Abstract
Studies suggest that inflammation might be involved in the pathogenesis of depression. Individuals with human immunodeficiency virus (HIV) have a higher risk of depression and elevated inflammatory profiles. Despite this, research on the link between inflammation and depression among this high-risk population is limited. We examined a sample of men who have sex with men from the Multicenter AIDS Cohort Study in prospective analyses of the association between inflammation and clinically relevant depression symptoms, defined as scores >20 on Center for Epidemiological Studies Depression Scale. We included 1,727 participants who contributed 9,287 person-visits from 1984 to 2010 (8,218 with HIV (HIV+) and 1,069 without (HIV−)). Exploratory factor analysis (EFA) was used to characterize underlying inflammatory processes from 19 immune markers. Logistic regression with generalized estimating equations was used to evaluate associations between inflammatory processes and depressive symptoms stratified by HIV serostatus. Three EFA-identified inflammatory processes (EIPs) were identified. EIP-1 scores—described by soluble tumor necrosis factor receptor 2 (sTNF-R2), soluble interleukin-2 receptor α (sIL-2Rα), sCD27, B-cell activating factor, interferon γ-induced protein 10 (IP-10), soluble interleukin-6 receptor (sIL-6R), sCD14, and sGP130—were significantly associated with 9% higher odds of depressive symptoms in HIV+ participants (odds ratio = 1.09; 95% confidence interval: 1.03, 1.16) and 33% higher odds in HIV− participants (odds ratio = 1.33; 95% confidence interval: 1.09, 1.61). Findings suggest that immune activation might be involved in depression risk among both HIV+ and HIV− men who have sex with men.
Keywords: biomarkers, depression, HIV, immune activation, inflammation
Depression occurs in nearly 1 in 4 women and 1 in 6 men during their lifetimes (1, 2), with the majority of individuals having recurrent episodes of depression. Furthermore, many people with depression never receive diagnoses or treatment, and only about 30%–35% of adults achieve remission using current therapeutic approaches, which leaves nearly two-thirds of the disease burden remaining (3–6). Hence, taken together, depression is estimated to be the fourth-leading cause of overall disease burden and the leading cause of nonfatal disease burden worldwide (7).
A heightened risk of depression is associated with the presence of somatic or physical disease conditions, and this elevated risk is especially evident in persons infected with human immunodeficiency virus (HIV). Indeed, major depression is more than 3–4 times more prevalent in HIV-positive (HIV+) persons than in persons who are not infected with HIV (HIV−), with an estimated lifetime prevalence as high as 22%–45% in persons with HIV (8, 9). Given that an estimated 37.9 million people worldwide are infected with HIV (10), and that treatment with antiretroviral therapy (ART) (11) and control of HIV replication and reductions in HIV viral load (12) does not diminish the risk of depression in persons with HIV, there is an urgent need to identify factors that might be targeted to prevent and treat depression in persons who are HIV+.
Inflammation, as indexed by circulating markers such as C-reactive protein (CRP), is elevated in persons with HIV, and this increase in systemic inflammation occurs even in HIV+ persons whose viral loads are well suppressed (13, 14). Whether inflammation is associated with heightened risk of depression in persons who are HIV+ is not known, although patients with major depressive disorder exhibit increased expression of proinflammatory cytokines and their receptors, as well as increases in acute phase reactants such as CRP. Furthermore, experimental data show that acute activation of inflammatory signaling and the induction of inflammatory cytokines causes depressive symptoms in otherwise nondepressed adults (15–18). Moreover, a blockade of inflammatory pathways reduces depressive symptoms in patients with inflammatory disorders such as rheumatoid arthritis, psoriasis, and cancer, as well as in patients with major depressive disorder who have high levels of inflammation prior to treatment (19). Together, these findings raise the possibility that increases in inflammation are involved in the pathogenesis of depression, and epidemiologic studies demonstrate that higher levels of markers of systemic inflammation such as interleukin-6 and CRP prospectively predict the development of depressive symptoms in otherwise healthy adults (20–22). Despite the increased prevalence of depression, and of inflammation, in HIV+ persons, there is limited research that has interrogated this link, and no study to our knowledge has examined whether underlying inflammatory processes correlate with depressive symptoms in HIV+ persons.
To address this gap, we examined men who have sex with men (MSM), a population at heightened risk of depression (23), who are also at substantial risk of HIV infection. MSM account for more than half of all new HIV infections in the United States annually (24). In this study, we examined the prevalence of depressive symptoms and heightened inflammation between HIV+ and HIV− MSM, as well as the prospective association between inflammation and depression among HIV+ MSM as compared with HIV− MSM.
METHODS
Study design and population
The Multicenter AIDS Cohort Study (MACS) is an ongoing prospective study, established in 1984, of HIV+ and HIV− MSM from 4 US sites (Baltimore, Maryland/Washington, DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania). Details of the MACS design have been described elsewhere (25). Briefly, data are collected semiannually using standardized questionnaires, physical examinations, and blood samples for laboratory testing and storage. MACS is approved by the institutional review boards at the 4 study sites, and each participant provided informed consent.
For the current study, MACS participants were included if they completed at least 1 study visit at which depressive symptoms and proximate inflammatory biomarkers were available. All person-visits were used after enrollment that had both inflammatory measures and depression symptom assessment.
Measurement of inflammatory markers
Twenty-four inflammatory markers were available that were measured at selected study visits from 1984 through 2010 from an inflammatory biomarker substudy (26–28). Of these, 19 markers with <10% of samples below the lower limit of detection were included in the analyses. The excluded markers (granular-macrophage colony-stimulating factor, interferon-γ, interleukin-2, interleukin-1β, and interleukin-12p70) had high levels of missingness that could bias final results. The remaining 19 markers were CRP, soluble tumor necrosis factor receptor 2 (sTNF-R2), soluble interleukin-2 receptor α (sIL-2Rα), sGP130, sCD27, B-cell activating factor, interferon γ-induced protein 10 (IP-10), soluble interleukin-6 receptor (sIL-6R), sCD14, interleukin-6, interleukin-8, tumor necrosis factor-α, macrophage inflammatory protein 1 β (MIP-1β), monocyte chemoattractant protein 1 (MCP-1), MCP-4, eotaxin, B-lymphocyte chemoattractant/B-cell-attracting chemokine 1 (BLC/BCA-1), interleukin-10, and thymus and activation-regulated chemokine.
