Abstract
A higher level of physical activity (PA) is associated with decreased risk of mortality, dementia, and depression, yet the mechanisms involved are not well understood, and little evidence exists for Mexican Americans. With data from the Sacramento Area Latino Study on Aging (1998–2007), we used Cox proportional hazards regression to separately evaluate associations of baseline PA level with mortality, dementia/cognitive impairment without dementia (CIND), and depressive symptoms, and we estimated the mediating effects of inflammatory markers in additive hazard models. A low level of PA (<35 metabolic equivalent of task–hours/week) was associated with increased mortality (hazard ratio (HR) = 1.50, 95% confidence interval (CI): 1.20, 1.88), dementia/CIND (HR = 1.37, 95% CI: 0.96, 1.96), and depressive symptoms (HR = 1.23, 95% CI: 1.00, 1.52). A low PA level added 512 (95% CI: −34, 1,058) cases of dementia/CIND per 100,000 person-years at risk (direct effect), while, through a mediating path, interleukin 6 (IL-6) added another 49 (95% CI: 5, 94) cases, or 9% of the total effect. For mortality, 8%–10% of the PA total effect was mediated through IL-6, tumor necrosis factor α (TNF-α), or TNF-α receptors. None of the inflammatory markers mediated the association between PA and depressive symptoms. Our results suggest that antiinflammation (especially as assessed by IL-6 and TNF-α levels) may partly explain how PA protects against dementia/CIND and mortality.
Keywords: dementia, depression, inflammation, mediation, mortality, physical activity
Dementia and depression are the most common mental disorders in older persons and represent a large social and economic burden globally (1). With the rapid growth of the elderly population, prevention of aging-related dementia and late-life depression is of growing importance. Epidemiologic evidence is accumulating that regular physical activity (PA) and an active lifestyle have many health benefits, including lowering mortality, preventing some major chronic diseases, and improving physical, psychological, and social functioning (2). Although it has been shown that exercise induces an antiinflammatory response and reduces atherosclerosis and metabolic dysregulation (3), much less is known about whether exercise induces an antiinflammatory effect that maintains better brain function.
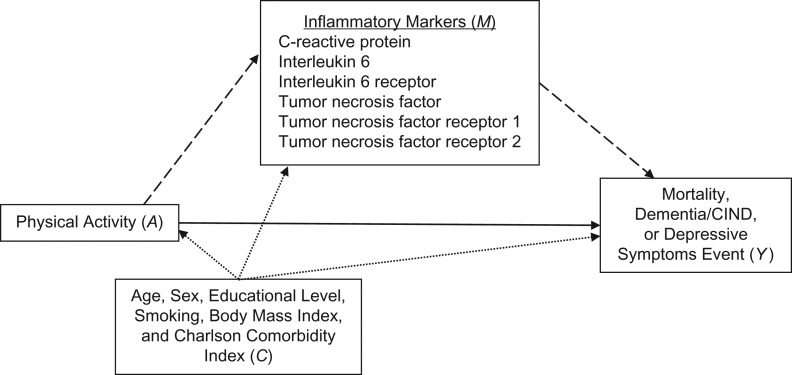
Transient inflammation is a protective response that stimulates healing processes after injury and infection, yet failure to completely terminate the immune response in a timely manner may cause a systemic state of low-grade chronic inflammation that contributes to dementia and depressive disorders in the elderly (4–8). Several studies have found elevated levels of C-reactive protein (CRP), interleukin 6 (IL-6), and/or tumor necrosis factor α (TNF-α) to be related to the deterioration of cognitive function (5, 6, 9). Recent meta-analyses also suggested that inflammation (peripheral elevation of CRP and IL-6 levels) may lead to depression (10–12). Inverse associations between PA and markers of inflammation, reflected by increased concentrations of CRP and other proinflammatory cytokines, have also been reported (5, 13, 14). However, to our knowledge, no prior studies have examined whether inflammatory markers mediate the association between PA and cognition or mood. Here we examine whether CRP, IL-6, or TNF-α explains associations between PA and dementia/cognitive impairment without dementia (CIND), depressive symptoms, or mortality among elderly Mexican Americans (Figure 1), the most rapidly growing segment of elderly persons living in the United States and a group that is also at greater risk of developing dementia or depression than non-Hispanic whites (15–17). To date, no single best or summary biomarker of chronic inflammation has been established; thus, we relied on a suite of different inflammatory markers and assumed that they share a common explanatory factor inflammatory response. We hypothesized that some of these biomarkers may be better or worse proxies for the concept of chronic inflammation as measured in the blood, and this may also vary by outcome considered.
Figure 1.
Assumed causal structure of relationships of physical activity (exposure; A), inflammatory markers (mediators; M), and mortality, incident dementia/cognitive impairment without dementia (CIND), or incident depressive symptoms (outcomes; Y) with measured confounders (C). Inflammatory markers are intermediate variables on the causal pathway between exposure (physical activity) and outcomes (mortality, incident dementia/CIND, or incident depressive symptoms). The direct effect is represented by the solid arrow, and indirect effects are represented by dashed arrows; confounder pathways are depicted as dotted arrows.
