4 |
| C-reactive protein, mg/L | 2.2 ± 3.9 | 3.8 ± 5.2 |
For continuous and categorical variables, respectively, means ± SDs and percentages are presented. A case–cohort design was undertaken in which the subcohort and incident cases of type 2 diabetes were independently sampled from the eligible participants in the EPIC cohort. By design, 774 incident cases were also in the subcohort. EPIC, European Prospective Investigation into Cancer and Nutrition.
Definitions of each type, percentage of nonconsumers and of those consuming >250 g/d, and characteristics by beverage consumption are presented in Supplemental Tables 2 and 3.
Calculated among women.
Not available in 2 research centers and thus evaluated among 8850 adults in the subcohort and 6752 cases.
Consumption levels in total alcoholic beverages were calculated in each cohort. One serving was defined as 150 mL.
Positive associations with incidence of T2D were seen for SSBs and for milk, whereas inverse or null associations were seen for coffee, tea, and fruit juice (Table 2). HRs of SSBs indicated 44% higher T2D incidence (95% CI: 28%, 62%) per 250 g/d increment with adjustment for demographic characteristics only. The association was attenuated but still significant after adjustment for socioeconomic and lifestyle covariates and for adiposity measures. In the most adjusted model, milk consumption was weakly but positively associated with T2D incidence, whereas coffee consumption and tea consumption were inversely associated. Fruit juice was not significantly associated. There was no significant evidence of departure from linearity (Supplemental Figure 2).
TABLE 2.
Prospective associations between habitual consumption of beverages and incidence of type 2 diabetes in 8 countries in the EPIC-InterAct case–cohort study (n = 27,662)1
| Sugar-sweetened beverages | Fruit juice | Milk | Coffee | Tea | |
|---|---|---|---|---|---|
| HR (95% CI) per 250 g/d2 | |||||
| Adjusted for demographic variables3 | 1.44 (1.28, 1.62) | 0.99 (0.91, 1.07) | 1.06 (0.96, 1.17) | 0.97 (0.93, 1.01) | 0.90 (0.88, 0.93) |
| + The other potential confounders | 1.25 (1.14, 1.37) | 0.98 (0.91, 1.05) | 1.10 (1.02, 1.18) | 0.92 (0.89, 0.95) | 0.90 (0.86, 0.95) |
| + BMI, waist circumference | 1.18 (1.08, 1.28) | 1.06 (0.96, 1.17) | 1.10 (1.02, 1.19) | 0.91 (0.89, 0.94) | 0.93 (0.87, 0.98) |
| Rate difference (95% CI) comparing 250 g/d with 0 serving/d4 | |||||
| Adjusted for demographic variables3 | +13 (+8.7, +17) | −3.2 (−7.2, +0.9) | +1.0 (−0.9, +2.9) | −2.0 (−3.5, −0.5) | −4.8 (−7.8, −1.9) |
| + The other potential confounders | +8.9 (+2.1, +16) | −0.5 (−6.1, +5.0) | +3.5 (+0.7, +6.2) | −3.3 (−5.8, −0.8) | −3.6 (−6.9, −0.3) |
| + BMI, waist circumference | +7.4 (−1.6, +16) | +1.1 (−5.6, +7.8) | +4.3 (+0.8, +7.7) | −4.0 (−7.6, −0.4) | −3.1 (−7.6, +1.5) |
EPIC, European Prospective Investigation into Cancer and Nutrition.
12,333 cases were evaluated. From the InterAct subcohort, 774 cases/192,287 person-years contributed. Five beverages were evaluated simultaneously using country-specific Prentice-weighted Cox proportional hazard regression models. Country-specific estimates were pooled by multivariable random-effects meta-analysis.
Five beverages were adjusted mutually for each other. Demographic covariates included recruitment centers, age, and sex. Further adjustment for potential confounders included education, marital status, hormone replacement therapy, menopausal status, history of oral contraceptive use, hypertension, dyslipidemia, family history of diabetes, prevalent diseases (coronary heart disease and stroke), smoking, physical activity, alcohol consumption, dietary supplement use, and dietary consumption (total energy intake, vegetables, fruits, nuts, cheese, yogurt, red meats, processed meats, fish, confectionary, and cereals).
