ABSTRACT
Background
Childhood obesity continues to be a global health problem. Previous research suggests that linear growth retardation or stunting during early childhood increases the risk of obesity, but others have reported that rapid linear growth poses a greater concern than early nutritional status.
Objective
The objective of this study was to determine if growth trajectories are associated with body composition at age 8–10 y.
Methods
Study participants consisted of 255 girls and 281 boys who participated in a follow-up of the Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development (POSGRAD) Study. Sex-specific latent height class (LHC) trajectories were derived from 11 measures of height from birth to 5 y of age and used to calculate 3 distinct growth classes for boys (low, intermediate, and high) and 2 distinct classes for girls (low and high). Body composition at age 8–10 y was estimated using bioelectrical impedance analysis. Multivariable linear regression analysis was used to determine the relationship between growth trajectory classes and fat mass (FM) and fat-free mass (FFM) in late childhood, controlling for confounding factors.
Results
In girls, there were no significant associations between LHC and FM or FFM. In boys, relative to the intermediate LHC, the low LHC had higher FM (β = 0.69 kg; 95% CI: 0.26−1.11 kg) and the high LHC had lower FM (β = −0.40 kg; 95% CI: −0.76 to −0.05 kg). Boys in the low LHC had significantly less FFM (β = −0.69 kg; 95% CI: −1.11 to −0.26 kg), and boys in the high LHC had more FFM (β = 0.40 kg; 95% CI: 0.05−0.76 kg) compared with the intermediate LHC.
Conclusion
Gain in height among boys, but not girls, in early childhood was associated with lower adiposity in late childhood compared with children with a slower rate of growth. Clinical trial registration number: NCT00646360
Keywords: growth, body composition, latent class analysis, adiposity, double burden of malnutrition
Introduction
The global prevalence of obesity has more than doubled since the 1980s (1). In Latin America, upward of 25% of children <18 y of age were overweight or obese between 2008 and 2013 (2), and ∼58% of adults in Latin America are overweight or obese (3). Being overweight or obese as a child is a serious public health concern as it is associated with a number of chronic diseases, including hypertension, dyslipidemia, insulin resistance, fatty liver disease, and psychosocial complications (4–6). It is unclear whether or not being classified as obese in childhood increases the risk of obesity in adolescence or adulthood (7–9) given the differences in cohorts studied as well as methodologic issues that do not always allow for consistent conclusions. However, rapid growth has been identified as a contributing factor to the development of obesity (10–12).
Children who grow poorly in utero or during early childhood, particularly those who are classified as stunted [height-for-age z score (HAZ) <−2 SD] (13), have been reported to be at a higher risk for obesity based on cross-sectional population studies (14, 15) and may contribute to the double burden of disease in low- and middle-income countries (LMICs) (16, 17). A study from Senegal that found that girls who were stunted at age 6–18 mo had greater truncal fat than nonstunted girls, independent of BMI [weight (kg)/height (m)2] (18). In Guatemala, stunted children had an adolescent BMI above the median for US children of the same age (19), and adults who were severely stunted as children had greater central fat, independent of total fat mass, compared with moderately or never-stunted counterparts (20). However, in prospective studies, stunting was associated with a decreased BMI or fat mass (FM) in childhood. For example, Walker et al. (21) reported that children who were stunted at 2 y of age had a lower BMI at ages 17–18 y compared with children who were not stunted. Results from the Birth to 20 cohort in South Africa suggest that children who were stunted early in life did not have a BMI z score greater than those who were not stunted (22). As well, a 6-y prospective study of children in Bolivia found that stunting was associated with a lower BMI z score and arm muscularity (23). Divergences in these conclusions may be attributed to differences in methodologies or environmental factors that vary from epigenetic changes to modifications in the microbiome, topics that have been covered in great depth in recent reviews (24–27). Regardless, the lack of consensus on this topic suggests that a more nuanced understanding of how growth patterns influence adiposity is critical to develop appropriate interventions to reduce the prevalence of childhood obesity.
Regarding early growth patterns and obesity, a recent study of early weight gain reported an increased incidence of obesity in later childhood among children who entered kindergarten overweight (9). Longitudinal studies have also reported an increased risk of obesity from excess weight gain as early as the first 6 mo of life (28). Yet, a prospective study of children in Jamaica found that those who were chronically stunted or “recovered” from stunting had a lower BMI than nonstunted children after a 5-y follow-up period (29). The lack of agreement between studies may be explained by the different methods used to assess obesity, as past studies used BMI as a surrogate measure of adiposity without the ability to distinguish between FM and fat-free mass (FFM).
Currently, >70% of the adult population in Mexico is either overweight or obese, and the prevalence of obesity for school-aged children and adolescents is 33% and 36%, respectively (30). The need to determine how growth patterns may contribute to the high prevalence of childhood obesity is of great public health importance, especially as Mexico continues to experience the nutrition transition (31). To address this question, we studied the relationship between growth patterns and adiposity in late childhood, using latent class growth analysis (LCGA) to explore the heterogeneity in gain of height over the life course, in children living in Cuernavaca, Mexico.
Methods
Study participants were offspring born to women who participated in the Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development (POSGRAD) Study, a double-blind, randomized, placebo-controlled trial designed to assess the effect of prenatal supplementation with DHA on offspring growth and development, described in detail elsewhere (NCT00646360) (32). The intervention trial was conducted in Mexico from 2004 to 2006, during which time 1094 women were randomly assigned to receive 400 mg/d of algal DHA or placebo from 18 to 22 wk of gestation through delivery. Birth outcomes (968 live births and 5 stillbirths) were obtained within 24 h of delivery, and follow-up of the offspring for growth and development has been ongoing (33). Most recently, the POSGRAD birth cohort has been followed up at 8–10 y, and body composition measurements were obtained in a subsample.