METHODS
Study population
The Sacramento Area Latino Study on Aging (SALSA) was a prospective cohort study of community-dwelling Mexican Americans who resided in California’s Sacramento Valley (details are provided elsewhere) (18). Of 1,789 persons aged 60–101 years recruited in 1998–1999, home visits were conducted every 12–15 months, including clinical and cognitive assessments ending in 2007; semiannual phone calls between home visits obtained updated information on medications, health events, and some other factors. The annual attrition rate was 5%, including mortality and loss to follow-up between baseline and 2007 (19).
Here we excluded persons who met one or more of the following criteria: missing data for physical activity (n = 113) or inflammatory markers (n = 227); no follow-up data (n = 92); and prevalent dementia/CIND or depressive symptoms at baseline for the analyses in which we targeted dementia/CIND or depressive symptoms. A total of 1,459 participants constituted the final sample for analyses of mortality, 1,397 comprised the sample for incident dementia/CIND, and 935 comprised the sample for incident depressive symptoms (see Web Figure 1 and Web Table 1, available at https://academic.oup.com/aje). All participants gave informed consent, and ethical approval was obtained from the institutional review boards of the University of Michigan and the San Francisco, Davis, and Los Angeles campuses of the University of California.
Measures
Physical activity
Baseline information on PA was assessed from self-reports as previously described (20). Participants were asked to report the average number of hours per week they spent engaging in 18 different types of common activities among older adults during a regular week. Eight activities that required a 3-fold or more increase over the metabolic equivalent of task (MET) energy expenditure required by quiet sitting (≥3 METs) were summed to generate weekly measures of moderate-to-vigorous PA (MET-hours/week); participants were classified into a “low” or “high” PA group on the basis of the first tertile (<35 MET-hours/week vs. ≥35 MET-hours/week).
Mortality
Deaths among participants were identified by 1) interviews with family members when tracking participants who could not be reached for annual home visits or interim 6-month telephone follow-ups, 2) online surveillance of death notices, and 3) review of the Social Security Death Index, the National Death Index, and vital statistics data files of the state of California. The cause of death was classified according to the International Classification of Diseases, Tenth Revision.
Dementia/CIND
Procedures used to classify dementia and CIND have been extensively described elsewhere (18). Briefly, at each visit, participants underwent 2 cognitive evaluations (the Modified Mini-Mental State Examination (21) and a delayed word recall trial from the Spanish English Verbal Learning Test). If scores on the Modified Mini-Mental State Examination or the Spanish English Verbal Learning Test fell below the 20th percentile or decreased from baseline by more than 8 points or more than 3 points, respectively, the participant was referred for a neuropsychological test battery and a standard neuropsychological examination. A team of neurologists and a neuropsychologist reviewed all referred participants and classified them as demented, CIND, or cognitively normal based on the standard diagnostic criteria for dementia (22), Alzheimer disease (23), and vascular dementia (24). Participants who were not diagnosed as having dementia or CIND but died during the study period were reclassified if any of the following causes of death were listed on the death certificate: dementia of Alzheimer type, vascular dementia, other dementia, or unspecified dementia. For this analysis, dementia and CIND were combined into one outcome: dementia/CIND.
Depressive symptoms
We evaluated depressive symptoms using the Center for Epidemiologic Studies Depression Scale (CES-D), which has been widely used and validated in older adults (25). Among persons who did not report any depression at baseline, those with a CES-D score greater than or equal to 16 or who used an antidepressant prescription drug during follow-up were considered to have developed depressive symptoms or to have initiated antidepressant therapy during follow-up (hereafter referred to as “incident depressive symptoms”) (26, 27). Prescription drug use was derived from a medicine cabinet inventory during home visits and phone follow-ups. Antidepressant medications consisted predominantly of selective serotonin reuptake inhibitors and tricyclic antidepressants, with fewer participants taking atypical antipsychotic agents, noradrenergic and specific serotonergic antidepressants, norepinephrine-dopamine reuptake inhibitors, and serotonin-norepinephrine reuptake inhibitors. These classifications were reviewed by a neurologist and a psychiatrist.
Inflammatory markers
Baseline serum samples were collected from each participant and processed/stored at the Medical Center Clinical Laboratory at the University of California, Davis. CRP levels were measured with the CRP Ultra Wide Range Reagent Kit latex-enhanced immunoassay (Equal Diagnostics, Exton, Pennsylvania); levels of IL-6 and TNF-α and their receptors were determined using the Quantiglo Chemiluminescent Immunoassay (R&D Systems, Minneapolis, Minnesota). Since the distributions of inflammatory markers were found to be nonnormal and highly skewed, for each marker, we used the quartile 3 cutpoint as a cutoff for the marker among participants who had baseline CRP levels less than 10 mg/L (CRP: 5.1 mg/L; IL-6: 5.2 pg/mL; IL-6 receptor: 44,009.2 pg/mL; TNF-α: 4.8 pg/mL; TNF-α receptor 1: 1,822.1 pg/mL; TNF-α receptor 2: 2,880.9 pg/mL). Participants who had baseline CRP levels greater than or equal to 10 mg/L were placed in the high-CRP group, under the assumption that a CRP concentration greater than or equal to 10 mg/L indicates clinically relevant acute inflammation.