As previously described (26), serum concentrations of sCD14, sCD27, sGP130, soluble interleukin-6 receptor alpha, soluble interleukin-6 receptor, soluble tumor necrosis factor receptor 2, B-cell activating factor, and BLC/BCA-1 were measured using the multiplexed Luminex platform with a single lot of assay kits (R&D Systems, Minneapolis, Minnesota). Concentrations of interleukin-6, interleukin-8, interleukin-10, tumor necrosis factor-α, MCP-1, macrophage inflammatory protein 1 β (MIP-1β), eotaxin, MCP-4, thymus and activation-regulated chemokine, and interferon γ-induced protein 10 (IP-10) were assessed using an electrochemiluminescence-based multiplexed Meso Scale Discovery (Meso Scale Diagnostics LLC, Rockville, Maryland) assay system. For each plate of serum samples tested, a plate-specific lower limit of detection was defined for each biomarker. An additional biomarker, CRP, was measured by Quest Diagnostics laboratory (Secaucus, New Jersey).
Assessment of depressive symptoms
Depressive symptoms were measured at every study visit using the Center for Epidemiology Studies Depression Scale (CES-D), a 20-item scale designed to assess the frequency of self-reported depressive symptoms experienced over the past week (29). The scale, ranging from 0 to 60, is a valid and reliable instrument, and it has been widely used in studies of MSM and HIV-infected populations (30, 31). Higher scores indicate higher severity of depressive symptoms. For the present study, CES-D responses with at least 16 nonmissing items were included, with score summaries up-weighted to reflect a full 20-item scale. We categorized the total CES-D score at each visit into 3 categories: CES-D >20 represented evidence of clinically relevant depressive symptoms; a score of 12–20 represented milder or domain-specific symptoms; and a score of <12 represented evidence of no depressive symptoms. Whereas a threshold of 16 is generally considered to be indicative of depressive symptomatology, these thresholds were chosen to improve sensitivity and specificity in an MSM cohort (32, 33).
Because depressive symptoms could be mitigated with antidepressant treatment without necessarily affecting an underlying inflammatory mechanism, an alternative definition of depression considered either current antidepressant use or CES-D >20, to capture both current and a recent history of depressive symptoms. A CES-D score of <12 and no antidepressant use was considered evidence of no current or recent depressive symptoms.
Covariate measurements
Age, race/ethnicity, educational level, height, weight, smoking status, alcohol intake, and cocaine use since last visit were self-reported at each visit. Body mass index was calculated as weight (kilograms) divided by height (meters) squared. Hepatitis C virus (HCV) infection was defined by a positive HCV RNA test at each visit. In statistical analysis, we considered age (continuous), black race (versus nonblack), college education (any versus none), obesity (body mass index: >30 versus ≤30), hepatitis C infection (current positive versus negative HCV RNA), current smoking (versus former/never), an alcohol intake indicator (none, 1–3 drinks/week, 4–13 drinks/week, and more than 13 drinks/week), and cocaine use (yes versus no) as potential confounders. HIV-related factors included ART use and viral suppression (HIV RNA <50 copies/mL). Covariate values including HIV serostatus were treated as time-varying and assessed at each included visit.
Statistical analysis
Imputation below the lower limit of detection
Biomarker values from the full study sample were natural log-transformed. Among the 19 markers retained in analyses with detectability >90%, 11 had at least 1 value below the lower limit of detection across the included person-visits. We imputed values for the 1,392 missing values of these 11 markers (0.8% of the total number of biomarker values) using a single stochastic imputation from truncated log-normal distributions fitted to the data for each marker. Specifically, we fitted a normal distribution to the log-transformed data, extrapolated to the tail of the distribution below the limit of detection, and drew values randomly to impute missingness below the limit of detection. This method maintained the distributional shape of the data for exploratory factor analysis (EFA).
Exploratory factor analysis
EFA was used to identify patterns of covariance between the set of 19 inflammatory biomarkers that might signify involvement in similar underlying processes (34). First, we standardized all biomarker values across all visits (on the log scale) to a mean of 0 and a standard deviation of 1. Maximum likelihood estimation was used to estimate correlations (referred to as factor loadings) between each inflammatory biomarker and underlying inflammatory processes. Orthogonal rotation was used to optimize separation between highly correlated and poorly correlated markers to discriminate primary contributors to each inflammatory process from biomarkers minimally involved. Inflammatory biomarkers with low factor loadings (<0.4) on all EFA-identified inflammatory processes (EIPs) were removed from the final EFA analysis. For each visit, the factor loadings were used as weights in weighted linear combinations of the biomarkers to estimate the magnitude of each inflammatory process (referred to as factor scores) for each individual, creating new composite variables representing underlying inflammatory processes. Thus the individual inflammatory marker levels were replaced as exposures by a smaller number of composite variables representing underlying inflammatory processes (e.g., immune activation, proinflammation, and cell recruitment and migration).
Regression analysis of inflammatory processes and depressive symptoms
The estimated standardized continuous factor scores from each EIP were used as independent variables in logistic regressions to evaluate associations between inflammatory processes and depressive symptoms over time, stratified by HIV serostatus. Models adjusted for age, black race, college education, current smoking, obesity, HCV infection, alcohol intake, and cocaine use. Generalized estimating equations (GEE) were used to account for multiple contributing observations from each participant. The primary estimate of interest was the relative odds of having clinically relevant depressive symptoms compared with having no depressive symptoms per standard-deviation increase in EIP scores. Terms for interaction between HIV serostatus and the EIPs were tested using Wald tests. We refitted models evaluating the relative odds of having mild depressive symptoms per standard-deviation increase in EIP scores, and we repeated the analysis using a standard CES-D threshold of 16 to allow for comparison with other literature.