A total of 545 children completed the body composition measures at age 8–10 y with a total of 9 repeated measurements for height. The lowest number of repeated measures was 3, the minimum value to maintain model stability when using LCGA (34). The final sample included 536 participants (281 boys, 255 girls) as 9 measures were excluded due to excess movement during the body composition measurement (Supplemental Table 1).
Data collection
Anthropometric measurements
Birth weight (to the nearest 10 g) and length (to the nearest 1 mm) were measured with the use of a pediatric scale and a portable length measurement board following standard procedures (35). Weight and length at ages 1, 3, 6, 9, 12, and 18 mo were measured with the same equipment and procedures. Weight and standing height were measured at 24, 36, 48, and 60 mo and at their 8- to 10-y follow-up with a Tanita scale and a Seca stadiometer. Waist circumference (WC) was measured using a Gulick fiberglass tape (Creative Health Products, Inc.) accurate to within 0.1 cm. Trained study personnel at the Mexican Social Security Institute's Hospital General I in Cuernavaca, Mexico, performed data collection. The exact age in days at the 8- to 10-y follow-up was calculated by subtracting date of birth from the date of measurement and used to calculate age-specific z-scores relative to school-aged children and adolescent WHO standards (36).
Body composition
Body composition was estimated with a tetrapolar bioelectrical impedance analyzer (BIA) (ImpediMed DF50; ImpediMed) and validated equations for raw values of resistance Ω and reactance Ω for Mexican children (37). Briefly, using the equation that was validated against deuterium dilution, the raw values for resistance (R) were entered into the equation 0.661 × Ht²/R + 0.200 × Wt – 0.320 to estimate the FFM, after which FM was calculated as the difference between body weight and FFM. Trained personnel took all the measurements using a standardized protocol. Briefly, distal and proximal electrodes were placed 5 cm apart and all measurements were made on the right wrist and the right ankle with the participant in a supine position. We took the average of 2 trials (between 4 min and 4 min 59 s) as the final impedance value. Maximum allowable differences between 2 measurements were 3 Ω for both resistance (R) and reactance (Xc) (37). Mothers were instructed to bring the child after a 4-h fast (no caffeinated beverages or food), and 500 mL of sweet juice drink was offered 60 min before testing to ensure proper hydration for children 9 y of age who chose to provide a venous blood sample and come in after an overnight fast. All children were instructed to restrict strenuous physical activity for >8 h and void before the measurement.
Covariates
Maternal age, education, and socioeconomic status (SES) were obtained at recruitment, and SES was calculated using a list of assets obtained by interview (32). The Emory University Institutional Review Board and the National Institute of Public Health Biosafety, Investigation, and Ethics Committees both approved the protocol. Written informed consent was obtained from participating mothers after they received a detailed explanation of the study at baseline and during their offspring's follow-up, as well as assent from the children.
Statistical methods
The mean and standard deviation for continuous variables were calculated for the entire sample stratified by sex, and Student t test and χ2 tests were used to assess differences between sex. To test the main hypotheses, LCGA models were used to identify homogeneous subpopulations with distinct growth patterns within the larger cohort. In non-latent class type growth modeling, a single curve would be estimated for the whole sample, which can potentially hide heterogeneity within the sample (38). LCGA allows individuals with similar growth characteristics to be grouped together and provides each latent class its own growth curve (39). Sex-specific latent class height trajectories (LCHTs) were estimated from 11 possible measures of length/height, including measures at birth and at 1, 3, 6, 9, 12, 18, 24, 36, 48, and 60 mo. Less than 1% of participants had only 3 measurements, and 96% had 6 measurements or more. Sex-specific trajectories were modeled to accommodate potential sex differences in growth during infancy (40).
LCGA was used to develop a series of models with 2–4 classes using all available data and a robust maximum likelihood estimation and 200 random start values to avoid local solutions, generating a curve that represents the global maximum solution (34). As there are no definitive criteria for selecting the optimal number of classes, a combination of statistical criteria and interpretability was used (41). Briefly, we assessed the model fit using the Bayesian information criterion, the bootstrap likelihood ratio test, and the Lo-Mendell-Rubin likelihood ratio test and also took the interpretability of classes into account when determining the final model (34). Entropy (higher value indicates greater classification accuracy, range 0–1) and posterior probabilities (probability of assigning observations to groups given the data) were used to assess the quality of the classification (38–42). Finally, each group had an adequate sample size of N > 25 per group (43). Sex-specific LCHTs were derived using MPlus v.7.3 (Muthén & Muthén).
Means and SDs for continuous variables were calculated according to LCHT membership, and Student's t test or ANOVA was used to assess differences between LCHT groups. Multivariable linear regression analysis was used to determine the relationship between growth trajectory classes and FM (kg) and FFM (kg) in late childhood, controlling for current body weight (kg), SES (low, medium, and high), parity, and maternal education (y). It should be noted that body weight was included as a covariate for these analyses as the boys and girls in both “high” latent classes had a higher body weight compared with the low-growth classes. Latent class analyses were conducted using M-plus (Muthén & Muthén) while means and regression analyses were conducted using STATA 15 (StataCorp LLC), and statistical significance was determined at P < 0.05.
Results
Summary characteristics of the study participants are presented by sex in Table 1. There were no significant differences by sex for age, weight, height, and WC at follow-up (Table 1). Boys had significantly greater FFM compared with girls (P < 0.01), and girls had greater FM compared with boys (P < 0.05). There was a higher percentage of boys classified as obese, compared with girls (P < 0.05), using WHO cutoffs (36) (Table 1). With regard to maternal and household characteristics, there were no differences between boys and girls for maternal age at recruitment, maternal education, or SES.