Covariates
At the baseline interview, participants reported their age, sex, education (years), and smoking status (never, current, or ever smoker). Body mass index was calculated as weight (kg) divided by the square of height (m2) using measured height and weight. To control for the effect of chronic health conditions on mortality and other outcomes, we created a modified Charlson comorbidity index by assigning points for a history of myocardial infarction, congestive heart failure, stroke, dementia, liver disease, diabetes, renal disease, any malignancy, and leukemia or lymphoma and summing points across these items (28).
Statistical analysis
We summarized the participants’ characteristics according to their baseline level of PA. The associations of PA with mortality, dementia/CIND, and incident depressive symptoms were assessed separately using Cox proportional hazards regression models, adjusting for participants’ age, sex, education, body mass index, smoking, and modified Charlson comorbidity index. Mediation analysis was performed to determine whether inflammatory markers at least partly explained associations between physical activity and these outcomes. Given that the hazard ratios cannot be related to absolute numbers of events and the assumptions of a Cox model are not fully satisfied in mediation analysis (29), we employed the Aalen additive hazard model to assess direct and indirect effects in a survival analysis setting (29, 30).
A marginal structural modeling approach based on a counterfactual framework was applied to estimate the natural direct effect (NDE) of baseline PA on mortality, risk of dementia/CIND, or incident depressive symptoms, as well as the natural indirect effect (NIE) of PA via the inflammatory pathway with biomarkers as potential mediators (30, 31) (Figure 1). We first conducted logistic regression analysis to estimate the associations of PA with each of the inflammatory markers (mediator) adjusted for confounding factors. Next, we created a new data set by duplicating each original observation and generating additional counterfactual exposure variables (i.e., assigning the opposite of the observed PA value) in the extended data set. Weights were derived from the logistic regression models by regressing the inflammatory markers on PA and confounding factors (Web Table 2). The final step was to estimate the effect of both PA level (low vs. high) and each inflammatory marker on mortality, onset of dementia/CIND, or incident depressive symptoms by fitting the additive hazards model and adjusting for confounders. These estimates can be interpreted as the number of additional events per 100,000 person-years at risk, when comparing low-PA subjects with high-PA subjects to generate the NDE and NIE of baseline PA on each of the outcomes. The proportion mediated is given by the ratio of the estimated indirect effect to the total effect. Ninety-five percent confidence intervals for the NDE and the NIE were extracted directly from the model, while 95% confidence intervals for the mediated proportion were computed by bootstrapping using 10,000 samples. Because of the mutual dependence between all inflammatory markers conditional on exposure (PA) and covariates, we did not consider all inflammatory markers in the same model but relied on 1 model for each marker.
Sensitivity analyses were conducted 1) based solely on the CES-D score to define incident depressive symptoms, 2) removing deaths and cases of dementia/CIND or depressive symptoms from the first 1 or 2 years of follow-up, and 3) based on Fine and Gray’s competing-risk regression models (32). Given that competing risks arise in time-to-event data when the event of interest cannot be observed because of a preceding event, we applied Fine and Gray’s subdistribution hazard model to address the effects of death (competing events) occurring before dementia/CIND or incident depressive symptoms. To assess the interaction between PA and inflammatory markers on the multiplicative scale, we added a PA × biomarker product term to the model for each inflammatory marker and calculated the relative excess risk due to interaction as a measure of additive interaction (33). Mediation analysis was carried out using R 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria), and all other analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina). Effect estimates presented here may be considered formally statistically significant if the null value is not included in the 95% confidence interval.
RESULTS
We included only participants with a blood sample and data for at least 1 inflammatory marker. Demographic and baseline health characteristics in this group did not differ from those of the SALSA population overall (Web Table 1). At baseline, participants with low levels of PA were more likely to be female, more likely to be smokers, and had a higher body mass index, a higher prevalence of chronic diseases, and higher levels of inflammatory markers (Table 1). During follow-up, 331 participants died, 129 participants developed dementia/CIND, and 398 participants developed depressive symptoms. Low levels of PA were associated with increased mortality (hazard ratio (HR) = 1.68, 95% confidence interval (CI): 1.35, 2.09), incident dementia/CIND (HR = 1.45, 95% CI: 1.02, 2.06), and incident depressive symptoms (HR = 1.23, 95% CI: 1.00, 1.51); adjusting for covariates had little effect (Table 2).
Table 1.