Per 1000 persons × 10 y (on average, 39.5/10,000 person-years; 95% CI: 36.8, 42.5).
In sensitivity analyses, the findings for SSBs, coffee, and tea were similar to results from the main analyses, whereas findings for milk and fruit juice were different (Supplemental Tables 4 and 5). Milk consumption was not associated with T2D, after excluding under- or overreporters based on estimated energy intake and energy requirement, calibrating for measurement error, or censoring events in the first 6 y. After accounting for measurement error, fruit juice consumption was positively associated with T2D. In analyses assessing whether or not physiological markers mediated an observed association, the findings for milk and tea were attenuated toward the null, potentially mediated by the physiological markers, whereas findings for SSBs and coffee remained significant without marked changes.
Modeling comparative associations of beverage consumption with T2D, substituting coffee or tea for SSBs was estimated to lower T2D incidence significantly (Figure 1, Table 3). In analyses adjusted for potential confounders and adiposity measures, substituting coffee for SSBs by 250 g/d was estimated to lower the incidence of T2D by 21% (95% CI: 12%, 29%) or lower cases by 12.0 (95% CI: 5.0, 20.0) per 10,000 person-years of adults consuming SSBs ≥250 g/d. Similarly, substituting tea for SSBs was estimated to lower T2D incidence by 22% (95% CI: 15%, 28%) or T2D events by 11.0 (95% CI: 2.6, 20.0) per 10,000 person-years. When considering 5- and 20-y follow-up instead of 10 y, RDs were halved and doubled, respectively, as would be expected (Supplemental Table 6). The other estimates of substitution effects were not significant: as exception, the substitution of coffee or tea for milk was significantly associated with lower incidence (Table 3).
FIGURE 1.
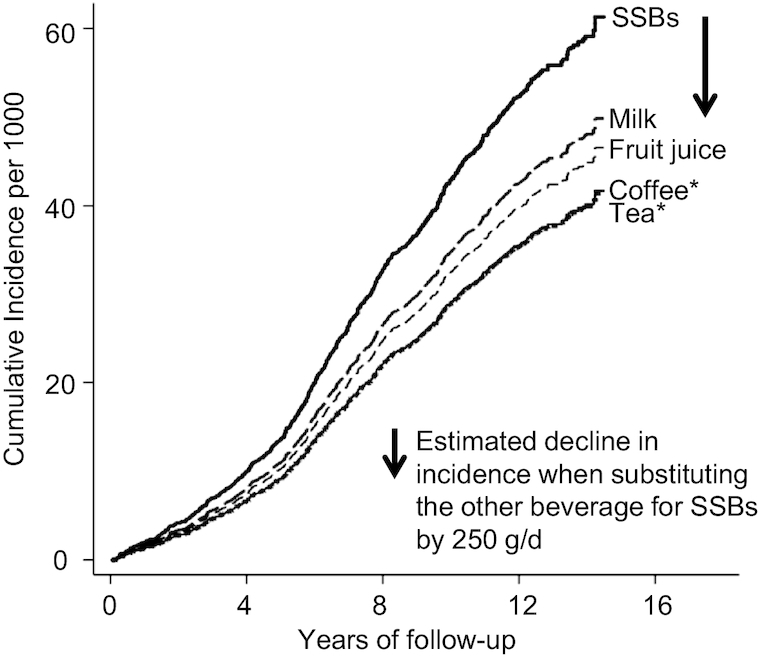
Prospective associations of substituting alternative beverages for SSBs with incidence of type 2 diabetes in the EPIC–InterAct case–cohort study. Cumulative incidence curves are displayed for adults consuming >250 g/d of SSB and adults modeled to replace SSB with 1 alternative beverage (milk, fruit juice, coffee, or tea) by 250 g/d: those for coffee and tea were almost identical. Baseline hazards of the subcohort and regression estimates (Table 3) derived from multivariable-adjusted Prentice-weighted Cox regression including the 5 variables simultaneously, demographic factors (recruitment centers, age, and sex), education, marital status, hormone replacement therapy, menopausal status, history of oral contraceptive use, hypertension, dyslipidemia, family history of diabetes, prevalent diseases (coronary heart disease or stroke), BMI, waist circumference, smoking, physical activity, alcohol consumption, dietary supplement use, and dietary consumption (total energy intake, vegetables, fruits, nuts, cheese, yogurt, red meats, processed meats, fish, confectionary, and cereals). *P < 0.001. EPIC, European Prospective Investigation into Cancer and Nutrition; SSB, sugar-sweetened beverage.