Among HIV+ men, the impact of ART use and HIV viral suppression on the association between inflammation and clinically relevant depressive symptoms was evaluated using interaction terms and stratified logistic regression models.
To evaluate the consistency of the relationship between inflammatory marker and depressive symptoms with those reported in the literature, we also examined the association between CRP and depressive symptoms in our data.
Sensitivity analyses
We conducted sensitivity analyses to evaluate the assumption of a linear relationship between the log odds of depressive symptoms and the degree of inflammation. The continuous EIP scores were categorized into tertiles and used as indicator variables in place of continuous scores in logistic models. Given the long duration of follow-up, spanning across eras before and after combination ART (cART) (35, 36), separate analyses stratified by calendar time (before and after July 1, 1996, for before cART and the cART era, respectively) to evaluate the presence of heterogeneity of effect. We also examined a more homogeneous group of ART-naive participants at baseline.
All analyses were performed using SAS, version 9.3 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Description of the study sample
There were 1,727 participants with 9,287 person-visits (1,069 HIV− and 8,218 HIV+) at which both CES-D and measurements of inflammation markers were available. There were 147 seroconverters who contributed both HIV+ and HIV− person-visits. Across visits, the CES-D score was internally consistent (Cronbach’s α = 0.93). Table 1 presents characteristics of MACS participants in the full study sample. There was a median of 4 visits (interquartile range, 3–6) per participant. HIV− and HIV+ individuals over time had similar age distributions, with a median of 44 years. However, HIV− individuals were more likely to be black, current smokers, or HCV-infected.
Table 1.
Demographic and Other Characteristics of the Full Sample of 1,727 Participants With 9,287 Person-Visits for a Study of Inflammation and Risk of Depression, Multicenter AIDS Cohort Study, United States, 1984–2010
| Characteristic | HIV-Positivea | HIV-Negativea | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | Median (IQR) | No. | % | Median (IQR) | No. | % | Median (IQR) | |
| Number of individuals contributing visitsb | 1,476 | 398 | 1,727 | ||||||
| CES-D score of >20 | 1,450 | 18 | 180 | 17 | 1,630 | 18 | |||
| CES-D score of >20 or antidepressant use | 2,636 | 32 | 277 | 26 | 2,913 | 31 | |||
| Age at visit, years | 44 (38–50) | 44 (38–51) | 44 (38–50) | ||||||
| Black race | 1,628 | 20 | 373 | 35 | 2,001 | 22 | |||
| BMI at visitc | 24.4 (22.4–27.1) | 25.2 (23.1–28.0) | 24.5 (22.5–27.1) | ||||||
| At least some college education | 7,007 | 85 | 859 | 80 | 7,866 | 85 | |||
| Current smoking | 2,582 | 31 | 426 | 40 | 3,008 | 32 | |||
| Alcohol use since last visit, drinks per week | |||||||||
| 0 | 1,613 | 20 | 204 | 19 | 1,817 | 19 | |||
| 1–3 | 3,839 | 47 | 449 | 42 | 4,288 | 46 | |||
| 4–13 | 2,173 | 26 | 308 | 29 | 2,481 | 27 | |||
| >13 | 593 | 7 | 108 | 10 | 701 | 8 | |||
| Cocaine use since last visit | 1,155 | 14 | 212 | 20 | 1,367 | 15 | |||
| HCV-positive at visit | 592 | 7 | 201 | 19 | 793 | 9 | |||
| Social support at visitd | 7,869 | 96 | 999 | 93 | 8,868 | 95 | |||
| HIV-related factors | |||||||||
| CD4+ T-cell count (cells/μL) | 506 (331–710) | ||||||||
| HIV RNA <50 copies/mLe | 3,115 | 39 | |||||||
| In cART eraf | 5,922 | 72 | |||||||
| ART useg | 5,884 | 75 | . | ||||||
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; cART, combination antiretroviral therapy; CES-D, Center for Epidemiologic Studies Depression Scale; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
a All characteristics except for number of individuals contributing visits were calculated on the basis of total person-visits. There were 8,218 person-visits among HIV-positive participants and 1,069 person-visits among HIV-negative participants.
b There were 147 seroconverters who contributed both HIV-seropositive and -seronegative person-visits.
c Weight (kg)/height (m)2.
d Social support was assessed using 1-item question at each visit “Is there someone you can talk to about things that are important to you—someone you can count on for understanding or support” (yes (1 or more) versus no (none)).
e Among 8,218 HIV-seropositive person-visits, 7,907 (96%) had HIV viral load available.
f The cART era was defined as time after July 1, 1996.
g Among 8,218 HIV seropositive person-visits, 7,800 (95%) had nonmissing ART use report.
Inflammation and depression among HIV+ and HIV− MSM
Depression according to HIV status
Antidepressant use was more prevalent in HIV+ individuals over time, at 21% compared with 13% for HIV− individuals (P < 0.0001). The prevalence of clinically relevant depressive symptoms was 18% among HIV+ and 17% among HIV− individuals (Table 1). When treatment for depression was considered in the definition of clinically relevant depressive symptoms, the prevalence rose to 32% among HIV+ participants and 26% among HIV− participants (Table 1).
