Abstract
Prenatal maternal smoking is a risk factor for lower birth weight. We performed epigenome-wide association analyses of placental DNA methylation (DNAm) at 720,077 cytosine-phosphate-guanine (CpG) sites and prenatal maternal smoking among 441 mother-infant pairs (2010–2014) and evaluated whether DNAm mediates the association between smoking and birth weight using mediation analysis. Mean birth weight was 3,443 (standard deviation, 423) g, and 38 mothers (8.6%) reported smoking at a mean of 9.4 weeks of gestation. Prenatal maternal smoking was associated with a 175-g lower birth weight (95% confidence interval (CI): −305.5, −44.8) and with differential DNAm of 71 CpGs in placenta, robust to latent-factor adjustment reflecting cell types (Bonferroni-adjusted P < 6.94 × 10−8). Of the 71 CpG sites, 7 mediated the association between prenatal smoking and birth weight (on MDS2, PBX1, CYP1A2, VPRBP, WBP1L, CD28, and CDK6 genes), and prenatal smoking × DNAm interactions on birth weight were observed for 5 CpG sites. The strongest mediator, cg22638236, was annotated to the PBX1 gene body involved in skeletal patterning and programming, with a mediated effect of 301-g lower birth weight (95% CI: −543, −86) among smokers but no mediated effect for nonsmokers (β = −38 g; 95% CI: −88, 9). Prenatal maternal smoking might interact with placental DNAm at specific loci, mediating the association with lower infant birth weight.
Keywords: DNA methylation, epigenetics, mediation, smoking
Editor’s note: An invited commentary on this article appears on page 1887, and the authors’ response appears on page 1890.
Prenatal maternal smoking is an established risk factor for lower birth weight and reduced fetal growth, and it is associated with increased risk of major childhood diseases such as asthma, obesity, and cancers (1–3). A complex chemical mixture of over 7,000 compounds make up cigarette smoke, including known reproductive toxicants such as carbon monoxide, arsenic, lead, cadmium, and nicotine. Although prenatal maternal cigarette smoke is a well-recognized risk factor for reduced fetal growth, mechanisms are only partially understood and might include both direct effects on the fetus and morphological and functional changes to the placenta (4).
DNA methylation (DNAm), occurring mostly at cytosine-phosphate-guanine dinucleotides (CpG sites), can involve extensive erasure, rewriting, and reprogramming during embryogenesis and cellular differentiation (5). This reprogramming phase might pose a susceptibility window for early nutritional and environmental cues to program future health trajectories of newborns by altering cellular structure, lineage commitment, physiology, and metabolic functions, a hypothesis known as fetal programming (6). For instance, prenatal smoking has been consistently associated with altered cord blood DNAm of several genes (7–10). Previous studies have shown that cord blood DNAm variability associated with prenatal smoking partially mediates the association of smoking and lower birth weight (11, 12). While multiple studies have looked at maternal smoking and DNAm in cord blood, few have investigated placental DNAm variation, and even fewer have used an agnostic epigenome-wide approach (13–16).
Methylation of DNA is vastly cell-type specific, and early environmental influences on the epigenome are likely tissue-and cell-type specific (17). Thus, investigating the role of target tissue in epigenetic studies is critical (18). The placenta is the master regulator of the fetal environment, with active endocrine and metabolic functions, synthesizing hormones and regulating nutrient and waste exchange (19). Nicotine, a parasympathomimetic stimulant and primary constituent of tobacco smoke, acts as a vasoconstrictor reducing intervillous placental blood flow, increases hypoxia, and interferes with placental development (20, 21). Additionally, many other reproductive toxicants present in cigarette smoke cross the placenta, exposing the fetus, and some accumulate within placental structures (22). Consequently, the placenta might be particularly susceptible to prenatal tobacco smoke and a target tissue to mediate associations with fetal growth. In this study, we hypothesized that DNAm variability of the placenta associated with prenatal maternal smoking early in pregnancy would mediate the established association of prenatal smoking and lower infant birth weight.
METHODS
Study population
Study participants were selected from the Genetics of Glucose Regulation in Gestation and Growth (Gen3G) cohort, a prospective prebirth cohort recruited between January 2010 and February 2014 in Sherbrooke, Canada. We recruited expecting mothers during the first trimester of pregnancy. They were eligible for enrollment if they were 18 years of age or older, had a singleton pregnancy, did not have prepregnancy diabetes based on medical history and screening, and planned to deliver at the Centre Hospitalier Universitaire de Sherbrooke. For the present study, mother-infant pairs were selected if they consented to placental tissue collection for DNA isolation and had >37 weeks of gestation at delivery.
The Gen3G cohort has been previously described (23). Mothers provided written informed consent prior to enrollment in accordance with the Declaration of Helsinki. Study protocols were approved by the ethical review board from the Center Hospitalier Universitaire de Sherbrooke.
Placental tissue and DNA
Placental tissue from the fetal side was collected by trained staff within 30 minutes of delivery. A 1-cm3 sample of placental tissue was collected approximately 5 cm from the umbilical cord insertion. Placental samples were subsequently stored at −80°C until DNA extraction occurred. We purified DNA from placental samples using the All Prep DNA/RNA/Protein Mini Kit (Qiagen, Germantown, Maryland). Purity of extracted DNA was evaluated using a spectrophotometer (Ultrospec 2000 UV/Visible; Pharmacia Biotech, Piscataway, New Jersey).