TABLE 3.
Prospective associations of beverage consumption with incident type 2 diabetes, modeled to estimate incidence rates if participants substituted 1 beverage for the other: EPIC-InterAct case–cohort study (n = 27,662)1
| Incidence per 10,000 person-years2 | Alternative beverages substituted for the beverage in the row (by 250 g/d) | |||||
|---|---|---|---|---|---|---|
| Beverage estimated to be substituted for an alternative beverage (per 250 g/d) | Sugar-sweetened beverages | Fruit juice | Milk | Coffee | Tea | |
| HR (95% CI)3 | ||||||
| Sugar-sweetened beverages | 39.5 | 1.00 (reference) | 0.89 (0.74, 1.07) | 0.91 (0.82, 1.02) | 0.79 (0.72, 0.88) | 0.78 (0.72, 0.85) |
| Fruit juice | 1.00 (reference) | 1.03 (0.9, 1.18) | 0.89 (0.83, 0.95) | 0.89 (0.76, 1.05) | ||
| Milk | 1.00 (reference) | 0.85 (0.78, 0.92) | 0.82 (0.74, 0.91) | |||
| Coffee | 1.00 (reference) | 1.02 (0.94, 1.11) | ||||
| Tea | 1.00 (reference) | |||||
| Rate differences (95% CI) per 10,000 person-years in adults with ≥250 g/d for each beverage4 | ||||||
| Sugar-sweetened beverages | 48.3 | 0.0 (reference) | −5.8 (−16.0, 4.4) | −2.0 (−8.5, 4.4) | −12.0 (−20.0, −5.0) | −11.0 (−20.0, −2.6) |
| Fruit juice | 25.2 | 4.1 (−3.9, 12.0) | 0.0 (reference) | 2.2 (−2.8, 7.2) | −3.4 (−8.2, 1.4) | −2.8 (−7.9, 2.4) |
| Milk | 41.3 | 3.6 (−5.2, 12.0) | −2.8 (−10.0, 4.4) | 0.0 (reference) | −8.2 (−13, −3.7) | −7.3 (−13.0, −1.4) |
| Coffee | 38.4 | 12.0 (2.5, 21.0) | 5.4 (−2.2, 13.0) | 8.2 (3.7, 13.0) | 0.0 (reference) | 0.9 (−4.6, 6.5) |
| Tea | 28.5 | 11.0 (0.6, 21.0) | 4.5 (−4.0, 13.0) | 7.3 (1.4, 13.0) | −0.9 (−6.5, 4.6) | 0.0 (reference) |
12,333 cases were evaluated, along with the subcohort of the total (774 cases/192,287 person-years). Prentice-weighted Cox proportional hazard regression was modeled for each country. Country-specific estimates were pooled by multivariable random-effects meta-analysis. EPIC, European Prospective Investigation into Cancer and Nutrition.
Calculated from the subcohort.
In each model, all beverages were mutually adjusted for each other. Demographic covariates included recruitment centers, age, and sex. Further adjustment for potential confounders included education, marital status, hormone replacement therapy, menopausal status, history of oral contraceptive use, hypertension, dyslipidemia, family history of diabetes, prevalent diseases (coronary heart disease and stroke), BMI, waist circumference, smoking, physical activity, alcohol consumption, dietary supplement use, and dietary consumption (total energy intake, vegetables, fruits, nuts, cheese, yogurt, red meats, processed meats, fish, confectionary, and cereals).