Underlying inflammatory processes in MSM
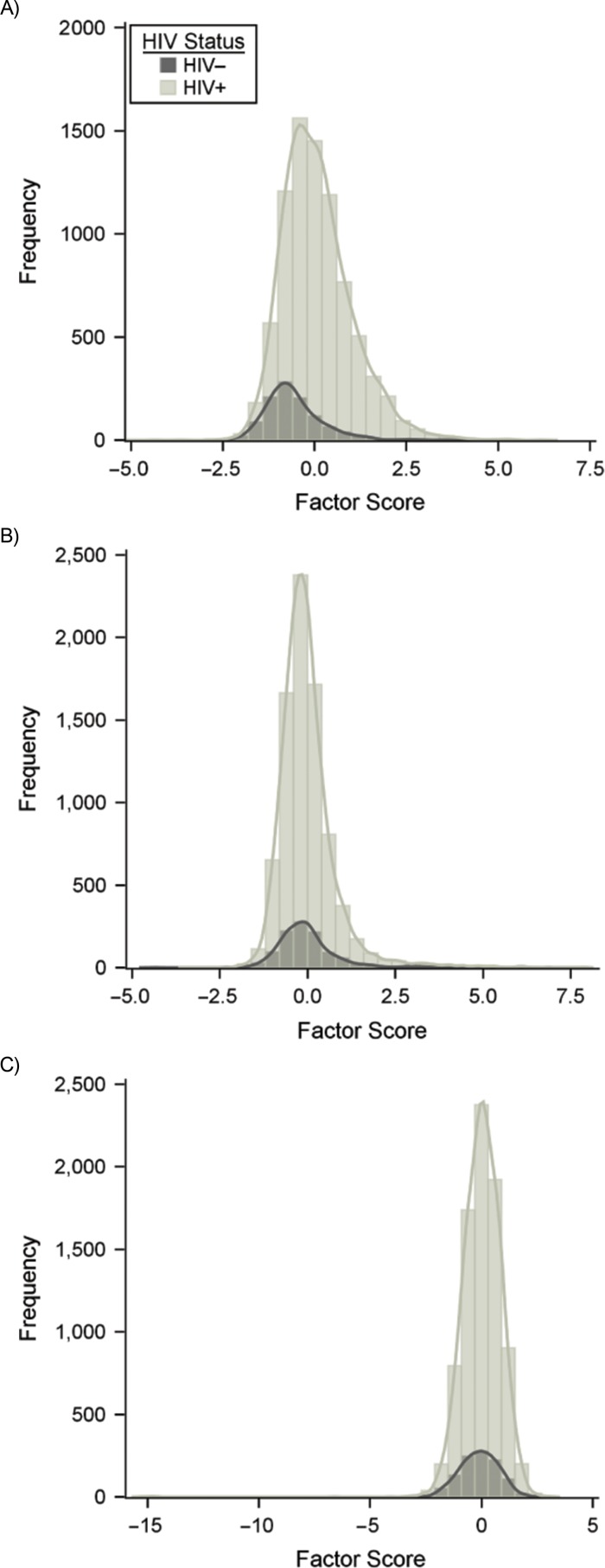
The EFA results are presented in Web Table 1 (available at https://academic.oup.com/aje). The repeatability of EFA biomarker results across samples and time points in the MACS has been examined previously and found to be high (26, 37). Utilizing all person-visits from the full study sample, 3 EIPs were identified, accounting for 57% of the total variance in the biomarker data. EIP-1 was characterized by markers of immune activation including sTNF-R2, soluble interleukin-2 receptor α (sIL-2rα), sCD27, B-cell activating factor, interferon γ-induced protein 10 (IP-10), sIL-6R, sCD14, and sGP130; EIP-2 was characterized by markers of proinflammation including interleukin-6, interleukin-8, tumor necrosis factor-α, and macrophage inflammatory protein 1 β (MIP-1β) levels; EIP-3 was characterized markers of cell recruitment, migration, and infiltration, including MCP-1, eotaxin, and MCP-4 levels (38). The distributions of estimated EIP scores are presented in Figure 1, stratified by HIV serostatus. Biomarker levels were generally higher among HIV+ individuals, particularly for EIP-1 (Figure 1 and Web Table 2).
Figure 1.
Factor score distribution for each exploratory factor analysis–identified inflammatory process (EIP) according to HIV serostatus, using person-visits in the Multicenter AIDS Cohort Study, United States, 1984–2010. A) Factor score distribution of EIP-1, which was characterized mainly by soluble tumor necrosis factor receptor 2 (sTNFR2), soluble interleukin-2 receptor α (sIL-2Rα), sCD27, B-cell activating factor, interferon γ-induced protein 10, soluble interleukin-6 receptor (sIL-6R), sCD14, and sGP130; B) factor score distribution of EIP-2, characterized by interleukin-6, interleukin-8, tumor necrosis factor-α, and macrophage inflammatory protein 1 β (MIP-1β); C) factor score distribution of EIP-3, characterized by monocyte chemoattractant protein 1 (MCP-1), eotaxin, and MCP-4.
Relationship between inflammatory processes and depressive symptoms
Markers of immune activation and clinically relevant depressive symptoms
Among HIV+ participants, 1 standard-deviation higher EIP-1 was significantly associated with a 9% higher odds of depressive symptoms (odds ratio (OR) = 1.09, 95% confidence interval (CI): 1.03, 1.16). Among HIV− participants, 1 standard-deviation increase in EIP-1 scores was significantly associated with a 33% higher odds of clinically relevant depressive symptoms (OR = 1.33, 95% CI: 1.09, 1.61). Associations between EIP-2 and EIP-3 and clinically relevant depressive symptoms were weaker and not statistically significant. Results are shown in Table 2, stratified by HIV serostatus. Although the magnitude of the difference in effect size between HIV+ and HIV− participants was large, the P for interaction was >0.05. Inclusion of self-reported antidepressant use in the definition of clinically relevant depressive symptoms did not substantially change the results.
Table 2.
Adjusted Logistic Regressiona of Depressive Symptoms on Continuous Estimates of Exploratory Factor Analysis–Identified Inflammatory Processes Among Person-Visits in the Multicenter AIDS Cohort Study, Baltimore, Maryland, 1984–2010
| EIP and Definition of Depression | Clinically Relevant Depressive Symptoms | |||
|---|---|---|---|---|
| HIV-Positive | HIV-Negative | |||
| ORb | 95% CI | OR | 95% CI | |
| EIP-1 | ||||
| CES-D onlyc | 1.09 | 1.03, 1.16 | 1.33 | 1.09, 1.61 |
| CES-D + antidepressant used | 1.11 | 1.05, 1.18 | 1.25 | 1.05, 1.49 |
| EIP-2 | ||||
| CES-D only | 1.04 | 0.99, 1.10 | 1.09 | 0.95, 1.26 |
| CES-D + antidepressant use | 1.02 | 0.97, 1.08 | 1.04 | 0.91, 1.18 |
| EIP-3 | ||||
| CES-D only | 0.96 | 0.88, 1.04 | 1.10 | 0.90, 1.35 |
| CES-D + antidepressant use | 1.03 | 0.96, 1.10 | 1.07 | 0.89, 1.30 |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; EIP, exploratory factor analysis–identified inflammatory process; GEE, generalized estimating equations; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio.
a Adjusted for age, black race, college education, current smoking, obesity, HCV infection, alcohol intake, and cocaine use since last visit; GEE used for analyses.
b Estimate for 1 standard-deviation increase in standardized EIP factor scores.
c Clinically relevant depressive symptoms defined as CES-D >20 compared with having not depressive symptoms defined as CES-D <12.
d Clinically relevant depressive symptoms defined as CES-D >20 or having antidepressant use compared with having not depressive symptoms defined as CES-D <12 and having no antidepressant use.