Prenatal maternal smoking and birth weight
During the first research visit, occurring at a mean of 9.4 (standard deviation (SD), 2.3) weeks of gestation, trained staff administered standardized questionnaires to participating women to collect medical and lifestyle history, including smoking behavior. Participants were classified as current smokers or not current smokers (which included past smokers) based on self-report at this visit. Within 2 hours of delivery, birth weight was measured in grams with an electronic scale in the hospital, following standard clinical protocol, by obstetrical nurses.
Placental DNA methylation
Epigenome-wide DNAm measurements were performed on placental DNA and quantified using the Infinium MethylationEPIC BeadChip (Illumina, San Diego, California), measuring over 850,000 CpGs at a nucleotide resolution. Data processing and cleaning have been described previously and in the Web Appendix (available at https://academic.oup.com/aje) (24).
Statistical analyses
We report demographic characteristics using means and standard deviations or proportions. We conducted epigenome-wide association studies (EWAS) of self-reported prenatal maternal smoking behavior by fitting linear regression models using limma for each CpG on the β-value scale. Confounding factors were selected a priori based on EWAS recommendations (25). We controlled for exposure-mediator (maternal age), mediator-outcome (sex, parity, gestational age, and cell-type/DNAm heterogeneity), and exposure-outcome (maternal body mass index, calculated as weight (kg)/height (m2)) confounding (Web Figure 1). We examined models adjusting for cell-type/DNAm heterogeneity estimated using latent-factors from ReFACTor (26), a reference-free adjustment method, using the first 10 principal components selected by visual inspection (Web Figure 2). We used quantile-quantile plots of P values from EWAS to inspect genomic inflation and calculated genomic inflation factors (λ). We used Manhattan and volcano plots to report EWAS results. We selected CpGs as candidate mediators if they were associated with prenatal maternal smoking or birth weight in both crude and ReFACTor-adjusted models (Bonferroni-adjusted P < 6.94 × 10−8). We selected candidate CpG mediators found in both crude and ReFACTor-adjusted models to ensure reproducibility, given that reference-free methods have varying assumptions (27).
For each individual candidate CpG mediator, we fitted 2 linear regression models: 1) the mediator model, with DNAm of the CpG as the outcome and prenatal smoking as a predictor, adjusting for maternal age, body mass index, parity, gestational age, sex, and the 10 principal components from ReFACTor; and 2) the outcome model, with birth weight as the outcome and prenatal smoking as a predictor, adjusting for the mediator, DNAm of the CpG, and the covariates from the first model. In mediation analyses, we adjusted for multiple comparisons using a false discovery rate (FDR) of 5% (<0.05) for the average causal mediated effect tests. Among the CpGs selected (FDR < 0.05), we tested for the exposure-mediator interaction using P < 0.10. To test for statistical mediation, we used a counterfactual framework to estimate the direct and indirect effects. This method is implemented in the mediation package in R (R Foundation for Statistical Computing, Vienna, Austria) (28). We used 100,000 simulations to report estimates and 95% confidence intervals.
Additionally, we tested for mediation among 3 CpGs previously reported to mediate the association of smoking and birth weight in placenta (16). For mediators found in our placenta analyses, we tested for mediation on 437 paired cord blood samples from our same cohort also measured with the MethylationEPIC array.
Our approach to mediation analysis assumes the sequential ignorability assumption: that there is no unmeasured confounding of the exposure-outcome, mediator-outcome, exposure-mediator relationships and that none of the mediator-outcome confounders are affected by the exposure (29). We visually inspected violation of this assumption by estimating correlations (ρ) between the residuals of the mediator and outcome regression for which direct and indirect effects would be zero. We checked for outliers and assumptions of linear regression models for mediation using regression diagnostic plots; all models reasonably met assumptions.
Among all candidate CpG mediators, we conducted KEGG biological pathway enrichment analyses using missMethyl with probe density bias correction (30). We also performed enrichment of candidate CpG mediators to test the likely tissue/cell of origin of findings using eFORGE (https://eforge.altiusinstitute.org/). We performed data analyses using R, version 3.5.1 (R Foundation for Statistical Computing).
RESULTS
We analyzed a total of 441 mother-infant pairs. At enrollment, mean maternal age was 28.3 (SD, 4.3) years and body mass index was 25.4 (SD, 5.6). All pregnant women were white, 48.5% were primiparous, and 38 (8.6%) reported currently smoking at the first research visit. Of the newborns, 52.6% were male; the mean gestational age was 39.5 (SD, 1.04) weeks, and mean birth weight was 3,443 (SD, 422.7) g (Table 1). Prenatal maternal smoking was associated with a 175-g lower birth weight (95% confidence interval (CI): −305.5, −44.8) after adjustment for maternal age, body mass index, parity, gestational age, sex, and cell-type/DNAm heterogeneity. Results remained consistent in crude and partially adjusting models or when using birth-weight-for-gestational-age z scores (Web Table 1).
Table 1.
Participant Characteristics From the Genetics of Glucose Regulation in Gestation and Growth Study (n = 441), Sherbrooke, Canada, 2010–2014
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| Maternal age, years | 28.3 (4.3) | ||
| Maternal body mass indexa | 25.4 (5.6) | ||
| Primiparous | 214 | 48.5 | |
| White ethnicity | 441 | 100 | |
| Smoking during pregnancy | |||
| No | 403 | 91.4 | |
| Yes | 38 | 8.6 | |
| Child sex | |||
| Male | 232 | 52.6 | |
| Female | 209 | 47.4 | |
| Gestational age, weeks | 39.5 (1.04) | ||
| Birth weight, g | 3,443 (422.7) | ||
| Birth weight for gestational age, z score | 0.39 (0.93) | ||
a Weight (kg)/height (m)2.