Baseline Characteristics of Study Participants According to Physical Activity Level, Sacramento Area Latino Study on Aging, 1998–2007
| Characteristic | Physical Activity Level | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total (n = 1,465) | High (≥35 MET-Hours/Week) (n = 912) | Low (<35 MET-Hours/Week) (n = 553) | |||||||
| No. of Persons | % | Median (IQR) | No. of Persons | % | Median (IQR) | No. of Persons | % | Median (IQR) | |
| Age, yearsa | 70.1 (6.6) | 69.8 (6.2) | 70.7 (7.1) | ||||||
| Male sex | 607 | 41 | 424 | 47 | 183 | 33 | |||
| Education, yearsa | 7.5 (5.4) | 7.8 (5.4) | 7.2 (5.3) | ||||||
| Body mass indexa,b | 29.8 (5.9) | 29.5 (5.3) | 30.2 (6.8) | ||||||
| Charlson comorbidity indexa | 0.9 (1.2) | 0.8 (1.1) | 1.1 (1.3) | ||||||
| Chronic disease | |||||||||
| Diabetes | 479 | 33 | 275 | 30 | 204 | 37 | |||
| Stroke | 127 | 9 | 63 | 7 | 64 | 12 | |||
| Cardiovascular disease | 528 | 36 | 304 | 33 | 224 | 41 | |||
| Smoking status | |||||||||
| Never smoker | 683 | 47 | 412 | 45 | 271 | 49 | |||
| Former smoker | 615 | 42 | 396 | 43 | 219 | 40 | |||
| Current smoker | 167 | 11 | 104 | 11 | 63 | 11 | |||
| APOE ε4 carrier | 207 | 14 | 135 | 15 | 72 | 13 | |||
| 3MSE score | 89 (81–94) | 89 (82–94) | 88 (80–93) | ||||||
| CES-D score | 6 (2–16) | 5 (2–13) | 8 (2–18) | ||||||
| Inflammatory marker | |||||||||
| C-reactive protein, mg/L | 3.3 (1.3–7.0) | 3.0 (1.2–6.5) | 4.2 (1.5–8.4) | ||||||
| Interleukin 6, pg/mL | 3.8 (2.5–5.7) | 3.5 (2.4–5.3) | 4.2 (2.8–6.4) | ||||||
| Interleukin 6 receptor, pg/mL | 35,538 (27,953–43,801) | 34,839 (27,441–42,948) | 36,426 (29,211–44,703) | ||||||
| TNF-α, pg/mL | 3.8 (2.9–4.8) | 3.7 (2.8–4.6) | 3.9 (3.1–5.1) | ||||||
| TNF-α receptor 1, pg/mL | 1,508 (1,284–1,843) | 1,476 (1,269–1,758) | 1,603 (1,327–1,988) | ||||||
| TNF-α receptor 2, pg/mL | 2,392 (1,978–2,942) | 2,320 (1,957–2,809) | 2,542 (2,078–3,227) | ||||||
Abbreviations: APOE, apolipoprotein E gene; CES-D, Center for Epidemiologic Studies Depression Scale; IQR, interquartile range; MET, metabolic equivalent of task; 3MSE, Modified Mini-Mental State Examination; TNF-α, tumor necrosis factor α.
a Values are expressed as mean (standard deviation).
b Weight (kg)/height (m)2.
Table 2.
Associations of Physical Activitya With Mortality, Dementia/Cognitive Impairment Without Dementia, and Depressive Symptoms (Cox Proportional Hazards Regression Model), Sacramento Area Latino Study on Aging, 1998–2007
| Outcome | No. of Events | Total No. of Participants | Model 1b | Model 2c | ||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Mortality | 331 | 1,459 | 1.68 | 1.35, 2.09 | 1.50 | 1.20, 1.88 |
| Dementia/CIND | 129 | 1,397 | 1.45 | 1.02, 2.06 | 1.37 | 0.96, 1.96 |
| Depressive symptoms | 398 | 935 | 1.23 | 1.00, 1.51 | 1.23 | 1.00, 1.52 |
Abbreviations: CI, confidence interval; CIND, cognitive impairment without dementia; HR, hazard ratio; MET, metabolic equivalent of task.
a Physical activity exposure was defined as low (<35 MET-hours/week) versus high (≥35 MET-hours/week).
b Results were adjusted for age, sex, and education.
c Results were adjusted for age, sex, education, smoking, body mass index, and Charlson comorbidity index.
We examined mediation through inflammation by adding biomarkers to each outcome model, adjusting for age, sex, and education (Web Table 3) and further coadjusting for body mass index, smoking, and modified Charlson comorbidity index (Table 3). Specifically, comparing participants with low levels of PA to those with high levels, we estimated that approximately 10% of the total effect on mortality was mediated through IL-6 (8%, 95% CI: 3, 18), TNF-α (8%, 95% CI: 4, 18), TNF-α receptor 1 (9%, 95% CI: 4, 20), or TNF-α receptor 2 (10%, 95% CI: 5, 23). In absolute terms, 122 (95% CI: 51, 194) additional deaths per 100,000 person-years at risk were mediated through changes in IL-6, 126 deaths (95% CI: 61, 191) were mediated through TNF-α, 147 deaths (95% CI: 62, 231) were mediated through TNF-α receptor 1, and 154 deaths (95% CI: 69, 240) were mediated through TNF-α receptor 2. For dementia/CIND, 9% (95% CI: −2, 57) of the total effect was mediated through IL-6, 12% (95% CI: −80, 116) through TNF-α receptor 1, and 11% (95% CI: −95, 129) through TNF-α receptor 2. For incident depressive symptoms, the mediated effects, especially for IL-6 and TNF-α receptors, disappeared (decreased from 8% to 3%) after further coadjustment for body mass index, smoking, and modified Charlson comorbidity index.