TABLE 1.
Background characteristics of women and children in the POSGRAD cohort1
| Boys (52.43%) | Girls (47.57%) | P value | |
|---|---|---|---|
| n | 281 | 255 | |
| Age, y | 8.7 ± 0.5 | 8.8 ± 0.5 | 0.57 |
| Weight, kg | 31.8 ± 7.6 | 31.4 ± 7.7 | 0.59 |
| Height, cm | 132.3 ± 6.1 | 131.6 ± 7.0 | 0.22 |
| Waist circumference, cm | 67.1 ± 10.0 | 67.5 ± 9.6 | 0.58 |
| FFM, kg | 22.3 ± 3.9 | 21.2 ± 4.0 | <0.01 |
| FM, kg | 9.4 ± 4.2 | 10.2 ± 4.2 | 0.03 |
| FM, % | 28.5 ± 6.4 | 31.5 ± 5.9 | <0.01 |
| BMI z score | 0.7 ± 1.5 | 0.6 ± 1.3 | 0.35 |
| Child overweight,2n (%) | 47 (17) | 55 (22) | 0.15 |
| Child obese,2n (%) | 70 (25) | 43 (17) | 0.02 |
| HAZ | 0.05 ± 0.90 | −0.05 ± 1.05 | 0.26 |
| Parity, n (%) | |||
| First child | 107 (38) | 96 (38) | 0.92 |
| Second child or more | 174 (62) | 159 (62) | |
| Maternal age at birth, y | 27 ± 4.72 | 26.38 ± 4.68 | 0.17 |
| Maternal education, y | 12.1 ± 3.48 | 11.85 ± 3.59 | 0.42 |
| SES, n (%) | |||
| Low | 80 (29) | 78 (30) | 0.59 |
| Medium | 91 (32) | 88 (35) | |
| High | 110 (39) | 89 (35) | |
Values are means ± SDs unless otherwise stated. P values are for χ2 tests (categorical variables) or t tests (continuous variables). FFM, fat free mass; FM, fat mass; HAZ, height-for-age z score; POSGRAD, Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development; SES, socioeconomic status (based on tertiles of study sample).
WHO cutoffs (36): overweight: > +1 SD (equivalent to BMI 25 kg/m2 at 19 y); obesity: > +2 SD (equivalent to BMI 30 kg/m2 at 19 y).
Height trajectories
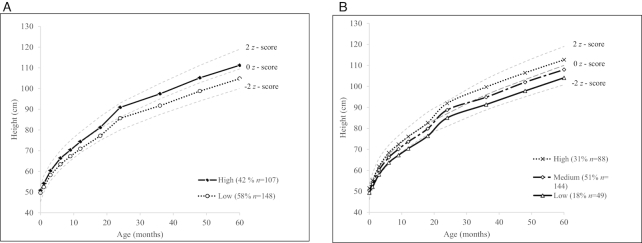
The best-fitting LCGT for height, based on model fit and quality of classification, identified 2 latent classes in girls (low and high) (Figure 1A) and 3 classes in boys (low, medium, and high) (Figure 1B) (Supplemental Table 2). The 2-class model for girls and the 3-class model for boys had the highest entropy (>0.79), indicating successful convergence. Classes also had the highest posterior probabilities of candidate models (>0.92), suggesting high class separation. An additional class did not improve the fit, suggested by the Lo-Mendell-Rubin likelihood ratio test and the bootstrap likelihood ratio test (Supplemental Table 2). Among the girls’ height trajectories, by the time they reached 24 mo, the difference between classes was >5 cm and increased to 6.4 cm by 60 mo of age. For boys, the difference between the highest and the lowest trajectory reached 5 cm by 9 mo, rising to 8.6 cm by 60 mo of age.
FIGURE 1.
Sex-specific height trajectories derived from 11 measures of height in the first 5 y of life of study participants at follow-up in the Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development (POSGRAD) Study. Mean height (cm) by latent class group in girls (A) and boys (B) and reference birth to 5 y length/height-for-age WHO z scores in the background.
Relationship between LCHT and body composition at follow-up visit
Anthropometric characteristics of the sample by LCHT membership are shown in Table 2. There was no significant difference in age between the classes for both girls and boys. In both sexes, weight, height, waist circumference, FM (kg), FFM (kg), and HAZ score were significantly different between classes (P < 0.05). The results for FM were similar for both girls and boys, and higher class membership meant higher FM compared with their shorter counterparts (P < 0.01). For percent FM, there was no statistical difference between any of the classes for boys, but girls in the high class had a higher percent FM compared with those in the low class.
TABLE 2.