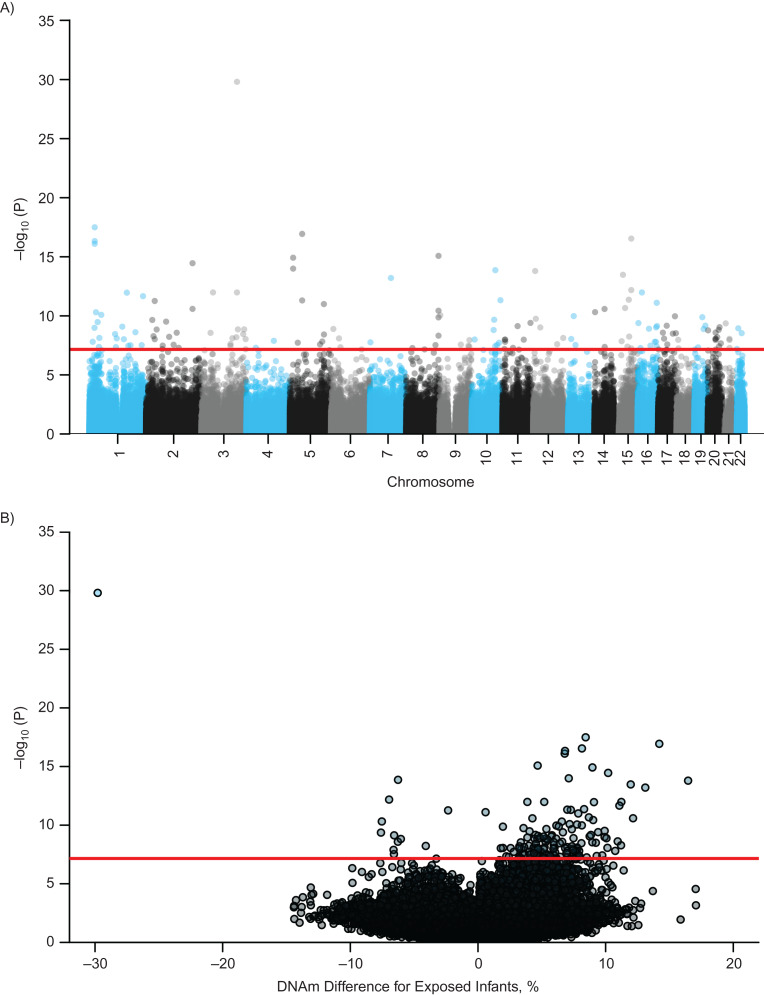
Prenatal maternal smoking was associated with placental DNAm at 89 CpGs (Bonferroni-adjusted P < 6.94 × 10−8) in models adjusting for maternal age, body mass index, parity, gestational age, and sex. Further adjustment for cell-type/DNAm heterogeneity yielded 153 differentially methylated CpGs (Bonferroni-adjusted P < 6.94 × 10−8) (Figure 1A; Web Figure 3). Effect sizes ranged from 29.8% lower DNAm (cg27402634; the leucine, glutamate, and lysine rich 1 gene (LEKR1)) to 16.5% greater DNAm (cg11051192; polyhomeotic homolog 1 (PHC1)) in the placenta of prenatal smokers compared to nonsmokers (Figure 1B).
Figure 1.
Manhattan plot (A) and volcano plot (B) for epigenome-wide association analyses of prenatal maternal smoking and placental DNA methylation (DNAm), adjusted for confounders and cell-type/DNA methylation heterogeneity, Sherbrooke, Canada, 2010–2014. The red line is the Bonferroni threshold for statistical significance.
For mediation analyses, we tested 71 CpGs individually as candidate mediators found to be differentially methylated (Bonferroni-adjusted P < 6.94 × 10−8) in crude models and models adjusting for cell-type/DNAm heterogeneity. The 71 CpGs were annotated to genes enriched (FDR < 0.05) within the notch signaling pathway, pathways in cancer, and the human papillomavirus infection pathway (Web Table 2). The 71 CpGs associated with smoking were enriched in DNAse I hypersensitivity sites of fetal placental tissues (Web Figure 4). Genomic annotation and summary results from fully adjusted EWAS analyses of prenatal smoking for the 71 CpGs considered as candidate mediators are reported in Web Table 3.
We found 7 CpGs mediating the association between prenatal maternal smoking and birth weight (FDR < 0.05; adjusted for 71 comparisons). Among these 7 CpGs, we observed exposure-mediator interaction for 5 (P < 0.10), as shown in Table 2. As shown in Table 3, among mediators interacting with prenatal smoking, cg14160212 (located within 151 base pairs of the myelodysplastic syndrome 2 translocation-associated protein (MDS2) gene) had a mediated effect of 280-g lower birth weight (95% CI: −478, −102) for smokers but no mediating effect for nonsmokers. Similarly, cg22638236 annotated to the pre-B-cell leukemia homeobox 1 protein gene (PBX1) had a mediated effect of 301-g lower birth weight (95% CI: −543, −86) for smokers but no mediated effect for nonsmokers. DNAm of cg20385913 (within 5,569 base pairs of the cytochrome P450 family 1 subfamily a member 2 (CYP1A2) gene) had a mediated effect of 244-g lower birth weight (95% CI: −457, −49) for smokers and no mediating effect for nonsmokers. DNAm of cg11280108 (located within 3,609 base pairs of Vpr (HIV-1) binding protein (VPRBP)) had a mediated effect of 185-g lower birth weight (95% CI: −363, −25) for smokers and no mediated effect in nonsmokers. Last, cg01883283 annotated to the WW domain binding protein 1-like gene (WBP1L) had a mediated effect of 135-g lower birth weight (95% CI: −246, −34) among smokers only. As noted in Table 2, no exposure-mediator interaction was observed for cg23878024, with an average mediated effect of 86-g lower birth weight (95% CI: −144, −34), and cg24005876 annotated to the cyclin dependent kinase 6 (CDK6) gene body, with an average mediated effect of 91-g lower birth weight (95% CI: −149, −41).