Analysis was performed by estimating incidence rate of adults consuming ≥250 g/d of the beverage of each row and by estimating rate differences representing effects of replacing the beverage of each row with the beverage of each column. The second column presents crude rates per 10,000 person-years of adults consuming ≥250 g/d of each beverage.
In analyses examining subtypes of beverages among subsets, consumption of sweetened milk beverages (separated from SSBs) was positively associated with incident T2D with an HR of 2.56 (95% CI: 1.04, 6.29) per 250 g/d (Supplemental Table 7). Other subgroups of beverages (decaffeinated coffee, vegetable juice, ASBs, and water) were not significantly associated with incident T2D. The HR for vegetable juice was not estimated precisely (95% CI: >10). Substituting decaffeinated coffee, ASBs, or water for SSBs was estimated to lower T2D incidence by 23% (95% CI: 15%, 30%), 22% (95% CI: 17%, 26%), or 13% (95% CI: −1.0%, 24%), respectively. Heterogeneity by country was moderate to substantial overall. For example, I2 varied from 39.7% for tea to 77.3% for replacing SSBs with another type of beverage (Supplemental Figure 3). The heterogeneity was not explained by average age, proportion of men or women, average BMI, or absolute incidence (P > 0.05 in meta-regression). Of prespecified variables, sex was identified to be a significant effect modifier for fruit juice (P-interaction = 0.02; P > 0.2 for others; Supplemental Figure 3). In men, but not in women, substituting fruit juice for SSBs was estimated to reduce T2D risk (HR: 0.69; 95% CI: 0.53, 0.90). This association in men and the heterogeneity by sex were not significant (P > 0.2 for each) in a post hoc analysis censoring events during the first 6 y of follow-up.
Discussion
Substituting consumption of coffee or tea for consumption of SSBs was associated with an ∼20% lower incidence of T2D across 8 European populations. In secondary analyses, substitution of decaffeinated coffee or ASBs for SSBs was also associated with lower incidence of T2D. By contrast, fruit juice and milk appeared unlikely to be suitable alternatives to SSBs for the prevention of T2D. Our study suggests the potential benefits of alternative beverages to SSBs for the primary prevention of T2D.
Our analysis provides the first evidence for populations of multiple European countries that coffee consumption was inversely associated with T2D incidence, whereas the association has been well established in individual countries across global regions (7); consumption of water or vegetable juice was not significantly associated with T2D incidence; and consumption of sweetened milk beverages was significantly positively associated with T2D incidence. Potential effects of substituting 1 beverage for another on T2D have been examined in only a few studies in the United States or the United Kingdom (16–18). We were unable to distinguish between sweetened and unsweetened tea or coffee, whereas EPIC-Norfolk evaluated detailed 7-d dietary records and highlighted positive associations of sweetened coffee or tea with T2D risk (18). Available evidence from this study and others indicates that T2D risk may be reduced by substituting unsweetened coffee or tea for SSBs but not by using fruit juice or milk as the replacement beverages.
Our secondary analysis of substituting ASBs for SSBs by 1 serving/d in the subset produced a stronger estimate (Supplemental Table 7) than that from the Nurses’ Health Study (HR: 0.95; 95% CI: 0.90, 0.98) (16) or the EPIC-Norfolk study (its study population partly overlapped with the population in EPIC-InterAct) (HR: 0.95; 95% CI: 0.80, 1.08) (17, 18). The heterogeneity between these results may have reflected different degrees of population-specific residual confounding (38, 39) or the use of different dietary assessment methods. Accounting for the heterogeneity and the uncertainty of potential adverse effects of ASBs on appetite, gut microbiota, and metabolic risks (38), evidence remains weak for the benefit of substituting ASBs for SSBs for T2D prevention. In modeling substitution of water for SSBs, the potential reduction in T2D incidence appeared quantitatively similar to those in EPIC-Norfolk (HR: 0.86; 95% CI: 0.74, 0.99) (no overlapping population with EPIC-InterAct) (18) and the Nurses’ Health Study (HR: 0.93; 95% CI: 0.89, 0.97) (16). Despite the consistency, results on consumption of water, as well as ASBs, should be interpreted cautiously partly because of residual confounding due to health consciousness. Based on our findings and other evidence, further research using controlled clinical studies and population-based studies is needed to better understand the efficacy of consumption of ASBs and water (or hydration) on the development of T2D (4, 40). Although this is beyond the scope of our study, ASBs and water may serve as favored alternatives (4, 40, 41) to SSBs because ASBs and water have low or zero energy content, and SSBs, ASBs, and water share a similar context of consumption as cold beverages. Effectiveness is likely to be related to such practical considerations, and relevant behaviors remain to be studied as well.