Comparison of anthropometric characteristics by latent height class membership of children in the POSGRAD cohort at follow-up 8–10 y of age1
| Latent height classes | |||||||
|---|---|---|---|---|---|---|---|
| Boys, n = 281 (52.4%) | Girls, n = 255 (47.6%) | ||||||
| Class | High | Medium | Low | P value | High | Low | P value |
| n | 88 | 144 | 49 | 107 | 148 | ||
| Age, y | 8.8 ± 0.5 | 8.92 ± 0.5 | 8.9 ± 0.5 | 0.26 | 8.9 ± 0.5 | 8.8 ± 1.1 | 0.13 |
| Weight, kg | 35.0 ± 8.3 | 30.9 ± 7.0 | 28.6 ± 6.0 | <0.01 | 35.3 ± 7.5 | 28.6 ± 6.5 | <0.01 |
| Height, cm | 136.8 ± 4.5 | 131.6 ± 5.1 | 126.4 ± 5.0 | <0.01 | 136.4 ± 5.6 | 128.2 ± 5.8 | <0.01 |
| Waist circumference, cm | 70.0 ± 10.2 | 66.2 ± 9.1 | 64.2 ± 11.2 | <0.01 | 71.6 ± 9.2 | 64.6 ± 8.9 | <0.01 |
| FFM, kg | 24.2 ± 4.0 | 21.9 ± 3.6 | 20.2 ± 2.9 | <0.01 | 23.1 ± 3.6 | 19.8 ± 3.7 | <0.01 |
| FM, kg | 10.8 ± 4.8 | 9.0 ± 3.9 | 8.4 ± 3.5 | <0.01 | 12.1 ± 4.4 | 8.8 ± 3.4 | <0.01 |
| FM, % | 29.5 ± 6.8 | 28.0 ± 6.2 | 28.4 ± 6.2 | 0.22 | 33.5 ± 5.7 | 30.1 ± 5.6 | <0.01 |
| Child overweight,2n (%) | 14 (16) | 26 (18) | 7 (14) | — | 32 (30) | 26 (18) | — |
| Child obese,2n (%) | 27 (31) | 30 (21) | 13 (27) | — | 23 (22) | 17 (11) | — |
| BMI z score | 0.9 ± 1.6 | 0.6 ± 1.5 | 0.7 ± 1.4 | 0.20 | 1.0 ± 1.3 | 0.4 ± 1.2 | <0.01 |
| HAZ score | 0.9 ± 0.6 | −0.1 ± 0.7 | −0.9 ± 0.6 | <0.01 | 0.7 ± .1 | −0.6 ± 0.8 | <0.01 |
Latent height class membership allows individuals with similar growth characteristics to be grouped together such that 3 groups (high, medium, and low) were determined for all boys and 2 classes (high and low) were determined for girls. Values are means ± SDs unless otherwise stated. P values are for χ2 tests (categorical variables) or t tests (continuous variables). FFM, fat free mass; FM, fat mass; HAZ, height-for-age z score; POSGRAD, Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development.
WHO cutoffs (36): overweight: > +1 SD (equivalent to BMI 25 kg/m2 at 19 y); obese: > +2 SD (equivalent to BMI 30 kg/m2 at 19 y).
Results from the linear regression analyses for the relationship between LCHT and FM and FFM are summarized in Tables 3 and 4, respectively. In girls, LCHT was not statistically associated with either FM or FFM, relative to current body weight, regardless of the model used. In boys, relative to the intermediate LCHT, the low class had higher FM (P < 0.001) and the high class had lower FM (P < 0.05). As a consequence, for FFM, boys in the low LCHT had significantly less FFM (P < 0.001), and boys in the high LCHT had more FFM (P < 0.03) compared with the intermediate group.
TABLE 3.
Multivariable linear regression analyses on the relationship between latent height class membership and fat mass in the POSGRAD cohort at follow-up 8–10 y of age1
| Boys | Girls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 12 | Model 23 | Model 12 | Model 23 | |||||||||
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| Latent class | ||||||||||||
| High | −0.40 | (−0.75 to 0.05) | 0.03 | −0.40 | (−0.76 to 0.05) | 0.03 | −0.11 | (−0.48 to 0.27) | 0.57 | −0.17 | (−0.54 to 0.21) | 0.19 |
| Medium | — | — | — | — | — | — | — | — | — | — | — | — |
| Low | 0.66 | (0.24–1.08) | <0.01 | 0.69 | (0.26–1.11) | <0.01 | — | — | — | — | — | — |
| Weight, kg | 0.54 | (0.52–0.56) | <0.01 | 0.54 | (0.52–0.56) | <0.01 | 0.52 | (0.49–0.54) | <0.01 | 0.52 | (0.49–0.54) | <0.01 |
| SES | ||||||||||||
| Low4 | — | — | — | — | — | — | — | — | — | — | — | — |
| Medium | — | — | — | 0.04 | (−0.26 to 0.44) | 0.84 | — | — | — | −0.37 | (−0.46 to 0.38) | 0.86 |
| High | — | — | — | −0.16 | (−0.57 to 0.24) | 0.43 | — | — | — | 0.40 | (−0.03 to 0.07) | 0.07 |
| Parity | — | — | — | −0.06 | (−.021 to 0.10) | 0.49 | — | — | — | −0.11 | (−0.27 to 0.05) | 0.19 |
| Maternal education, y | — | — | — | 0.002 | (−0.05 to 6.5) | 0.91 | — | — | — | 0.02 | (0.03 to 0.07) | 0.48 |
Latent height class membership allows individuals with similar growth characteristics to be grouped together such that 3 groups (high, medium, and low) were determined for all boys and 2 classes (high and low) were determined for girls, with the medium group as the reference group for boys and the low group as the reference group for girls. POSGRAD, Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development; SES, socioeconomic status (based on tertiles of study sample).
Values in model 1 are regression coefficients adjusted for weight (kg) at follow-up (8–10 y).
Values in model 2 are regression coefficients adjusted for weight (kg) at follow-up (8–10 y), SES (low, medium, and high), parity, and maternal education (years).
Reference group.
TABLE 4.