Table 2.
Placental DNA Methylation Mediators for the Association of Prenatal Maternal Smoking and Infant Birth Weight With a Significant Average Mediated Effect, Sherbrooke, Canada, 2010–2014
| Annotation | Average Direct Effects | Average Mediated Effects | Exposure Mediator Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CpG Site | Chromosome | Position | Gene | Distance in Base Pairsa | β | 95% CI | P | β | 95% CI | FDRb | P Value |
| cg14160212 | 1 | 23907834 | MDS2a | 151 | −14 g | −179, 156 | 0.87 | −153 g | −260, −57 | 0.05 | 0.005 |
| cg22638236 | 1 | 164759974 | PBX1 | Body | −11 g | −180, 165 | 0.89 | −169 g | −297, −58 | 0.05 | 0.02 |
| cg20385913 | 15 | 75054510 | CYP1A2a | 5,569 | −44 g | −207, 122 | 0.60 | −139 g | −253, −36 | 0.03 | 0.04 |
| cg11280108 | 3 | 51537627 | VPRBPa | 3,609 | −69 g | −221, 85 | 0.37 | −115 g | −211, −30 | 0.03 | 0.09 |
| cg01883283 | 10 | 104512669 | WBP1L | Body | −86 g | −230, 57 | 0.24 | −86 g | −152, −27 | 0.04 | 0.09 |
| cg23878024 | 2 | 204488580 | CD28a | 82,618 | −89 g | −228, 49 | 0.21 | −86 g | −144, −34 | 0.03 | 0.12 |
| cg24005876 | 7 | 92303485 | CDK6 | Body | −84 g | −221, 53 | 0.23 | −91 g | −149, −41 | 0.04 | 0.30 |
Abbreviations: CD28, CD28 molecule; CDK6, cyclin dependent kinase 6; CI, confidence interval; CpG, cytosine-phosphate-guanine; CYP1A2, cytochrome P450 family 1 subfamily A member 2; FDR, false discovery rate; MDS2, myelodysplastic syndrome 2 translocation-associated protein; PBX1, pre-B-cell leukemia homeobox 1; VPRBP, Vpr (HIV-1) binding protein; WBP1L, WW domain binding protein 1-like.
a Not within the gene; therefore, annotated distance to nearest gene.
b FDR-adjusted P value.
Table 3.
Estimates for the Direct and Causal Mediated Effects Among Cytosine-Phosphate-Guanine Sites With Evidence of Exposure-Mediator Interaction According to Prenatal Maternal Smoking Status, Sherbrooke, Canada (2010–2014)
| Annotation | Prenatal Maternal Smoking | No Prenatal Maternal Smoking | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Direct Effects | Mediated Effects | Direct Effects | Mediated Effects | ||||||||||
| CpG Site | Gene | β | 95% CI | P Value | β | 95% CI | P Value | β | 95% CI | P Value | β | 95% CI | P Value |
| cg14160212 | MDS2 | −141 g | −296, 11 | 0.07 | −280 g | −478, −102 | 0.001 | 114 g | −110, 339 | 0.31 | −25 g | −83, 30 | 0.38 |
| cg22638236 | PBX1 | −142 g | −302, 14 | 0.07 | −301 g | −543, −86 | 0.005 | 121 g | −125, 369 | 0.33 | −38 g | −88, 9 | 0.11 |
| cg20385913 | CYP1A2 | −148 g | −302, 2 | 0.05 | −244 g | −457, −49 | 0.01 | 61 g | −169, 291 | 0.60 | −35 g | −94, 22 | 0.23 |
| cg11280108 | VPRBP | −139 g | −285, 6 | 0.06 | −185 g | −363, −25 | 0.02 | 1 g | −198, 201 | 0.99 | −45 g | −96, 2 | 0.06 |
| cg01883283 | WBP1L | −135 g | −278, 8 | 0.06 | −135 g | −246, −34 | 0.008 | −38 g | −203, 127 | 0.65 | −38 g | −97, 18 | 0.19 |
Abbreviations: CI, confidence interval; CpG, cytosine-phosphate-guanine; CYP1A2, cytochrome P450 family 1 subfamily A member 2; MDS2, myelodysplastic syndrome 2 translocation-associated protein; PBX1, pre-B-cell leukemia homeobox 1 protein; VPRBP, Vpr (HIV-1) binding protein; WBP1L, WW domain binding protein 1-like.
Distribution of DNAm levels among the 7 mediating CpGs are shown in raincloud plots (Web Figure 5) and according to smoking status on boxplots (Web Figure 6). Mediation analysis using birth-weight-for-gestational-age z scores as the outcome, instead of birth weight, yielded consistent results (Web Table 4). Using 437 paired cord blood samples, we did not observe mediated effects at the 7 CpGs (Web Table 5).