Our study indicated that milk consumption was positively associated with T2D but not after accounting for under- or overreporters of dietary consumption, measurement error, or reverse causation. These findings and prior nonsignificant findings in meta-analyses of low-fat or high-fat milk and T2D (23) indicate that milk is unlikely to be a healthy alternative to SSBs for the prevention of T2D. Clinical trials have suggested a benefit of milk consumption through its insulinogenic and antioxidative properties (42) with diversity in comparators (e.g., water or other beverages) and types of milk (e.g., full-fat or skim). Sweetened milk beverages have been little studied but are likely to be harmful based on biological plausibility related to added sugars (18) and this study using FFQs and the EPIC-Norfolk study using 7-d food records (18). In summary, specific types of milk remain to be evaluated, but consumption of milk or milk beverages is unlikely to contribute to the primary prevention of T2D.
Policy implications from this work deserve discussion. Our findings indicate the possible benefit of explicitly recommending alternatives to SSBs. This corroborates with the principle of beverage guidelines proposed in 2006 for the US population (4). The importance of such guidelines has been further supported by our estimates of T2D risk differences in the order of 10s per 10,000 person-years. This indicates that a benefit for individuals may be too small, but a population-level benefit could be meaningful, particularly in countries in which prevalence of T2D is alarming (43) and millions of adults are consuming SSBs [e.g., 50% of adults in the United States (5) and up to two-thirds in European countries (10)]. Our findings for consumption of milk and sweetened milk beverages also provide an implication for dietary guidelines. Dairy consumption has been widely recommended in federal dietary guidelines as a source of calcium, vitamin D, and other nutrients, and this recommendation has been questioned (44). By contrast, our finding does not support a presumed benefit, whereas sweetened milk is likely to be harmful at least in the context of the primary prevention of T2D. Further clinical, modeling, and policy research are warranted to explore the utility of explicitly recommending substitution of specific beverages for SSBs in diverse populations.
Despite no direct evidence, our work should stimulate further research on the effectiveness and efficacy of substituting alternative beverages for SSBs. For individuals who are at high risk of T2D and consume SSBs habitually, advice to consume other beverages instead of SSBs is reasonable to consider, rather than advice to reduce SSBs solely. Its benefit has been indicated in a trial demonstrating improvement of a diet by substituting ASBs or water for SSBs (45). Reducing SSB consumption may be easier to achieve than modifying food consumption because SSBs are often consumed in isolation rather than in combination after being cooked, for instance, with other foods. To strengthen these considerations with empirical evidence, behavioral effects of promoting consumption of healthy alternatives to SSBs should be established in future intervention studies.