Multivariable linear regression analyses on the relationship between latent height class membership and lean body mass in the POSGRAD cohort at follow-up 8–10 years of age1
| Boys | Girls | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 12 | Model 23 | Model 12 | Model 23 | |||||||||
| β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | β | 95% CI | P value | |
| Latent class | ||||||||||||
| High | 0.40 | (0.05–0.75) | 0.03 | 0.40 | (0.05– 0.76) | 0.03 | 0.11 | (−0.27 to 0.48) | 0.57 | 0.17 | (−0.21 to 0.54) | 0.38 |
| Medium | — | — | — | — | — | — | — | — | — | — | — | — |
| Low | −0.66 | (−1.08 to −0.24) | <0.01 | −0.69 | (−1.11 to −0.26) | <0.01 | — | — | — | — | — | — |
| Weight, kg | 0.46 | (0.44–0.48) | <0.01 | 0.46 | (0.44–0.48) | <0.01 | 0.48 | (0.46–0.51) | <0.01 | 0.48 | (0.46–0.51) | <0.01 |
| SES | ||||||||||||
| Low4 | — | — | — | — | — | — | — | — | — | — | ||
| Medium | — | −0.04 | (−0.44 to 0.36) | 0.84 | — | — | — | 0.04 | (−0.38 to 0.46) | 0.86 | ||
| High | — | 0.16 | (−0.24 to 0.57) | 0.43 | — | — | — | −0.40 | (−0.83 to 0.03) | 0.07 | ||
| Parity | — | 0.06 | (−0.10 to 0.21) | 0.49 | — | — | — | 0.11 | (−0.05 to 0.27) | 0.19 | ||
| Maternal education, y | — | −0.003 | (−0.05 to 0.05) | 0.91 | — | — | — | −0.02 | (0.07 to 0.03) | 0.48 | ||
Latent height class membership allows individuals with similar growth characteristics to be grouped together such that 3 groups (high, medium, and low) were determined for all boys and 2 classes (high and low) were determined for girls, with the medium group as the reference group for boys, and the low group as the reference group for girls. POSGRAD, Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development; SES, socioeconomic status (based on tertiles of study sample).
Values in model 1 are regression coefficients adjusted for weight (kg) at follow-up (8–10 y).
Values in model 2 are regression coefficients adjusted for weight (kg) at follow-up (8–10 y), SES (low, medium, and high), parity, and maternal education (y).
Reference group.
Discussion
As the global prevalence of childhood overweight and obesity continues to increase, especially in LMICs, it remains important to improve our understanding of how early linear growth faltering may influence the risk of obesity later in life. In our study, boys in the lowest height trajectory class had greater FM and lower FFM, after adjusting for differences in body weight, compared with boys in the intermediate height trajectory. At the same time, no such relationship was determined for girls. Our results support the hypothesis that poor or delayed growth in early life has a negative influence on body composition later in life. However, the fact that there may be some influence of sexual dimorphism is consistent with other studies (20) and merits additional investigation in similar cohorts.
Previous research has reported that rapid growth during infancy and early childhood was associated with early BMI rebound (10, 44). It has also been found that early adiposity rebound is a risk factor for adult obesity (45, 46). However, few studies have investigated growth in relation to body composition (FFM and FM) (47, 48). In our study, boys with lower linear growth trajectories at 5 y of age had significantly greater FM and lower FFM in later childhood compared with boys in the middle growth trajectory. Boys in the higher linear growth trajectories at 5 y of age had lower FM and higher FFM compared with boys in the middle growth trajectory. Findings from a recent study by de Beer et al. (47), in which linear growth was analyzed separately from relative weight gain, suggest that faster weight gain, only when accompanied with rapid linear gain, is associated with healthier childhood body composition. As well, data from LMICs suggest that conditional height at 2 y of age and midchildhood has a positive association with FFM (44). However, studies that provide divergent results were based on infant weight gain and are from high-income countries that show a predominant positive correlation between postnatal weight gain and later FM (49–51). In addition, rapid weight gain as a result of linear growth produces a greater increase in lean mass than fat mass, whereas rapid fat mass accrual during infancy is a better predictor of childhood obesity (52). Overall, these results suggest that early childhood may be a critical period for obesity development.
A number of studies have addressed whether or not poor growth is associated with excess adiposity in adolescence and adulthood (22, 53). For example, results from the Fels Longitudinal Study suggested that rapid weight gain from infancy to age 2 y was associated with increased FM, measured using MRI and DXA (54). As well, relative weight or height gain, but not birth weight, was positively associated with body size and fat mass in children from the Birth to Twenty Plus Cohort (Bto20) (55). A cohort study in Peru found that the rate of weight gain, but not size at birth, was positively associated with BMI, adjusted for age and sex (56). To our knowledge, our study is the first to investigate the influence of specific linear growth trajectories using LCGA in early childhood on body composition in later childhood. A number of factors differentiate our study from previous ones, including the use of longitudinal analyses instead of change in BMI z score or weight, the use of body composition variables rather than BMI, and adjusting for current weight to distinguish body composition compartments instead of simply fat mass. Perhaps more important, by using LCGA, the data were expressed as the relative growth rate for a group and not as individual changes, such as growing from short to average or from average to tall.
Stunting, a more severe form of linear growth retardation, has been reported to increase the risk of obesity (14, 15). One large cross-sectional study of several countries (Brazil, Russia, and South Africa) reported that stunted adults had an increased risk of obesity (14). Yet, a longitudinal cohort study in Bolivia found that stunting is negatively associated with BMI z score and fatness, assessed using skinfold measurements (23). Similar results were reported from a longitudinal cohort study in Jamaica, except that it was also found that stunted children who grew more rapidly during childhood had a higher BMI at age 17 y compared with those who grew less rapidly (21). Finally, stunting at age 2 y was not associated with obesity in the Bto20 (22). Although most Mexican children in our study (>80%) were not classified as stunted at age 2 y, boys who were shorter than their peers early in childhood and remained shorter for their first 5 y of life had a greater FM relative to their current weight compared with boys in the intermediate or high LCHT. These results are of particular concern as it has been suggested that growth-retarded children may be predisposed to developing obesity later in life, within specific environmental conditions (29, 57–59).