For comparability, we examined CpGs previously reported to mediate smoking–birth weight associations by placental DNAm in, to our knowledge, the only EWAS performed to date (16) and observed a consistent average causal mediated effect of 145-g lower birth weight (95% CI: −258, −36) for cg27402634 annotated to the leucine, glutamate, and lysine rich 1 gene (LEKR1). Additionally, we observed DNAm × smoking interaction on birth weight for this site. The mediated effect among smokers was 276-g lower birth weight (95% CI: −483, −76), with no mediated effect for nonsmoking mothers. We did not find a significant mediated effect for the other 3 CpGs reported by Morales et al. (16), possibly due to single-nucleotide polymorphisms within the probes (cg20340720, cg12294026) that might differ by minor allele frequency (Web Tables 6 and 7). Another important difference is that we used the MethylationEPIC array covering over 400,000 new CpGs located mostly at regulatory elements compared to the 450K previously used (31).
In sensitivity analyses of the mediated effect estimates, the sequential ignorability assumption might be violated for residual correlations of the mediator and outcome regressions far from the observed estimated mediated effects for all 7 CpGs (Web Figure 7).
DISCUSSION
Prenatal maternal smoking is an established risk factor for lower birth weight. Here we report our finding that placental DNAm of genes associated with prenatal maternal smoking mediated the association with reduced birth weight. We observed interaction with 5 of the 7 CpGs found. This resulted in a strong mediated effect among smokers and null, attenuated effects for nonsmokers for 5 out of the 7 CpGs. Our results suggest that DNAm variability in the placenta associated with prenatal maternal smoking might play an active role in the well-established association of prenatal smoking and low birth weight. Our results also highlight the need to consider exposure-mediator interactions in epigenetic studies as a relevant biological and statistical model.
We observed several exposure-mediator interactions suggesting that prenatal maternal smoking must be present for the mediator, placental DNAm, to affect birth weight. This is a prime example of a mediated interaction, plausible for biological mechanisms and models (32). A mediated-interaction epigenetic model is supported by previous studies that demonstrate epigenetic malleability associated with environmental exposures. For example, we have found that epigenetic variability associated with prenatal environmental exposures at birth might be attenuated as children age (33, 34). Additionally, in a randomized trial it was demonstrated that DNAm variability associated with prenatal maternal smoking in placenta, cord blood and buccal cells can be restored to “normal” DNAm levels comparable to that of infants born to nonsmoking mothers after vitamin-C supplementation (35). This reversibility of the mediated effect supports the hypothesis that prenatal maternal smoking must be present for DNAm to affect birth weight. This concept of mediated interaction has been proposed to more accurately reflect phenomena observed in models of disease (32). We propose that a mediated-interaction model is highly relevant to epigenetic studies.
The strongest CpG mediator was annotated to PBX1. PBX1 encodes a nuclear protein that belongs to the PBX homeobox family of transcriptional factors, widely transcribed throughout the developing murine embryo and essential for skeletal patterning and programming (36). The PBX1 gene regulates osteoblast differentiation by altering chromatin structures and regulating transcription of bone specific genes (37). A previous EWAS of cord blood reported greater DNAm of a genomic region in PBX1 to be strongly associated with higher birth weight for gestational age (38). In adults, higher urinary nicotine equivalents among smokers were associated with greater leukocyte DNAm at CpGs near PBX1 (39). In a large study of adults, PBX1 DNAm of leukocytes associated with current body mass index across multiple cohorts, and this association was replicated for DNAm of adipose tissue (40). Whether or not PBX1 DNAm of placenta at birth is relevant for future child adiposity remains to be tested.
Prenatal smoking was also associated with 6.3% lower DNAm at cg01883283 annotated to WBP1L and mediated associations with birth weight. Placental DNAm of this gene has been linked to prenatal smoking at a nearby CpG within 145 base pairs of our site (8.7% lower DNAm at cg20340720) (16). However, in this previous study, no evidence of a mediated effect between prenatal smoking and birth weight was found for cg20340720. Another CpG mediator, cg24005876, annotated to the cell division protein kinase 6 (CDK6) gene, is member of the serine/threonine protein kinases family and a critical regulator of cell cycle. The CDK6 gene has been shown to play an important role in the development of polyploidy as well as stromal decidualization (41). In a study of human term placentas, CDK6 mRNA was modestly correlated with infant birth weight (r = 0.33) (42). This gene also covers a susceptibility loci for lung cancer shown to interact with smoking status/history (43).
CYP1A2 is involved in the metabolism of pharmacological compounds as well as polycyclic aromatic hydrocarbons (PAHs) present in cigarette smoke (44). The CYP1A2 enzyme is induced by tobacco smoke, shown to be primarily mediated by aryl hydrocarbon receptor ligands like polycyclic aromatic hydrocarbons (45). Placental CYP1A2 expression has been detected in first trimester but not full-term placentas (46). From candidate gene studies, lower placental DNAm of the CYP1A1 gene, another CYP family member, has been consistently associated with prenatal smoking (47–49). Additionally, prenatal maternal smoking has been consistently reported to be associated with differential DNAm near CYP1A1 in cord blood analyses, as well as in an EWAS of placenta (9, 16).