Table 3.
Natural Direct and Indirect Effects of Physical Activitya on Incidence of Mortality, Dementia/Cognitive Impairment Without Dementia, and Depressive Symptoms as Estimated by Levels of Inflammatory Biomarkers (Mediation Analysis Results From an Additive Hazards Model), Sacramento Area Latino Study on Aging, 1998–2007b
| Potential Mediator | Mortality | Dementia/CIND | Depressive Symptoms | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Incident Casesc | 95% CI | Proportion Mediated, % | 95% CI | No. of Incident Casesc | 95% CI | Proportion Mediated, % | 95% CI | No. of Incident Casesc | 95% CI | Proportion Mediated, % | 95% CI | |
| C-reactive protein | ||||||||||||
| NDE | 1,553 | 786, 2,319 | 573 | 31, 1,115 | 1,694 | −179, 3,567 | ||||||
| NIE | 2 | −53, 56 | 0 | −4, 4 | −23 | −56, 9 | N/Ad | 6 | −93, 105 | 0 | −14, 16 | |
| Interleukin 6 | ||||||||||||
| NDE | 1,405 | 648, 2,162 | 512 | −34, 1,058 | 1,708 | −211, 3,627 | ||||||
| NIE | 122 | 51, 194 | 8 | 3, 18 | 49 | 5, 94 | 9 | −2, 57 | 51 | −104, 205 | 3 | −16, 30 |
| Interleukin 6 receptor | ||||||||||||
| NDE | 1,552 | 747, 2,356 | 565 | 24, 1,106 | 1,833 | −88, 3,755 | ||||||
| NIE | 11 | −15, 36 | 1 | −1, 3 | −2 | −14, 9 | 0 | −5, 3 | −35 | −88, 19 | N/A | |
| TNF-α | ||||||||||||
| NDE | 1,412 | 611, 2,213 | 379 | −170, 929 | 1,987 | 40, 3,935 | ||||||
| NIE | 126 | 61, 191 | 8 | 4, 18 | 9 | −32, 49 | 2 | −37, 48 | −43 | −210, 124 | N/A | |
| TNF-α receptor 1 | ||||||||||||
| NDE | 1,401 | 623, 2,179 | 353 | −192, 899 | 1,760 | −113, 3,632 | ||||||
| NIE | 147 | 62, 231 | 9 | 4, 20 | 50 | −4, 104 | 12 | −80, 116 | 40 | −78, 158 | 2 | −11, 22 |
| TNF-α receptor 2 | ||||||||||||
| NDE | 1,356 | 573, 2,139 | 315 | −219, 849 | 1,805 | −116, 3,725 | ||||||
| NIE | 154 | 69, 240 | 10 | 5, 23 | 40 | −13, 93 | 11 | −95, 129 | 53 | −117, 223 | 3 | −17, 29 |
Abbreviations: CI, confidence interval; CIND, cognitive impairment without dementia; MET, metabolic equivalent of task; N/A, not applicable; NDE, direct effect; NIE, indirect effect; TNF-α, tumor necrosis factor α.
a Physical activity exposure was defined as low (<35 MET-hours/week) versus high (≥35 MET-hours/week).
b Baseline inflammation exposure was defined as less than the third quartile versus greater than or equal to the third quartile. The proportion mediated was defined as the ratio of the indirect effect to the total effect for a low versus high physical activity level. The model adjusted for age, sex, education, smoking, body mass index, and Charlson comorbidity index.
c Additional incident cases per 100,000 person-years at risk.
d N/A because of negative estimates.
While all associations remained unchanged in the first-year lagged analyses for dementia/CIND, incident depressive symptoms, and mortality (Web Table 4), the effect estimate for PA and incident depressive symptoms moved towards the null after we excluded 129 cases of depression that occurred during early follow-up (the first 2 years after baseline) (HR = 1.15, 95% CI: 0.89, 1.49). Compared with the Cox regression model results, accounting for competing risks of death did not substantially attenuate the estimates for any of the 3 outcomes (<10%; Web Table 5). We repeated the main analysis with incident depression symptoms based solely on the CES-D score and found our findings to be broadly consistent (Web Table 6). We also excluded persons with a CRP level greater than 10 mg/L, and the hazard ratio estimates were not markedly different from the main results for any of the 3 outcomes. Web Table 7 displays the PA × inflammation interaction in relation to mortality, dementia/CIND, and incident depressive symptoms on both the multiplicative and additive (i.e., relative excess risk due to interaction) scales. Both multiplicative and additive effects of interactions between PA and CRP on incidence of depressive symptoms were found.
DISCUSSION
In this analysis of data from SALSA, PA lowered the risk of death, dementia/CIND, and depressive symptoms among elderly Latino adults, corroborating the findings of previous studies (34, 35). Importantly, we found that the associations of PA with mortality and dementia/CIND, but not with depression, were partly mediated through well-known inflammatory pathways represented by IL-6 and/or TNF-α. Nevertheless, a large proportion of the effect of PA on mortality, cognition, and mood seems to be operating independently of the inflammatory pathways or the biomarkers we employed.