As with any study, certain limitations merit discussion to most fully appreciate the results presented. First, the trajectory classes developed were determined within the framework of LCGAs, allowing one to see variability within a population. Trajectory groups are latent strata (60), meaning that the groups developed are composed of individuals following approximately the same growth course. Individuals are assigned a probability of membership to the class, but they do not necessarily belong to a class. In this study, models were selected based on the highest posterior probability (>0.92) to assess the quality of classification. Simply, LCGA classes are not concrete but are sound statistical devices that allow one to see variabilities in distinct regions of distribution (61, 62). Second, it is not always possible to control for unknown confounding factors that were not measured, such as dietary intake, physical activity, energy expenditure, environmental toxins (e.g., endocrine disruptors), and other factors associated with energy balance. It is unclear if including any of these variables would have strengthened or weakened our data, but having data on energy balance or environmental exposures would allow for more nuanced conclusions to be made from our data. Finally, we did not have clinical data on pubertal development that may have influenced growth, including rapid changes in body size and composition. In fact, the sexually dimorphic differences between boys and girls may have influenced the regional distribution of body fat (63). Nonetheless, a number of important strengths to our study lend considerable credence to the results presented. For example, we successfully collected anthropometric data at 60 mo for >90% of the original birth cohort. As well, there were no significant differences in maternal and SES characteristics between the final subsample at follow-up and the original cohort. Finally, body composition was assessed using a valid and precise methodology (BIA), and FM was calculated from raw data using a prediction equation that had been validated for Mexican children. Furthermore, as the boys and girls in the higher growth trajectories had greater body weight compared with the low-growth trajectory, we controlled for body weight to best determine the relationship between body composition compartments and growth trajectories.
In summary, based on the results of this study, slower height gain during early childhood contributes to excess adiposity later in childhood. As the prevalence of childhood obesity continues to increase in many developing and transitional countries, a greater understanding of how growth contributes to the double burden of disease is warranted. In particular, future research needs to focus on discrete aspects of growth and the development of obesity to better understand how to prevent or reverse the double burden of disease.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—PLB and DJH: designed the overall research study; JAR, AB-V, LH-C, IR, and UR: conceived of and conducted the initial intervention study; PLB and RG-F: conducted the data collection; PLB: performed the statistical analysis; PLB and DJH: wrote the paper; DJH: had primary responsibility for final content; and all authors: have read and approved the final manuscript.
Notes
This study was funded by NIH grants HD-043099 and HD-058818; the March of Dimes Foundation; the Grant Sectoral Fund in Health and Social Security Research CONACYT 202062, 233903, and 262140; and the New Jersey Institute for Food, Nutrition, and Health.
Author disclosures: PLB, RG-F, JAR, AB-V, LH-C, IR, IG-C, UR, and DJH, no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: BIA, bioelectrical impedance analysis; Bto20, Birth to Twenty Plus Cohort; FFM, fat-free mass; FM, fat mass; HAZ, height-for-age z score; LCGA, latent class growth analysis; LCHT, latent class height trajectory; LHC, latent height class; LMIC, low- and middle-income country; POSGRAD, Prenatal Omega-3 Fatty Acid Supplementation and Child Growth and Development; SES, socioeconomic status; WC, waist circumference.
References
- 1. World Health Organization (WHO) Obesity and overweight: key facts [Internet]. 2018; [cited 4 December 2018]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 2. Rivera JA, de Cossio TG, Pedraza LS, Aburto TC, Sanchez TG, Martorell R. Childhood and adolescent overweight and obesity in Latin America: a systematic review. Lancet Diabetes Endocrinol. 2014;2(4):321–32. [DOI] [PubMed] [Google Scholar]
- 3. PAHO Health information and analysis. Health situation in the Americas: basic indicators 2017 [Internet]. Washington (DC): Pan American Health Organization; 2017. Available from: http://iris.paho.org/xmlui/bitstream/handle/123456789/34329/CoreIndicators2017_eng.pdf?sequence = 1&isAllowed = y&ua = 1. [Google Scholar]
- 4. Daniels SR. Complications of obesity in children and adolescents. Int J Obes. 2009;33(Suppl 1):S60–5. [DOI] [PubMed] [Google Scholar]
- 5. Stettler N, Iotova V. Early growth patterns and long-term obesity risk. Curr Opin Clin Nutr Metab Care. 2010;13(3):294–9. [DOI] [PubMed] [Google Scholar]
- 6. Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright CM, Marryat L, McColl J, Harjunmaa U, Cole TJ. Pathways into and out of overweight and obesity from infancy to mid-childhood. Pediatr Obes. 2018;13(10):621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Inter-relationships among childhood BMI, childhood height, and adult obesity: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28(1):10–16. [DOI] [PubMed] [Google Scholar]