EPIC-InterAct had the strengths of longitudinal design; a large number of T2D cases adjudicated in a standardized manner (19); the availability of covariates; 24-h recall dietary data in the subset; and a wide variety of beverage types, including water and milk beverages for which little evidence was available to date. A number of limitations of this study typical of observational research exist and limit a causal inference for a dietary effect on disease incidence. Thus, the substitution effects should be considered as estimates modeled from observational evidence. Relevant limitations include residual confounding due to unmeasured or imprecisely measured confounders. Although our calibration for measurement errors indicated robustness of our main conclusion, measurement errors were likely to exist heterogeneously across countries in estimating absolute amounts of beverage consumption and in estimating habitual consumption because dietary exposures were measured only at baseline. Bias during the follow-up could occur; for example, an increase in SSB consumption over years (10, 11) could have widened between-individual variance of SSB consumption and made HRs per 1 serving/d overestimated. Misclassification is also possible because InterAct (and many previous studies) had no data on sports drinks, sweetened coffee or tea, and other subtypes, although these subtypes of beverages may increase the risk of T2D (3, 4, 18, 46). Availability of data was limited on preparation methods for beverages; for instance, populations in Italy and the United Kingdom prepare (brew) coffee differently, which may have differential biological effects and contribute to heterogeneity in prospective associations. Last, generalizability is limited because we primarily studied white adults in Europe (7, 8). Because tea, coffee, SSBs, ASBs, and water are consumed globally (2, 3) in diverse cultural and culinary settings, future work is warranted to characterize healthy alternatives to SSBs in different regions of the world.
In conclusion, across 8 European countries, SSB consumption was positively associated, and consumption of coffee or tea was inversely associated, with the risk of developing T2D. In our modeling of substitution effects of different beverages, ∼20% of risk was estimated to be reduced by substituting coffee or tea for SSBs. This work provides implications for the primary prevention of T2D by reducing SSB consumption and increasing consumption of healthier beverage options.
Supplementary Material
Acknowledgments
We thank N Kerrison (MRC Epidemiology Unit, Cambridge, UK) for managing the data for the InterAct project. The authors’ responsibilities were as follows—NJW: chief investigator of the EPIC-InterAct study; SJS, CL, NGF, and NJW: coordinated the EPIC-InterAct study; FI, MBS, SJS, MG, DR, BB, and ES-F: conceived the current study; : EA, LA, DA, HG, CD, GF, PWF, HF, PJ, RK, K-TK, TK, FRM, GM, M-DC, PMN, KO, VMP, SP, AP-C, JRQ, FR, MR-B, OR, IS, MS, AMWS, AT, TYNT, RT, LETV, HAW, CL ER, NGF, and NJW: provided essential materials necessary for this current study; FI: performed statistical analyses and wrote the manuscript; FI, SJS, and NGF: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: contributed to interpretation of data, revised the article critically for important intellectual content, and read and approved the final manuscript.
Notes
The InterAct project was funded by the EU FP6 program (LSHM_CT_2006_037197). InterAct investigators acknowledge funding from the following agencies: Medical Research Council Epidemiology Unit Core Support (MC_UU_12015/1, MC_UU_12015/5); National Institute for Health Research, Biomedical Research Centre Cambridge: Nutrition, Diet, and Lifestyle Research Theme (IS-BRC-1215-20014); the German Federal Ministry of Education and Research, the German Center for Diabetes Research, the State of Brandenburg, Germany; Netherlands Agency grant IGE05012 and an Incentive Grant from the Board of the University Medical Center Utrecht (Netherlands); Dutch Ministry of Public Health, Welfare, and Sports, Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund, and Statistics Netherlands; Swedish Research Council, Novo Nordisk, Swedish Heart Lung Foundation, and Swedish Diabetes Association; Danish Cancer Society; Deutsche Krebshilfe; Associazione Italiana per la Ricerca sul Cancro; Asturias Regional Government; Navarra Regional Government, Health Research Fund of the Spanish Ministry of Health; CIBER Epidemiología y Salud Pública, Spain; Murcia Regional Government (grant 6236); and AIRE-ONLUS Ragusa, AVIS-Ragusa, and Sicilian Regional Government.
Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/World Health Organization.
Author disclosures: FI, MBS, SJS, MG, DR, BB, ES-F, EA, LA, DA, HB, CD, GF, PWF, HF, PJ, RK, K-TK, TK, FRM, GM, M-DC, PMN, KO, VMP, SP, AP-C, JRQ, FR, MR-B, OR, IS, MS, AMWS, AT, TYNT, RT, LETV, HAW, CL, ER, NGF, and NJW, no conflicts of interest.