Less is known about DNAm of cg11280108 within 3,611 base pairs of the Homo sapiens VPRBP gene but this region has been shown to be involved in cell proliferation, chromatin remodeling, and oocyte survival and programming (50–52). Much less is known about DNAm or placental expression of cg14160212 (myelodysplastic syndrome 2 translocation-associated protein (MDS2)) or cg23878024 (CD28), and these CpGs did not directly annotate to genes. The underlying genetic architecture could also influence observed results. However, only one of our mediators (cg01883283; WBP1L) was located within 500 kb of a birth-weight single-nucleotide polymorphism (rs74233809) (53).
A previous study performed an EWAS of prenatal maternal smoking and placental DNA, investigating the mediated effect of DNAm (16). In this study, cg27402634 (LEKR1) explained up to 36% of the association between prenatal smoking and lower birth weight. In our cohort, cg27402634 had the strongest association with prenatal smoking but did not reach an FDR < 0.05 for the average mediated effect. We did observe a similar mediated effect of 145-g lower birth weight post hoc, but contrary to the previous study, an exposure-mediator interaction was present at this site. Although we did not observe mediation for the other 2 CpGs reported by Morales et al. (16) (cg25585967 and cg12294026) within the trio rho guanine nucleotide exchange factor (TRIO) gene body, they were consistently associated with greater placental DNAm for smokers with similar magnitudes.
Our results highlight that prenatal maternal smoking signatures of placenta DNAm are distinct of cord blood signatures. For example, using paired cord blood samples from our study we found no evidence of mediation at the 7 discovered CpGs from placenta. We compared the 71 CpGs associated with maternal smoking in placenta to 6,073 CpGs (8) previously reported from meta-analyses of cord blood and found that only 1 CpG (cg21992501; tetratricopeptide repeat domain 27 (TTC27)) positively associated with smoking in our placenta samples and in the meta-analyses of cord blood.
Our study has several important limitations. In DNAm studies with exposure misclassification, it has been demonstrated that DNAm of CpGs could serve as better biomarkers of the exposure than self-report, thereby increasing the type I error rate, underestimating the direct effect, and overestimating mediated effects (54). We also did not collect objective exposure biomarkers, and it is possible that our estimated mediated effects are inflated. However, self-reported smoking during pregnancy had high sensitivity (97.6%) and specificity (100%) compared with plasma cotinine in a comparable Canadian population (55) and other large cohorts (56). Another important limitation is that we did not ascertain sustained smoking throughout pregnancy—women could have stopped smoking after the first research visit—or exposure to secondhand smoke. Future studies should consider the impact of periconceptional paternal smoking and its impact on germ cell DNAm and on the infant’s epigenome. Timing of exposure, mediator, and outcome ascertainment is an important limitation of our study—women could have continued to smoke throughout pregnancy and placental DNAm levels could have changed until birth, when birth weight was measured. However, in the general Canadian population, approximately 53% of women quit smoking by the third trimester of pregnancy (57), so it is likely that most women in our study quit by the third trimester. Additionally, placental DNAm profiles are largely established in early gestation. Our assumptions are that smoking in early pregnancy influences DNAm of placental genes that are established in early gestation, thereby influencing fetal growth. Nevertheless, it is possible that fetal growth influences placenta DNAm. Therefore, our results must be interpreted in light of the timing of exposure and DNAm measurements.
An important strength of our approach is the implementation of the counterfactual framework in mediation analysis to estimate effects in the presence of exposure-mediator interactions as a relevant biological model for epigenetic epidemiology. Nevertheless, statistical mediation might not accurately reflect underlying causal biological processes such as transcriptional memory or cell lineage commitment. Therefore, our results do not necessarily reflect a causal biological mechanism. Future studies could also develop epigenetic biosensors of prenatal smoking from placenta DNAm signatures that might serve as biomarkers of the prenatal environment.
In conclusion, in this cohort, we observed that placental DNAm variability at specific loci associated with prenatal maternal smoking might mediate the association with lower birth weight within biologically relevant genes. Placental DNAm mediators annotated to the PBX1 gene, involved in skeletal patterning and programming; CYP1A2 gene, whose activity is induced by polycyclic aromatic hydrocarbons found in cigarette smoke; CDK6, whose expression in placenta has been associated with birth weight and stromal decidualization; and the WBP1L gene, previously shown to be differentially methylated in the placenta of smokers. Finally, we report evidence of significant exposure × mediator interactions and propose this to be highly relevant model for epigenetic epidemiology.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Environmental Health Sciences, School of Public Health, University of California, Berkeley, Berkeley, California (Andres Cardenas); Department of Population Medicine, Harvard Pilgrim Health Care Institute, Harvard Medical School, Boston, Massachusetts (Andres Cardenas, Sharon M. Lutz, Marie-France Hivert); Department of Environmental Health, Rollins School of Public Health, Emory University, Atlanta, Georgia (Todd M. Everson); Centre de Recherche du Center Hospitalier Universitaire de Sherbrooke, Sherbrooke, Quebec, Canada (Patrice Perron, Luigi Bouchard, Marie-France Hivert); and Diabetes Unit, Massachusetts General Hospital, Boston, Massachusetts (Marie-France Hivert).
This work was supported by Fonds de Recherche du Québec en Santé (award 20697), Canadian Institute of Health Research (award MOP 115071), American Diabetes Association (accelerator award 1-15-ACE-26 to M.-F.H.), and the National Institutes of Health (grant K01HL125858 to S.M.L.).
Conflict of interest: none declared.