We aimed to examine whether PA acts on the outcomes via the inflammation pathway, reflected by the changes in levels of inflammation biomarkers we measured (i.e., CRP, IL-6, or TNF-α). Our findings of IL-6 and/or TNF-α acting as mediators of the PA-mortality and PA-cognition associations are supported by prior research in humans and animal models. In several elderly cohorts, inverse associations between PA levels and plasma levels of inflammatory markers have been found (5, 13). Inflammation is also associated with chronic medical conditions, including dementia and AD, and strongly predicts all-cause mortality independently of preexisting morbidity (4, 6, 9, 36). Regular PA is antiinflammatory and increases production and release of IL-6 and other cytokines by skeletal muscles (3, 37). During muscle contraction, plasma IL-6 level increases remarkably, but it declines in the postexercise period; the transient increase in IL-6 level subsequently triggers the antiinflammatory cytokines interleukin 10 and interleukin 1 receptor antagonists and suppresses TNF-α level, thus reducing inflammation (3, 37). Animal studies support the possibility of exercise-induced neuroimmune cytokine changes in the brain; for example, hippocampal interleukin 18 correlated positively with number of new neurons in aging rats, and in a mouse model of Alzheimer disease, exercise reduced brain levels of interleukin 1β (38, 39). Sedentary and inactive lifestyles cause visceral fat accumulation and activate inflammatory pathways (3), while regular exercise reduces proinflammatory cytokine levels and systemic inflammation; it thus presents a potential mechanism of protection against cognitive decline and early mortality.
To our knowledge, the English Longitudinal Study of Ageing is the only study to have previously suggested that inflammatory markers mediate the association between sedentary behavior and mortality (40). In that study, inflammatory markers (CRP and fibrinogen) explained about 16% of the association between sedentary behavior (hours of television viewing) and mortality after adjustment for physical activity; however, possible mediating effects of inflammation were not examined (41). We found approximately 10% of the PA-mortality association to be mediated by IL-6 or TNF-α, but not by CRP. In a 1-year intervention trial of moderate PA, the intervention group of elderly subjects showed reduced systemic concentrations of IL-6 but not CRP (42). Given that CRP is considered a downstream biomarker produced primarily by the liver in response to IL-6 and other inflammatory cytokines (3, 37), it may not be surprising to find that IL-6, production of which is stimulated by muscle contraction during activity, mediates effects of PA on inflammation. While higher CRP levels are observed in older Hispanics (43, 44), CRP appears not to be associated with cardiovascular disease events or mild cognitive impairment among Hispanics (43, 45).
Depression is a common psychiatric disorder in older US adults (46). In comparison with whites, Mexican Americans, especially immigrants, are more likely to report depressive symptoms but less likely to receive treatment (16, 17). Our findings for PA and depressive symptoms in Mexican Americans are in line with prior studies conducted in persons of other ethnicities (34). They suggest that from a health promotion perspective, increasing PA levels even late in life could be an important strategy for preventing depression. None of the biomarkers we employed (CRP, IL-6, and TNF-α), however, mediated the association between PA and depressive symptoms, which is consistent with a prior study (47). We observed both multiplicative and additive effects of interactions between PA and CRP on depression (Web Table 7), with the highest risk for depression being seen among participants with low baseline PA and a high CRP level (HR = 1.58, 95% CI: 1.17, 2.12). Moreover, inserting a time lag of 2 years (excluding 129 cases with incident depressive symptoms) removed associations between PA and depression, suggesting that PA may affect depression only in the short term, but reverse causation or lack of statistical power cannot be excluded as alternate explanations.
Our mediation results should be interpreted with some caution. First, we assumed positivity and no other unmeasured confounders and measurement error. On the basis of our directed acyclic graph, we assumed that the set of confounders for the exposure-mediator, mediator-outcome, and exposure-outcome effects were the same. To derive valid estimates of NDEs, our mediation analysis was further based on the assumption that there was no mediator-outcome confounder that was also affected by the exposure. These are necessary but restrictive assumptions. The dichotomization of PA level and levels of inflammatory markers may have introduced measurement error, which would have been nondifferential. PA level was measured only once and at the same time as the inflammatory markers; thus, temporality was not clearly established, and time-varying confounding factors were not adjusted for in the analysis (48). However, in a review article, Woods et al. (5) suggested that it is reasonable to hypothesize that a sedentary and inactive lifestyle increases plasma levels of these biomarkers, while we would not expect the reverse to be the case. In auxiliary analyses, we assessed associations 1) between PA and inflammatory markers (Web Table 8) and 2) between inflammatory markers and mortality, dementia/CIND, or depressive symptoms (Web Table 9). We found PA to be negatively associated with all markers and IL-6, TNF-α, and TNF-α receptors to be associated with mortality and dementia/CIND (results not shown), supporting our main mediation results—that is, that IL-6 and TNF-α partly mediate the estimated protective effects of PA on mortality and dementia/CIND. Finally, while we were able to control for major confounders, residual confounding is always a possibility.