Proinflammatory markers and mild depressive symptoms in HIV+
In contrast to analyses of those experiencing clinically relevant depressive symptoms, both EIP-1 and EIP-2 were associated with higher odds of having mild depressive symptoms (i.e., CES-D 12–20). One standard-deviation increase in EIP-1 scores was significantly associated with a 9% higher odds of mild depressive symptoms among HIV+ participants (OR = 1.09, 95% CI: 1.03, 1.16), while EIP-2 scores were significantly associated with an 11% higher odds of mild depressive symptoms among HIV+ participants (OR = 1.11, 95% CI: 1.05, 1.18) (Web Figure 1). These relationships were approximately null among HIV− participants (OR = 1.01, 95% CI: 0.83, 1.23; and OR = 0.97, 95% CI: 0.81, 1.15, respectively).
In secondary analyses, using a traditional threshold of 16 to define the presence of depressive symptoms yielded results similar to that found for mild depressive symptoms (Table 3). Stratification by current ART use and HIV suppression status did not indicate important subgroups (Table 4). Finally, analysis of the single inflammatory marker CRP—which has been previously found associated with depressive symptoms (20, 39–41)—resulted in weak associations with clinically relevant depressive symptoms among both HIV+ and HIV− participants (OR = 1.04, 95% CI: 1.00, 1.09; and OR = 1.10, 95% CI: 0.98, 1.25, respectively).
Table 3.
Adjusted Logistic Regressiona of Depressive Symptoms, Using a Standard Depression Score Threshold of 16, on Continuous Estimates of Exploratory Factor Analysis–Identified Inflammatory Processes in Person-Visits in the Multicenter AIDS Cohort Study, United States, 1984–2010
| EIP and Definition of Depression | Depressive Symptoms Using CES-D Score of ≥16 | |||
|---|---|---|---|---|
| HIV-Positive | HIV-Negative | |||
| ORb | 95% CI | OR | 95% CI | |
| EIP-1 | ||||
| CES-D onlyc | 1.08 | 1.02, 1.14 | 1.15 | 0.97, 1.38 |
| CES-D + antidepressant used | 1.09 | 1.03, 1.16 | 1.20 | 1.01, 1.42 |
| EIP-2 | ||||
| CES-D only | 1.07 | 1.01, 1.13 | 1.14 | 1.00, 1.31 |
| CES-D + antidepressant use | 1.04 | 0.99, 1.09 | 1.13 | 0.99, 1.30 |
| EIP-3 | ||||
| CES-D only | 1.00 | 0.92, 1.09 | 1.04 | 0.86, 1.25 |
| CES-D + antidepressant use | 1.05 | 0.98, 1.13 | 1.06 | 0.88, 1.26 |
Abbreviations: CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; EIP, exploratory factor analysis–identified inflammatory process; GEE, generalized estimating equations; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio.
a Adjusted for age, black race, college education, current smoking, obesity, HCV infection, alcohol intake, and cocaine use since last visit; GEE used for analyses.
b Estimate for 1 standard-deviation increase in standardized EIP factor scores.
c Depressive symptoms defined as CES-D ≥16 compared with having not depressive symptoms defined as CES-D <16.
d Depressive symptoms defined as CES-D ≥16 or having antidepressant use compared with having not depressive symptoms defined as CES-D <16 and having no antidepressant use.
Table 4.
Adjusted Logistic Regressiona of Depressive Symptoms on Continuous Estimates of Exploratory Factor Analysis–Identified Inflammatory Processes Among Seropositive Men Stratified by Antiretroviral Therapy Use and Viral Suppression in the Multicenter AIDS Cohort Study, United States, 1984–2010
| EIP and Definition of Depression | Clinically Relevant Depressive Symptoms, HIV-Positive Person-Visits | |||||
|---|---|---|---|---|---|---|
| Not on ART, HIV Virally Unsuppressed | On ART, HIV Virally Suppressed | On ART, HIV Virally Unsuppressed | ||||
| ORb | 95% CI | OR | 95% CI | OR | 95% CI | |
| EIP-1 | ||||||
| CES-D onlyc | 1.12 | 1.01, 1.24 | 1.04 | 0.94, 1.14 | 1.05 | 0.97, 1.15 |
| CES-D + antidepressant used | 1.19 | 1.04, 1.35 | 1.09 | 0.98, 1.21 | 1.09 | 1.00, 1.19 |
| EIP-2 | ||||||
| CES-D only | 1.10 | 0.94, 1.28 | 0.99 | 0.91, 1.08 | 1.05 | 0.98, 1.13 |
| CES-D + antidepressant use | 1.05 | 0.90, 1.23 | 0.99 | 0.92, 1.07 | 1.02 | 0.94, 1.10 |
| EIP-3 | ||||||
| CES-D only | 0.94 | 0.84, 1.05 | 0.97 | 0.85, 1.11 | 0.94 | 0.84, 1.06 |
| CES-D + antidepressant use | 1.01 | 0.90, 1.14 | 1.02 | 0.95, 1.09 | 1.01 | 0.90, 1.13 |
Abbreviations: ART, Antiretroviral Therapy; CES-D, Center for Epidemiologic Studies Depression Scale; CI, confidence interval; EIP, exploratory factor analysis–identified inflammatory process; GEE, generalized estimating equations; HCV, hepatitis C virus; HIV, human immunodeficiency virus; OR, odds ratio.
a Adjusted for age, black race, college education, current smoking, obesity, HCV infection, alcohol intake, and cocaine use since last visit; GEE used for analyses.
b Estimate for 1 standard-deviation increase in standardized EIP factor scores.
c Current depressive symptoms defined as CES-D >20 compared with having not depressive symptoms defined as CES-D <12.
d Current depressive symptoms defined as CES-D >20 or having antidepressant use compared with having not depressive symptoms defined as CES-D <12 and having no antidepressant use.