Abbreviations
- CDK6
cyclin dependent kinase 6
- CI
confidence interval
- CpG
cytosine-phosphate-guanine
- CYP1A2
cytochrome P450 family 1 subfamily A member 2
- DNAm
DNA methylation
- EWAS
epigenome-wide association studies
- FDR
false discovery rate
- Gen3G
Genetics of Glucose Regulation in Gestation and Growth
- PBX1
pre-B-cell leukemia homeobox 1 protein
- SD
standard deviation
- VPRBP
Vpr (HIV-1) binding protein
- WBP1L
WW domain binding protein 1-like
REFERENCES
- 1. Oken E, Levitan EB, Gillman MW. Maternal smoking during pregnancy and child overweight: systematic review and meta-analysis. Int J Obes (Lond). 2008;32(2):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. US Department of Health Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, Office on Smoking and Health; 2006. [Google Scholar]
- 3. DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113(4 suppl):1007–1015. [PubMed] [Google Scholar]
- 4. Jauniaux E, Burton GJ. Morphological and biological effects of maternal exposure to tobacco smoke on the feto-placental unit. Early Hum Dev. 2007;83(11):699–706. [DOI] [PubMed] [Google Scholar]
- 5. Perera F, Herbstman J. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol. 2011;31(3):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reichetzeder C, Dwi Putra SE, Li J, et al. Developmental origins of disease—crisis precipitates change. Cell Physiol Biochem. 2016;39(3):919–938. [DOI] [PubMed] [Google Scholar]
- 7. Joubert BR, Håberg SE, Nilsen RM, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect. 2012;120(10):1425–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joubert BR, Felix JF, Yousefi P, et al. DNA methylation in newborns and maternal smoking in pregnancy: genome-wide consortium meta-analysis. Am J Hum Genet. 2016;98(4):680–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyake K, Kawaguchi A, Miura R, et al. Association between DNA methylation in cord blood and maternal smoking: the Hokkaido study on environment and children’s health. Sci Rep. 2018;8:5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richmond RC, Simpkin AJ, Woodward G, et al. Prenatal exposure to maternal smoking and offspring DNA methylation across the lifecourse: findings from the Avon Longitudinal Study of Parents and Children (ALSPAC). Hum Mol Genet. 2014;24(8):2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Witt SH, Frank J, Gilles M, et al. Impact on birth weight of maternal smoking throughout pregnancy mediated by DNA methylation. BMC Genomics. 2018;19:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Küpers LK, Xu X, Jankipersadsing SA, et al. DNA methylation mediates the effect of maternal smoking during pregnancy on birthweight of the offspring. Int J Epidemiol. 2015;44(4):1224–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilhelm-Benartzi CS, Houseman EA, Maccani MA, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect. 2012;120(2):296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chhabra D, Sharma S, Kho AT, et al. Fetal lung and placental methylation is associated with in utero nicotine exposure. Epigenetics. 2014;9(11):1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suter M, Ma J, Harris A, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics. 2011;6(11):1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morales E, Vilahur N, Salas LA, et al. Genome-wide DNA methylation study in human placenta identifies novel loci associated with maternal smoking during pregnancy. Int J Epidemiol. 2016;45(5):1644–1655. [DOI] [PubMed] [Google Scholar]
- 17. Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. [DOI] [PubMed] [Google Scholar]
- 18. Bakulski KM, Fallin MD. Epigenetic epidemiology: promises for public health research. Environ Mol Mutagen. 2014;55(3):171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy VE, Smith R, Giles WB, et al. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–169. [DOI] [PubMed] [Google Scholar]
- 20. Larsen LG, Clausen HV, Jønsson L. Stereologic examination of placentas from mothers who smoke during pregnancy. Am J Obstet Gynecol. 2002;186(3):531–537. [DOI] [PubMed] [Google Scholar]
- 21. Holloway AC, Salomon A, Soares MJ, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Metab. 2014;306(4):E443–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Myllynen P, Pasanen M, Pelkonen O. Human placenta: a human organ for developmental toxicology research and biomonitoring. Placenta. 2005;26(5):361–371. [DOI] [PubMed] [Google Scholar]
- 23. Guillemette L, Allard C, Lacroix M, et al. Genetics of Glucose regulation in Gestation and Growth (Gen3G): a prospective prebirth cohort of mother–child pairs in Sherbrooke, Canada. BMJ Open. 2016;6(2):e010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cardenas A, Gagné-Ouellet V, Allard C, et al. Placental DNA methylation adaptation to maternal glycemic response in pregnancy. Diabetes. 2018;67(8):1673–1683. [DOI] [PubMed] [Google Scholar]
- 25. Breton CV, Marsit CJ, Faustman E, et al. Small-magnitude effect sizes in epigenetic end points are important in children’s environmental health studies: the Children’s Environmental Health and Disease Prevention Research Center’s Epigenetics Working Group. Environ Health Perspect. 2017;125(4):511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rahmani E, Zaitlen N, Baran Y, et al. Sparse PCA corrects for cell type heterogeneity in epigenome-wide association studies. Nat Methods. 2016;13(5):443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Teschendorff AE, Relton CL. Statistical and integrative system-level analysis of DNA methylation data. Nat Rev Genet. 2018;19(3):129–147. [DOI] [PubMed] [Google Scholar]
- 28. Tingley D, Yamamoto T, Hirose K, et al. Mediation: R package for causal mediation analysis. J Stat Softw 2014;59(5):1–38. [Google Scholar]
- 29. VanderWeele TJ. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25(5):749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Phipson B, Maksimovic J, Oshlack A. missMethyl: an R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics. 2016;32(2):286–288. [DOI] [PubMed] [Google Scholar]