The main strengths of our study were its longitudinal design, representative sample of the Mexican Americans in California, and 10-year-long follow-up with up to 7 interviews. For the first time, this study employed causal mediation analysis to examine the role plasma inflammatory markers may play in the associations between PA and dementia/CIND, incident depressive symptoms, and mortality. Mexican Americans are one of the fastest-growing segments of the elderly population in the United States, yet relatively few studies have investigated risk factors for dementia and depression in this population. Our results suggested that late-life PA, a modifiable lifestyle factor, protects against dementia and depressive symptoms in a population with more limited access to medical care (17). A lack of repeated PA assessments in our cohort precluded us from examining PA changes over time, and this may have caused exposure misclassification. However, with this prospective study design, misclassification of PA level at baseline was nondifferential and most likely biased our results towards the null. Since PA levels are associated with mortality, we further employed Fine and Gray’s competing-risk models to evaluate the association of PA with dementia/CIND or incident depressive symptoms (32). Although—as expected—risk estimates moved towards the null, accounting for competing risks of death did not substantially alter estimates of the effects of PA on dementia/CIND or incident depressive symptoms (<10%).
In summary, these findings confirm that being physically active, even at an older age, protects against cognitive decline, all-cause mortality, and possibly (in the short term) depressive symptoms in Mexican Americans. Importantly, plasma levels of inflammatory markers (IL-6 and TNF-α) may partly explain the protective effects of PA on dementia/CIND and mortality. Interventions that reduce inflammation may help to sustain cognitive function in older Mexican Americans.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (I-Fan Shih, Kimberly C. Paul, Yu Yu, Beate Ritz); Department of Epidemiology and Biostatistics, School of Medicine, University of California, San Francisco, San Francisco, California (Mary N. Haan); Department of Biostatistics, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California (Janet S. Sinsheimer); Departments of Human Genetics and Biomathematics, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Janet S. Sinsheimer); and Department of Neurology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, California (Beate Ritz).
This study was funded by the National Institute of Environmental Health Sciences (grant ES023451), the National Institute on Aging (grants AG012975 and AG033751), and the National Institute of Diabetes and Digestive and Kidney Diseases (grant DK060753).
We thank all the participants and staff of the Sacramento Area Latino Study on Aging for their dedication and commitment.
Conflict of interest: none declared.
Abbreviations
- CES-D
Center for Epidemiologic Studies Depression Scale
- CI
confidence interval
- CIND
cognitive impairment without dementia
- CRP
C-reactive protein
- HR
hazard ratio
- IL-6
interleukin 6
- MET
metabolic equivalent of task
- NDE
natural direct effect
- NIE
natural indirect effect
- PA
physical activity
- SALSA
Sacramento Area Latino Study on Aging
- TNF-α
tumor necrosis factor α
REFERENCES
- 1. World Health Organization Mental health of older adults. https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults. Published December 12, 2017. Accessed May 10, 2019.
- 2. Bauman A, Merom D, Bull FC, et al. Updating the evidence for physical activity: summative reviews of the epidemiological evidence, prevalence, and interventions to promote “active aging”. Gerontologist. 2016;56(suppl 2):S268–S280. [DOI] [PubMed] [Google Scholar]
- 3. Gleeson M, Bishop NC, Stensel DJ, et al. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. [DOI] [PubMed] [Google Scholar]
- 4. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 5. Woods JA, Wilund KR, Martin SA, et al. Exercise, inflammation and aging. Aging Dis. 2014;3(1):130–140. [PMC free article] [PubMed] [Google Scholar]
- 6. Bettcher BM, Kramer JH. Longitudinal inflammation, cognitive decline, and Alzheimer’s disease: a mini‐review. Clin Pharmacol Ther. 2014;96(4):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martínez-Cengotitabengoa M, Carrascón L, O’Brien JT, et al. Peripheral inflammatory parameters in late-life depression: a systematic review. Int J Mol Sci. 2016;17(12):Article 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deleidi M, Jäggle M, Rubino G. Immune aging, dysmetabolism, and inflammation in neurological diseases. Front Neurosci. 2015;9:Article 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jenny NS, French B, Arnold AM, et al. Long-term assessment of inflammation and healthy aging in late life: the Cardiovascular Health Study All Stars. J Gerontol A Biol Sci Med Sci. 2012;67(9):970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-α) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139(3):230–239. [DOI] [PubMed] [Google Scholar]
- 12. Smith KJ, Au B, Ollis L, et al. The association between C-reactive protein, interleukin-6 and depression among older adults in the community: a systematic review and meta-analysis. Exp Gerontol. 2018;102:109–132. [DOI] [PubMed] [Google Scholar]