Sensitivity analyses
Replacing continuous factor scores with tertiles of factor scores resulted in associations with the highest tertile of EIP-1 among HIV+ persons. Participants in the highest tertile of EIP-1 had 21% higher odds of clinically relevant depressive symptoms in HIV+ person-visits (Web Table 3). Results of stratified analyses based on calendar time showed similar associations in the cART era and in the pre-cART era (Web Table 4). Last, analysis was restricted to a more homogeneous group of 740 HIV+ ART-naive participants at baseline, and results were similar, with 1 standard-deviation higher EIP-1 significantly associated with a 13% higher odds of depressive symptoms (OR = 1.13, 95% CI: 1.05, 1.21).
DISCUSSION
Depression is a complex and multifaceted disorder. The fact that one-third of patients remain treatment-refractory (42), with the mechanism of action of nearly all of currently prescribed antidepressants being monoamine pathways, strongly suggests that other pathways to depression exist, including the possibility of an inflammatory subtype (43, 44). To our knowledge, this study is the first to broadly examine inflammation and depressive symptom relationships in an MSM population. Here we found that markers of immune activation were associated with clinically relevant depressive symptoms in both HIV+ and HIV− MSM, suggesting a positive relationship between T- and B-cell activation and the occurrence of depressive symptoms. Proinflammatory markers were associated with milder depression symptoms in HIV+ but not HIV− MSM, which might simply reflect the much higher levels of these markers in HIV+ MSM. While HIV+ persons appear to experience a higher burden of depressive symptoms compared with their HIV− counterparts, they are also more aggressively treated with antidepressants, resulting in similar burden of untreated or undertreated depressive symptoms.
Understanding how inflammation could affect the manifestation of depressive symptoms could have broad implications for our understanding of mechanisms of depression and the potential for treatment. HIV infection represents a context of heightened inflammation as noted in the present study. Higher levels of inflammatory markers in HIV+ individuals predict mortality (45) and are thought to be the link to the higher rates of age-related comorbidity observed among HIV+ participants (46). Similarly, an inflammatory depressive subtype could explain the noted high burden of depressive symptoms in HIV+, and our results provide some evidence that such a subtype might exist. While cytokine pathways are complex, this study examined a large number of markers representing several domains of function in more than 9,000 study visits from HIV+ and HIV− men.
Our results support prior suggestions of cell-mediated immune activation as a key feature of depression, and prior studies have found increased serum levels of sIL2r and sTNFr accompanying depressive symptoms (47). The immune marker sCD14, which contributed to EIP-1, is a macrophage activation marker that is thought to act as a coreceptor of the bacterial product lipopolysaccharides (LPS) (48). Microbial translocation has been posited to contribute to inflammation-related depressive symptoms (49, 50), consistent with the emerging concept of the microbiota-gut-brain axis (51–53). Proinflammatory markers such as interleukin-6, interleukin-8, and tumor necrosis factor-α have also been found to increase risk of depressive symptoms in the general population (39, 54, 55) and were associated with milder symptoms among HIV+ persons in the present study but not with more severe symptoms or more generally across MSM. Markers of cell recruitment, migration, and infiltration were not associated with depressive symptoms in MSM, but this EIP has been found to be less stable across samples in MACS (37) and might not well characterize an underlying inflammatory construct.
Inflammation in the context of HIV infection arises as a result of exposure to HIV viremia and can persist after effective antiretroviral therapy initiation. Controlling for ART use regardless of viral suppression status appeared to attenuate associations between markers of immune activation and clinically relevant depressive symptoms among HIV+ participants, as would be expected given that ART use is the primary driver of viral suppression, which can dampen viremia-induced immune activation (13). However, substantial differences in psychosocial and other factors also exist between ART users and nonusers (56), which makes comparisons across ART treatment groups challenging. Depression has been found to predict more rapid decline in CD4 counts in some studies of HIV-infected individuals (57), although this relationship has not been consistently demonstrated (58).
The strengths of our analysis include the broad panel of immune markers and large number of individual study visits, which allowed us to better describe complex inflammatory processes and their associations with depressive symptoms. Inflammatory marker measurements were available only through 2010; however, relationships between inflammatory markers and depressive symptoms are unlikely to have changed in subsequent years. We included all person-visits in the EFA, assuming measurement invariance across time; it is unlikely that the underlying biology of inflammatory processes would change over the duration of the study. The CES-D is not considered a clinical tool for diagnosing depression but has been consistently used in research to identify individuals experiencing depressive symptoms. However, thresholds for classifying levels of clinically relevant depressive symptoms using the CES-D are not clear for vulnerable populations such as MSM (59). It is also worth noting that our sample consisted of urban and mostly white men, and the number of HIV-seronegative men contributing HIV− visits was relatively small, increasing the variability in estimates, which might contribute to apparent differences in estimates between HIV+ and HIV− men.