- 31. Pidsley R, Zotenko E, Peters TJ, et al. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016;17:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ikram MA, VanderWeele TJ. A proposed clinical and biological interpretation of mediated interaction. Eur J Epidemiol. 2015;30(10):1115–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cardenas A, Rifas-Shiman SL, Godderis L, et al. Prenatal exposure to mercury: associations with global DNA methylation and hydroxymethylation in cord blood and in childhood. Environ Health Perspect. 2017;125(8):087022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardenas A, Rifas-Shiman SL, Agha G, et al. Persistent DNA methylation changes associated with prenatal mercury exposure and cognitive performance during childhood. Sci Rep. 2017;7:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shorey-Kendrick LE, McEvoy CT, Ferguson B, et al. Vitamin C prevents offspring DNA methylation changes associated with maternal smoking in pregnancy. Am J Respir Crit Care Med. 2017;196(6):745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Selleri L, Depew MJ, Jacobs Y, et al. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development. 2001;128(18):3543–3557. [DOI] [PubMed] [Google Scholar]
- 37. Gordon JA, Hassan MQ, Koss M, et al. Epigenetic regulation of early osteogenesis and mineralized tissue formation by a HOXA10-PBX1-associated complex. Cells Tissues Organs. 2011;194(2–4):146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Agha G, Hajj H, Rifas-Shiman SL, et al. Birth weight-for-gestational age is associated with DNA methylation at birth and in childhood. Clin Epigenetics. 2016;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Park SL, Patel YM, Loo LWM, et al. Association of internal smoking dose with blood DNA methylation in three racial/ethnic populations. Clin Epigenetics. 2018;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Demerath EW, Guan W, Grove ML, et al. Epigenome-wide association study (EWAS) of BMI, BMI change and waist circumference in African American adults identifies multiple replicated loci. Hum Mol Genet. 2015;24(15):4464–4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tan J, Raja S, Davis MK, et al. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev. 2002;111(1–2):99–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sedlmeier E-M, Brunner S, Much D, et al. Human placental transcriptome shows sexually dimorphic gene expression and responsiveness to maternal dietary n-3 long-chain polyunsaturated fatty acid intervention during pregnancy. BMC Genomics. 2014;15:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Deng Q, Guo H, Dai J, et al. Imputation-based association analyses identify new lung cancer susceptibility variants in CDK6 and SH3RF1 and their interactions with smoking in Chinese populations. Carcinogenesis. 2013;34(9):2010–2016. [DOI] [PubMed] [Google Scholar]
- 44. Hukkanen J. Induction of cytochrome P450 enzymes: a view on human in vivo findings. Expert Rev Clin Pharmacol. 2012;5(5):569–585. [DOI] [PubMed] [Google Scholar]
- 45. Pelkonen O, Turpeinen M, Hakkola J, et al. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 2008;82(10):667–715. [DOI] [PubMed] [Google Scholar]
- 46. Hakkola J, Raunio H, Purkunen R, et al. Detection of cytochrome P450 gene expression in human placenta in first trimester of pregnancy. Biochem Pharmacol. 1996;52(2):379–383. [DOI] [PubMed] [Google Scholar]
- 47. van Otterdijk SD, Binder AM, Michels KB. Locus-specific DNA methylation in the placenta is associated with levels of pro-inflammatory proteins in cord blood and they are both independently affected by maternal smoking during pregnancy. Epigenetics. 2017;12(10):875–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Janssen BG, Gyselaers W, Byun HM, et al. Placental mitochondrial DNA and CYP1A1 gene methylation as molecular signatures for tobacco smoke exposure in pregnant women and the relevance for birth weight. J Transl Med. 2017;15:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Suter M, Abramovici A, Showalter L, et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism. 2010;59(10):1481–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim K, Kim JM, Kim JS, et al. VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell. 2013;52(3):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakagawa T, Lv L, Nakagawa M, et al. CRL4 VprBP E3 ligase promotes monoubiquitylation and chromatin binding of TET dioxygenases. Mol Cell. 2015;57(2):247–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu C, Zhang YL, Pan WW, et al. CRL4 complex regulates mammalian oocyte survival and reprogramming by activation of TET proteins. Science. 2013;342(6165):1518–1521. [DOI] [PubMed] [Google Scholar]
- 53. Horikoshi M, Beaumont RN, Day FR, et al. Genome-wide associations for birth weight and correlations with adult disease. Nature. 2016;538(7624):248–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Valeri L, Reese SL, Zhao S, et al. Misclassified exposure in epigenetic mediation analyses. Does DNA methylation mediate effects of smoking on birthweight? Epigenomics. 2017;9(3):253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McDonald SD, Perkins SL, Walker MC. Correlation between self-reported smoking status and serum cotinine during pregnancy. Addict Behav. 2005;30(4):853–857. [DOI] [PubMed] [Google Scholar]
- 56. Kvalvik LG, Nilsen RM, Skjærven R, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. Pediatr Res. 2012;72(1):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Public Health Agency of Canada What Mothers Say: the Canadian Maternity Experiences Survey Ottawa, Canada: Maternal and Infant Health Section, Public Health Agency of Canada; 2009. (Public Health Agency of Canada publication no. 978-1-100-10828-5). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.