- 13. Hamer M, Sabia S, Batty GD, et al. Physical activity and inflammatory markers over 10 years: follow-up in men and women from the Whitehall II cohort study. Circulation. 2012;126(8):928–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen CS, Hasselbalch SG, Waldemar G, et al. Biochemical markers of physical exercise on mild cognitive impairment and dementia: systematic review and perspectives. Front Neurol. 2015;6:Article 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matthews KA, Xu W, Gaglioti AH, et al. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged ≥65 years. Alzheimers Dement. 2019;15(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerst K, Al-Ghatrif M, Beard HA, et al. High depressive symptomatology among older community-dwelling Mexican Americans: the impact of immigration. Aging Ment Health. 2010;14(3):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. González HM, Tarraf W, Whitfield KE, et al. The epidemiology of major depression and ethnicity in the United States. J Psychiatr Res. 2010;44(15):1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haan MN, Mungas DM, Gonzalez HM, et al. Prevalence of dementia in older Latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51(2):169–177. [DOI] [PubMed] [Google Scholar]
- 19. Haan MN, Al-Hazzouri AZ, Aiello AE. Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: the Sacramento Area Latino Study on Aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(suppl 1):i102–i110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shih IF, Paul K, Haan M, et al. Physical activity modifies the influence of apolipoprotein E ε4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimers Dement. 2018;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 22. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 23. McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS‐ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. [DOI] [PubMed] [Google Scholar]
- 24. Chui HC, Victoroff JI, Margolin D, et al. Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology. 1992;42(3):473–480. [DOI] [PubMed] [Google Scholar]
- 25. Guarnaccia PJ, Angel R, Worobey JL. The factor structure of the CES-D in the Hispanic Health and Nutrition Examination Survey: the influences of ethnicity, gender and language. Soc Sci Med. 1989;29(1):85–94. [DOI] [PubMed] [Google Scholar]
- 26. Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 27. Thomas JL, Jones GN, Scarinci IC, et al. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. Int J Psychiatry Med. 2001;31(1):25–40. [DOI] [PubMed] [Google Scholar]
- 28. Aiello AE, Haan MN, Pierce CM, et al. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63(6):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology. 2011;22(4):575–581. [DOI] [PubMed] [Google Scholar]
- 30. Nordahl H, Lange T, Osler M, et al. Education and cause-specific mortality: the mediating role of differential exposure and vulnerability to behavioral risk factors. Epidemiology. 2014;25(3):389–396. [DOI] [PubMed] [Google Scholar]
- 31. Lange T, Vansteelandt S, Bekaert M. A simple unified approach for estimating natural direct and indirect effects. Am J Epidemiol. 2012;176(3):190–195. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 33. Li R, Chambless L. Test for additive interaction in proportional hazards models. Ann Epidemiol. 2007;17(3):227–236. [DOI] [PubMed] [Google Scholar]
- 34. Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45(5):649–657. [DOI] [PubMed] [Google Scholar]
- 35. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta‐analysis of prospective studies. J Intern Med. 2011;269(1):107–117. [DOI] [PubMed] [Google Scholar]
- 36. Giovannini S, Onder G, Liperoti R, et al. Interleukin‐6, C‐reactive protein, and tumor necrosis factor‐alpha as predictors of mortality in frail, community‐living elderly individuals. J Am Geriatr Soc. 2011;59(9):1679–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25(5):811–816. [DOI] [PubMed] [Google Scholar]
- 38. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–472. [DOI] [PubMed] [Google Scholar]
- 39. Speisman RB, Kumar A, Rani A, et al. Daily exercise improves memory, stimulates hippocampal neurogenesis and modulates immune and neuroimmune cytokines in aging rats. Brain Behav Immun. 2013;28:25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamer M, Yates T, Demakakos P. Television viewing and risk of mortality: exploring the biological plausibility. Atherosclerosis. 2017; 263:151–155. [DOI] [PubMed] [Google Scholar]
- 41. Hamer M, de Oliveira C, Demakakos P. Non-exercise physical activity and survival: English Longitudinal Study of Ageing. Am J Prev Med. 2014;47(4):452–460. [DOI] [PubMed] [Google Scholar]
- 42. Nicklas BJ, Hsu FC, Brinkley TJ, et al. Exercise training and plasma C‐reactive protein and interleukin‐6 in elderly people. J Am Geriatr Soc. 2008;56(11):2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. O’Bryant SE, Johnson L, Edwards M, et al. The link between C-reactive protein and Alzheimer’s disease among Mexican Americans. J Alzheimers Dis. 2013;34(3):701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shah T, Newcombe P, Smeeth L, et al. Ancestry as a determinant of mean population C-reactive protein values: implications for cardiovascular risk prediction. Circ Cardiovasc Genet. 2010;3(5):436–444. [DOI] [PubMed] [Google Scholar]
- 45. Veeranna V, Zalawadiya SK, Niraj A, et al. Association of novel biomarkers with future cardiovascular events is influenced by ethnicity: results from a multi-ethnic cohort. Int J Cardiol. 2013;166(2):487–493. [DOI] [PubMed] [Google Scholar]
- 46. Fiske A, Wetherell JL, Gatz M. Depression in older adults. Annu Rev Clin Psychol. 2009;5:363–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hamer M, Molloy GJ, de Oliveira C, et al. Leisure time physical activity, risk of depressive symptoms, and inflammatory mediators: the English Longitudinal Study of Ageing. Psychoneuroendocrinology. 2009;34(7):1050–1055. [DOI] [PubMed] [Google Scholar]
- 48. Mansournia MA, Etminan M, Danaei G, et al. Handling time varying confounding in observational research. BMJ. 2017;359:j4587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.