Supplemental Figures 1–3, Supplemental Tables 1–7, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
NGF and NJW contributed equally to this work.
Abbreviations used: ASB, artificially-sweetened beverage; EPIC, European Prospective Investigation into Cancer and Nutrition; HbA1c, glycated hemoglobin; hsCRP, high-sensitivity C-reactive protein; RD, rate difference; SSB, sugar-sweetened beverage; T2D, type 2 diabetes.
References
- 1. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Singh GM, Micha R, Khatibzadeh S, Lim S, Ezzati M, Mozaffarian D. Estimated global, regional, and national disease burdens related to sugar-sweetened beverage consumption in 2010. Circulation. 2015;132(8):639–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diab Endocrinol. 2016;4(2):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006;83(3):529–42. [DOI] [PubMed] [Google Scholar]
- 5. Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huxley R, Lee CMY, Barzi F, Timmermeister L, Czernichow S, Perkovic V, Grobbee DE, Batty D, Woodward M, Man C et al.. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169(22):2053–63. [DOI] [PubMed] [Google Scholar]
- 8. Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: a meta-analysis of prospective studies. Eur J Nutr. 2014;53(1):25–38. [DOI] [PubMed] [Google Scholar]
- 9. Zheng M, Allman-Farinelli M, Heitmann BL, Rangan A. Substitution of sugar-sweetened beverages with other beverage alternatives: a review of long-term health outcomes. J Acad Nutr Diet. 2015;115(5):767–79. [DOI] [PubMed] [Google Scholar]
- 10. Euromonitor International. Passport global market information database. [Internet] 2014. Available from: http://www.portal.euromonitor.com/portal, (accessed 6 November, 2019). [Google Scholar]
- 11. European Food Safety Authority. Use of the EFSA comprehensive European food consumption database in exposure assessment. EFSA J. 2011;9(3):2097. [Google Scholar]
- 12. Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr. 2013;98(4):1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng M, Rangan A, Olsen NJ, Andersen LB, Wedderkopp N, Kristensen P, Grøntved A, Ried-Larsen M, Lempert SM, Allman-Farinelli M et al.. Substituting sugar-sweetened beverages with water or milk is inversely associated with body fatness development from childhood to adolescence. Nutrition. 2015;31(1):38–44. [DOI] [PubMed] [Google Scholar]
- 14. Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr. 2012;95(2):283–9. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein AM, de Koning L, Flint AJ, Rexrode KM, Willett WC. Soda consumption and the risk of stroke in men and women. Am J Clin Nutr. 2012;95(5):1190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95(6):1454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Koning L, Malik VS, Rimm EB, Willett WC, Hu FB. Sugar-sweetened and artificially sweetened beverage consumption and risk of type 2 diabetes in men. Am J Clin Nutr. 2011;93(6):1321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. O'Connor L, Imamura F, Lentjes MAH, Khaw K-T, Wareham NJ, Forouhi NG. Prospective associations and population impact of sweet beverage intake and type 2 diabetes, and effects of substitutions with alternative beverages. Diabetologia. 2015;58:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. The InterAct Consortium. Design and cohort description of the InterAct Project: an examination of the interaction of genetic and lifestyle factors on the incidence of type 2 diabetes in the EPIC study. Diabetologia. 2011;54(9):2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The InterAct Consortium. Tea consumption and incidence of type 2 diabetes in Europe: the EPIC-InterAct case–cohort study. PLoS One. 2012;7(5):e36910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sluijs I, Forouhi NG, Beulens JWJ, van der Schouw YT, Agnoli C, Arriola L, Balkau B, Barricarte A, Boeing H, Bueno-de-Mesquita HB et al.. The amount and type of dairy product intake and incident type 2 diabetes: results from the EPIC-InterAct study. Am J Clin Nutr. 2012;96(2):382–90. [DOI] [PubMed] [Google Scholar]
- 22. The InterAct Consortium. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–30. [DOI] [PubMed] [Google Scholar]
- 23. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose–response meta-analysis of observational studies. Am J Clin Nutr. 2016;103(4):1111–24. [DOI] [PubMed] [Google Scholar]