- 9. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014;370(5):403–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brisbois TD, Farmer AP, McCargar LJ. Early markers of adult obesity: a review. Obes Rev. 2012;13(4):347–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Corvalan C, Gregory CO, Ramirez-Zea M, Martorell R, Stein AD. Size at birth, infant, early and later childhood growth and adult body composition: a prospective study in a stunted population. Int J Epidemiol. 2007;36(3):550–7. [DOI] [PubMed] [Google Scholar]
- 12. McCarthy A, Hughes R, Tilling K, Davies D, Smith GD, Ben-Shlomo Y. Birth weight; postnatal, infant, and childhood growth; and obesity in young adulthood: evidence from the Barry Caerphilly Growth Study. Am J Clin Nutr. 2007;86(4):907–13. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization (WHO); WHO Multicentre Growth Reference Study Group WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): World Health Organization; 2006. [Google Scholar]
- 14. Popkin BM, Richards MK, Montiero CA. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr. 1996;126(12):3009–16. [DOI] [PubMed] [Google Scholar]
- 15. El Taguri A, Besmar F, Abdel Monem A, Betilmal I, Ricour C, Rolland-Cachera MF. Stunting is a major risk factor for overweight: results from national surveys in 5 Arab countries. East Mediterr Health J. 2009;15(3):549–62. [PubMed] [Google Scholar]
- 16. Rivera JA, Pedraza LS, Martorell R, Gil A. Introduction to the double burden of undernutrition and excess weight in Latin America. Am J Clin Nutr. 2014;100(6):1613S–6S. [DOI] [PubMed] [Google Scholar]
- 17. Tzioumis E, Adair LS.. Childhood dual burden of under- and overnutrition in low- and middle-income countries: a critical review. Food Nutr Bull. 2014;35(2):230–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benefice E, Garnier D, Simondon KB, Malina RM. Relationship between stunting in infancy and growth and fat distribution during adolescence in Senegalese girls. Eur J Clin Nutr. 2001;55(1):50–8. [DOI] [PubMed] [Google Scholar]
- 19. Schroeder DG, Martorell R.. Fatness and body mass index from birth to young adulthood in a rural Guatemalan population. Am J Clin Nutr. 1999;70(1):137S–44S. [DOI] [PubMed] [Google Scholar]
- 20. Schroeder DG, Martorell R, Flores R. Infant and child growth and fatness and fat distribution in Guatemalan adults. Am J Epidemiol. 1999;149(2):177–85. [DOI] [PubMed] [Google Scholar]
- 21. Walker SP, Chang SM, Powell CA. The association between early childhood stunting and weight status in late adolescence. Int J Obes. 2007;31(2):347–52. [DOI] [PubMed] [Google Scholar]
- 22. Cameron N, Wright MM, Griffiths PL, Norris SA, Pettifor JM. Stunting at 2 years in relation to body composition at 9 years in African urban children. Obes Res. 2005;13(1):131–6. [DOI] [PubMed] [Google Scholar]
- 23. Tanner S, Leonard WR, Reyes-Garcia V; TAPS Bolivia Study Team . The consequences of linear growth stunting: influence on body composition among youth in the Bolivian Amazon. Am J Phys Anthr. 2014;153(1):92–102. [DOI] [PubMed] [Google Scholar]
- 24. Hoffman D, Reynolds R, Hardy D. Developmental origins of health and disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75:951–70. [DOI] [PubMed] [Google Scholar]
- 25. Fleming TP, Watkins AJ, Velazquez MA, Mathers JC, Prentice AM, Stephenson J, Barker M, Saffery R, Yajnik CS, Eckert JJ et al.. Origins of lifetime health around the time of conception: causes and consequences. Lancet. 2018;391(10132):1842–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martorell R, Zongrone A.. Intergenerational influences on child growth and undernutrition. Paediatr Perinat Epidemiol. 2012;26(Suppl 1):302–14. [DOI] [PubMed] [Google Scholar]
- 27. Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6(6):2165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Taveras EM, Rifas-Shiman SL, Sherry B, Oken E, Haines J, Kleinman K, Rich-Edwards JW, Gillman MW. Crossing growth percentiles in infancy and risk of obesity in childhood. Arch Pediatr Adolesc Med. 2011;165(11):993–8. [DOI] [PubMed] [Google Scholar]
- 29. Walker SP, Gaskin PS, Powell CA, Bennett FI. The effects of birth weight and postnatal linear growth retardation on body mass index, fatness and fat distribution in mid and late childhood. Public Health Nutr. 2002;5(3):391–6. [DOI] [PubMed] [Google Scholar]
- 30. Hernández Ávila M, Rivera-Dommarco J, Shamah Levy T, Cuevas Nasu L, María GA, Gaona Pineda EB, Romero Martínez M, Gómez-Humarán IM, Hernández PS, Hernández SV et al.. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016 [Internet]. Cuernavaca (México): Instituto Nacional de Salud Pública (MX); 2016; [cited 10 March 2018]. Available from: http://transparencia.insp.mx/2017/auditorias-insp/12701_Resultados_Encuesta_ENSANUT_MC2016.pdf. [Google Scholar]
- 31. Kroker-Lobos MF, Pedroza-Tobias A, Pedraza LS, Rivera JA. The double burden of undernutrition and excess body weight in Mexico. Am J Clin Nutr. 2014;100(6):1652S–8S. [DOI] [PubMed] [Google Scholar]
- 32. Ramakrishnan U, Stein AD, Parra-Cabrera S, Wang M, Imhoff-Kunsch B, Juarez-Marquez S, Rivera J, Martorell R. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010;31(2 Suppl):S108–16. [DOI] [PubMed] [Google Scholar]
- 33. Gonzalez-Casanova I, Stein AD, Hao W, Garcia-Feregrino R, Barraza-Villarreal A, Romieu I, Rivera JA, Martorell R, Ramakrishnan U. Prenatal supplementation with docosahexaenoic acid has no effect on growth through 60 months of age. J Nutr. 2015;145(6):1330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Soc Pers Psychol Compass. 2008;2(1):302–17. [Google Scholar]
- 35. World Health Organization (WHO) Physical status: the use of and interpretation of anthropometry, report of a WHO Expert Committee. Geneva (Switzerland): World Health Organization; 1995. [PubMed] [Google Scholar]