In conclusion, our findings further support a role of inflammation and immune activation markers in the occurrence of depressive symptoms among MSM, a population with a high burden of depression and HIV infection. Subsequent studies should examine subgroups of treatment-refractory individuals with depression to understand whether inflammation might contribute to symptomology in this group—a finding that could pave the way for novel treatment approaches. Focused studies on novel markers of cellular immune response and bacterial infiltration such as kynurenine/tryptophan ratio and lipopolysaccharides would be key for understanding underlying mechanisms.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, Chapel Hill, North Carolina (Haidong Lu); Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Pamela J. Surkan); Cousins Center for Psychoneuroimmunology, Semel Institute for Neuroscience and Human Behavior, University of California, Los Angeles, Los Angeles, California (Michael R. Irwin, Elizabeth C. Breen); Department of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Michael R. Irwin, Elizabeth C. Breen); Department of Psychiatry and Behavioral Sciences, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Glenn J. Treisman); Department of Neurology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Ned Sacktor); Department of Behavioral and Community Health Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania (Ron Stall); Division of Infectious Diseases, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois (Steven M. Wolinsky); Department of Epidemiology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland (Lisa P. Jacobson, Alison G. Abraham); and Department of Ophthalmology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Alison G. Abraham).
This work was supported by the National Institute of Allergy and Infectious Diseases (grants U01-AI35039, U01-AI35040, U01-AI35041, U01-AI35042, and UM1-AI35043), the National Institute of Mental Health (grant R03-MH103961) and the Center for AIDS Research (CFAR) at Johns Hopkins Bloomberg School of Public Health.
We thank all the collaborators, staff, and participants of the Multicenter AIDS Cohort Study (MACS). Data in this manuscript were collected by the Multicenter AIDS Cohort Study with centers at Baltimore (U01-AI35042): Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Adrian Dobs, Michelle Estrella, W. David Hardy, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, John P. Phair, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (PI), Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Lawrence A. Kingsley, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): the Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (PI), Alison Abraham, Keri Althoff, Michael Collaco, Priya Duggal, Sabina Haberlen, Eithne Keelaghan, Heather McKay, Alvaro Muñoz, Derek Ng, Anne Rostich, Eric C. Seaberg, Sol Su, Pamela Surkan, Nicholas Wada. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The authors also thank the Center for AIDS Research (CFAR) at Johns Hopkins Bloomberg School of Public Health.
The Multicenter AIDS Cohort Study is funded primarily by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Cancer Institute, the National Institute on Drug Abuse, and the National Institute of Mental Health. Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute, and the National Institute on Deafness and Communication Disorders. Multicenter AIDS Cohort Study data collection is also supported by grant UL1-TR001079 (Johns Hopkins Institute for Clinical and Translational Research) from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research.
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health, Johns Hopkins Institute for Clinical and Translational Research, or National Center for Advancing Translational Sciences. The Multicenter AIDS Cohort Study website is located at http://aidscohortstudy.org/.
Conflict of interest: none declared.
Glossary
- ART
antiretroviral therapy
- cART
combination antiretroviral therapy
- CES-D
Center for Epidemiologic Studies Depression Scale
- CI
confidence interval
- CRP
C-reactive protein
- EFA
exploratory factor analysis
- EIP
EFA-based inflammatory process
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- MACS
the Multicenter AIDS Cohort Study
- MSM
men who have sex with men
- OR
odds ratio
- sTNF-R2
soluble tumor necrosis factor receptor 2
REFERENCES
- 1. Kessler RC, Birnbaum H, Bromet E, et al. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med. 2010;40(2):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289(23):3095–3105. [DOI] [PubMed] [Google Scholar]
- 3. Alexopoulos GS. Depression in the elderly. Lancet. 2005;365(9475):1961–1970. [DOI] [PubMed] [Google Scholar]
- 4. Andrews G, Issakidis C, Sanderson K, et al. Utilising survey data to inform public policy: comparison of the cost-effectiveness of treatment of ten mental disorders. Br J Psychiatry. 2004;184:526–533. [DOI] [PubMed] [Google Scholar]
- 5. Chisholm D, Sanderson K, Ayuso-Mateos JL, et al. Reducing the global burden of depression: population-level analysis of intervention cost-effectiveness in 14 world regions. Br J Psychiatry. 2004;184:393–403. [DOI] [PubMed] [Google Scholar]
- 6. Roose SP, Schatzberg AF. The efficacy of antidepressants in the treatment of late-life depression. J Clin Psychopharmacol. 2005;25(4 suppl 1):S1–S7. [DOI] [PubMed] [Google Scholar]
- 7. Üstün TB, Ayuso-Mateos JL, Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry. 2004;184:386–392. [DOI] [PubMed] [Google Scholar]
- 8. Nanni MG, Caruso R, Mitchell AJ, et al. Depression in HIV infected patients: a review. Curr Psychiatry Rep. 2015;17:530. [DOI] [PubMed] [Google Scholar]
- 9. Cruess DG, Evans DL, Repetto MJ, et al. Prevalence, diagnosis, and pharmacological treatment of mood disorders in HIV disease. Biol Psychiatry. 2003;54(3):307–316. [DOI] [PubMed] [Google Scholar]
- 10. UNAIDS Global HIV & AIDS statistics—2019 fact sheet; 2019. https://www.unaids.org/en/resources/fact-sheet. Accessed July 22, 2019.