- 24. Beulens JWJ, van der Schouw YT, Bergmann MM, Rohrmann S, Schulze MB, Buijsse B, Grobbee DE, Arriola L, Cauchi S, Tormo MJ et al.. Alcohol consumption and risk of type 2 diabetes in European men and women: influence of beverage type and body size—the EPIC-InterAct study. J Intern Med. 2012;272(4):358–70. [DOI] [PubMed] [Google Scholar]
- 25. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case–cohort study. Lancet Diab Endocrinol. 2014;2(10):810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J et al.. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5(6b):1113–24. [DOI] [PubMed] [Google Scholar]
- 27. Margetts BM, Pietinen P. European Prospective Investigation into Cancer and Nutrition: validity studies on dietary assessment methods. Int J Epidemiol. 1997;26(Suppl 1):S1–5. [DOI] [PubMed] [Google Scholar]
- 28. Slimani N, Deharveng G, Unwin I, Southgate DAT, Vignat J, Skeie G, Salvini S, Parpinel M, Møller A, Ireland J et al.. The EPIC Nutrient Database Project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61(9):1037–56. [DOI] [PubMed] [Google Scholar]
- 29. Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, Kroke A, Trichopoulos D, Trichopoulou A, Lauria C et al.. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5(6B):1125–45. [DOI] [PubMed] [Google Scholar]
- 30. Wareham NJ, Jakes RW, Rennie KL, Schuit J, Mitchell J, Hennings S, Day NE. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6(4):407–13. [DOI] [PubMed] [Google Scholar]
- 31. Mendez MA, Popkin BM, Buckland G, Schroder H, Amiano P, Barricarte A, Huerta J-M, Quirós JR, Sánchez M-J, González CA. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake–obesity relations. Am J Epidemiol. 2011;173(4):448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prentice RL. A case–cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73(1):1–11. [Google Scholar]
- 33. Yang J, Mao Q-X, Xu H-X, Ma X, Zeng C-Y. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4(7):e005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith-Warner SA, Spiegelman D, Ritz J, Albanes D, Beeson WL, Bernstein L, Berrino F, van den Brandt Pa, Buring JE, Cho E et al.. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol. 2006;163(11):1053–64. [DOI] [PubMed] [Google Scholar]
- 35. Langenberg C, Sharp SJ, Franks PW, Scott RA, Deloukas P, Forouhi NG, Froguel P, Groop LC, Hansen T, Palla L et al.. Gene–lifestyle interaction and type 2 diabetes: the EPIC interact case–cohort study. PLoS Med. 2014;11(5):e1001647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sterne JaC, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosner B, Spiegelman D, Willett WC. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136(11):1400–13. [DOI] [PubMed] [Google Scholar]
- 38. Borges MC, Louzada ML, de Sá TH, Laverty AA, Parra DC, Garzillo JMF, Monteiro CA, Millett C. Artificially sweetened beverages and the response to the global obesity crisis. PLoS Med. 2017;14(1):e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Popkin BM, D'Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev. 2010;68(8):439–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Toews I, Lohner S, Küllenberg de Gaudry D, Sommer H, Meerpohl JJ. Association between intake of non-sugar sweeteners and health outcomes: systematic review and meta-analyses of randomised and non-randomised controlled trials and observational studies. BMJ. 2019;5:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Visioli F, Strata A. Milk, dairy products, and their functional effects in humans: a narrative review of recent evidence. Adv Nutr. 2014;5(2):131–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387(10027):1513–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weaver CM. How sound is the science behind the dietary recommendations for dairy?. Am J Clin Nutr. 2014;99(5):1217S–22S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piernas C, Tate DF, Wang X, Popkin BM. Does diet-beverage intake affect dietary consumption patterns? Results from the Choose Healthy Options Consciously Everyday (CHOICE) randomized clinical trial. Am J Clin Nutr. 2013;97(3):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han E, Powell LM. Consumption patterns of sugar-sweetened beverages in the United States. J Acad Nutr Diet. 2013;113(1):43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