- 36. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramirez E, Valencia ME, Bourges H, Espinosa T, Moya-Camarena SY, Salazar G, Alemán-Mateo H. Body composition prediction equations based on deuterium oxide dilution method in Mexican children: a national study. Eur J Clin Nutr. 2012;66(10):1099–103. [DOI] [PubMed] [Google Scholar]
- 38. Nylund KL, Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: a Monte Carlo simulation study. Struct Equ Model. 2007;14(4):535–69. [Google Scholar]
- 39. Hoekstra T, Barbosa-Leiker C, Koppes LLJ, Twisk JWR. Developmental trajectories of body mass index throughout the life course: an application of latent class growth (mixture) modelling. Longit Life Course Stud. 2011;2(3):319–30. [Google Scholar]
- 40. Broere-Brown ZA, Baan E, Schalekamp-Timmermans S, Verburg BO, Jaddoe VW, Steegers EA. Sex-specific differences in fetal and infant growth patterns: a prospective population-based cohort study. Biol Sex Differ. 2016;7(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Collins LM, Lanza ST. Latent Class and Latent Transition Analysis: With Applications in the Social, Behavioral, and Health Sciences. Hoboken (NJ): Wiley; 2010. p. 179–224. [Google Scholar]
- 42. Berlin KS, Parra GR, Williams NA. An introduction to latent variable mixture modeling (part 2): longitudinal latent class growth analysis and growth mixture models. J Pediatr Psychol. 2014;39(2):188–203. [DOI] [PubMed] [Google Scholar]
- 43. Ram N, Grimm KJ.. Growth mixture modeling: a method for identifying differences in longitudinal change among unobserved groups. Int J Behav Dev. 2009;33(6):565–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SA et al.. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382(9891):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes 2005. 2006;30(Suppl 4):S11–17. [DOI] [PubMed] [Google Scholar]
- 46. Péneau S, González-Carrascosa R, Gusto G, Goxe D, Lantieri O, Fezeu L, Hercberg S, Rolland-Cachera MF. Age at adiposity rebound: determinants and association with nutritional status and the metabolic syndrome at adulthood. Int J Obes 2005. 2016;40(7):1150–6. [DOI] [PubMed] [Google Scholar]
- 47. de Beer M, Vrijkotte TG, Fall CH, van Eijsden M, Osmond C, Gemke RJ. Associations of infant feeding and timing of linear growth and relative weight gain during early life with childhood body composition. Int J Obes. 2015;39(4):586–92. [DOI] [PubMed] [Google Scholar]
- 48. Araujo de Franca GV, De Lucia Rolfe E, Horta BL, Gigante DP, Yudkin JS, Ong KK, Victora CG. Associations of birth weight, linear growth and relative weight gain throughout life with abdominal fat depots in adulthood: the 1982 Pelotas (Brazil) birth cohort study. Int J Obes. 2016;40(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ong KK, Emmett P, Northstone K, Golding J, Rogers I, Ness AR, Wells JC, Dunger DB. Infancy weight gain predicts childhood body fat and age at menarche in girls. J Clin Endocrinol Metab. 2009;94(5):1527–32. [DOI] [PubMed] [Google Scholar]
- 50. Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87(6):1776–84. [DOI] [PubMed] [Google Scholar]
- 51. Leunissen RW, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA. 2009;301(21):2234–42. [DOI] [PubMed] [Google Scholar]
- 52. Koontz MB, Gunzler DD, Presley L, Catalano PM. Longitudinal changes in infant body composition: association with childhood obesity. Pediatr Obes. 2014;9(6):e141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27(10):1274–82. [DOI] [PubMed] [Google Scholar]
- 54. Demerath EW, Reed D, Choh AC, Soloway L, Lee M, Czerwinski SA, Chumlea WC, Siervogel RM, Towne B. Rapid postnatal weight gain and visceral adiposity in adulthood: the Fels Longitudinal Study. Obesity. 2009;17(11):2060–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Prioreschi A, Munthali RJ, Kagura J, Said-Mohamed R, De Lucia Rolfe E, Micklesfield LK, Norris SA. The associations between adult body composition and abdominal adiposity outcomes, and relative weight gain and linear growth from birth to age 22 in the Birth to Twenty Plus cohort, South Africa. PLoS One. 2018;13(1):e0190483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sterling R, Miranda JJ, Gilman RH, Cabrera L, Sterling CR, Bern C, Checkley W. Early anthropometric indices predict short stature and overweight status in a cohort of Peruvians in early adolescence. Am J Phys Anthr. 2012;148(3):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hoffman D, Sawaya A, Coward W, Wright A. Energy expenditure of stunted and nonstunted boys and girls living in the shantytowns of São Paulo, Brazil. Am J Clin Nutr. 2000;72:1025–31. [DOI] [PubMed] [Google Scholar]
- 58. Lee SK, Nam SY, Hoffman DJ. Growth retardation at early life and metabolic adaptation among North Korean children. J Dev Orig Health Dis. 2015;6(4):291–8. [DOI] [PubMed] [Google Scholar]
- 59. Leonard WR, Sorensen MV, Mosher MJ, Spitsyn V, Comuzzie AG. Reduced fat oxidation and obesity risks among the Buryat of Southern Siberia. Am J Hum Biol. 2009;21(5):664–70. [DOI] [PubMed] [Google Scholar]
- 60. Nagin D. Group-based modeling of development. Cambridge (MA): Harvard University Press; 2005. [Google Scholar]
- 61. Nagin DS, Odgers CL.. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6(1):109–38. [DOI] [PubMed] [Google Scholar]
- 62. Tu YK, Tilling K, Sterne JA, Gilthorpe MS. A critical evaluation of statistical approaches to examining the role of growth trajectories in the developmental origins of health and disease. Int J Epidemiol. 2013;42(5):1327–39. [DOI] [PubMed] [Google Scholar]
- 63. Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31(6):192–200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.