- 11. Tao J, Vermund SH, Qian HZ. Association between depression and antiretroviral therapy use among people living with HIV: a meta-analysis. AIDS Behav. 2018;22(5):1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans DL, Ten Have TR, Douglas SD, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159(10):1752–1759. [DOI] [PubMed] [Google Scholar]
- 13. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29(4):463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cho JH, Irwin MR, Eisenberger NI, et al. Transcriptomic predictors of inflammation-induced depressed mood. Neuropsychopharmacology. 2019;44(5):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Irwin MR, Cole S, Olmstead R, et al. Moderators for depressed mood and systemic and transcriptional inflammatory responses: a randomized controlled trial of endotoxin. Neuropsychopharmacology. 2019;44(3):635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eisenberger NI, Moieni M, Inagaki TK, et al. In sickness and in health: the coregulation of inflammation and social behavior. Neuropsychopharmacology. 2017;42:242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Muscatell KA, Dedovic K, Slavich GM, et al. Greater amygdala activity and dorsomedial prefrontal-amygdala coupling are associated with enhanced inflammatory responses to stress. Brain Behav Immun. 2015;43:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol Bull. 2014;140(3):774–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gimeno D, Kivimäki M, Brunner EJ, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39(3):413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van den Biggelaar AH, Gussekloo J, de Craen AJ, et al. Inflammation and interleukin-1 signaling network contribute to depressive symptoms but not cognitive decline in old age. Exp Gerontol. 2007;42(7):693–701. [DOI] [PubMed] [Google Scholar]
- 22. Wium-Andersen MK, Ørsted DD, Nielsen SF, et al. Elevated C-reactive protein levels, psychological distress, and depression in 73,131 individuals. JAMA Psychiatry. 2013;70(2):176–184. [DOI] [PubMed] [Google Scholar]
- 23. Mills TC, Paul J, Stall R, et al. Distress and depression in men who have sex with men: the Urban Men’s Health Study [erratum appears in Am J Psychiatry 2004;161(4):776]. Am J Psychiatry. 2004;161(2):278–285. [DOI] [PubMed] [Google Scholar]
- 24. Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. [DOI] [PubMed] [Google Scholar]
- 26. McKay HS, Bream JH, Margolick JB, et al. Host factors associated with serologic inflammatory markers assessed using multiplex assays. Cytokine. 2016;85:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McKay HS, Bream JH, Margolick JB, et al. Data on serologic inflammatory biomarkers assessed using multiplex assays and host characteristics in the Multicenter AIDS Cohort Study (MACS). Data Brief. 2016;9:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McKay HS, Margolick JB, Martínez-Maza O, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine. 2017;90:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Radloff LS. A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 30. Salomon EA, Mimiaga MJ, Husnik MJ, et al. Depressive symptoms, utilization of mental health care, substance use and sexual risk among young men who have sex with men in EXPLORE: implications for age-specific interventions. AIDS Behav. 2009;13(4):811–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grov C, Golub SA, Parsons JT, et al. Loneliness and HIV-related stigma explain depression among older HIV-positive adults. AIDS Care. 2010;22(5):630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lyness JM, Noel TK, Cox C, et al. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies–Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157(4):449–454. [PubMed] [Google Scholar]
- 33. Vilagut G, Forero CG, Barbaglia G, et al. Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES-D): a systematic review with meta-analysis. PLoS One. 2016;11(5):e0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakkinen PA, Wahl P, Cushman M, et al. Clustering of procoagulation, inflammation, and fibrinolysis variables with metabolic factors in insulin resistance syndrome. Am J Epidemiol. 2000;152(10):897–907. [DOI] [PubMed] [Google Scholar]
- 35. Clifford DB, Evans S, Yang Y, et al. Impact of efavirenz on neuropsychological performance and symptoms in HIV-infected individuals. Ann Intern Med. 2005;143(10):714–721. [DOI] [PubMed] [Google Scholar]
- 36. Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk of suicidal ideation, attempted, or completed suicide: an analysis of trial data. Ann Intern Med. 2014;161(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Abraham AG, Darilay A, McKay H, et al. Kidney dysfunction and markers of inflammation in the Multicenter AIDS Cohort Study. J Infect Dis. 2015;212(7):1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fahey JL. Cytokines, plasma immune activation markers, and clinically relevant surrogate markers in human immunodeficiency virus infection. Clin Diagn Lab Immunol. 1998;5(5):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. [DOI] [PubMed] [Google Scholar]
- 40. Poudel-Tandukar K, Bertone-Johnson ER, Palmer PH, et al. C-reactive protein and depression in persons with human immunodeficiency virus infection: the Positive Living with HIV (POLH) Study. Brain Behav Immun. 2014;42:89–95. [DOI] [PubMed] [Google Scholar]
- 41. Irwin MR, Archer G, Olmstead R, et al. Increased risk of depression in non-depressed HIV infected men with sleep disturbance: prospective findings from the Multicenter AIDS Cohort Study. EBioMedicine. 2018;36:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. [DOI] [PubMed] [Google Scholar]
- 43. Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70(1):31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13(6):467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wada NI, Bream JH, Martínez-Maza O, et al. Inflammatory biomarkers and mortality risk among HIV-suppressed men: a Multisite Prospective Cohort Study. Clin Infect Dis. 2016;63(7):984–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60(suppl 1):S1–S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):664–675. [DOI] [PubMed] [Google Scholar]
- 48. Kitchens RL, Thompson PA. Modulatory effects of sCD14 and LBP on LPS-host cell interactions. J Endotoxin Res. 2005;11(4):225–229. [DOI] [PubMed] [Google Scholar]
- 49. Berk M, Williams LJ, Jacka FN, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maes M, Kubera M, Leunis JC, et al. In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatr Scand. 2013;127(5):344–354. [DOI] [PubMed] [Google Scholar]
- 51. Maes M, Kubera M, Leunis JC. The gut-brain barrier in major depression: intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinol Lett. 2008;29(1):117–124. [PubMed] [Google Scholar]
- 52. Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci. 2012;13(10):701–712. [DOI] [PubMed] [Google Scholar]
- 53. Maes M, Kubera M, Leunis JC, et al. Increased IgA and IgM responses against gut commensals in chronic depression: further evidence for increased bacterial translocation or leaky gut. J Affect Disord. 2012;141(1):55–62. [DOI] [PubMed] [Google Scholar]
- 54. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. [DOI] [PubMed] [Google Scholar]
- 55. Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. [DOI] [PubMed] [Google Scholar]
- 56. Vanable PA, Carey MP, Blair DC, et al. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS Behav. 2006;10(5):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Burack JH, Barrett DC, Stall RD, et al. Depressive symptoms and CD4 lymphocyte decline among HIV-infected men. JAMA. 1993;270(21):2568–2573. [PubMed] [Google Scholar]
- 58. Lyketsos CG, Hoover DR, Guccione M, et al. Depressive symptoms as predictors of medical outcomes in HIV infection. Multicenter AIDS Cohort Study. JAMA. 1993;270(21):2563–2567. [PubMed] [Google Scholar]
- 59. Armstrong NM, Surkan PJ, Treisman GJ, et al. Optimal metrics for identifying long term patterns of depression in older HIV-infected and HIV-uninfected men who have sex with men. Aging Ment Health. 2019;23(4):507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.