Abstract
Childhood blood pressure (BP) is a strong predictor of later risk of cardiovascular disease. However, few studies have assessed dynamic BP trajectories throughout the early-life period. We investigated the relationship between early-life factors and systolic BP (SBP) from infancy to adolescence using linear spline mixed-effects models among 1,370 children from Project Viva, a Boston, Massachusetts-area cohort recruited in 1999–2002. After adjusting for confounders and child height, we observed higher SBP in children exposed to gestational diabetes mellitus (vs. normoglycemia; age 3 years: β = 3.16 mm Hg (95% confidence interval (CI): 0.28, 6.04); age 6 years: β = 1.83 mm Hg (95% CI: 0.06, 3.60)), hypertensive disorders of pregnancy (vs. normal maternal BP; age 6 years: β = 1.39 mm Hg (95% CI: 0.10, 2.67); age 9 years: β = 1.84 mm Hg (95% CI: 0.34, 3.34); age 12 years: β = 1.70 mm Hg (95% CI: 0.48, 2.92)), higher neonatal SBP (per 10-mm Hg increase; age 3 years: β = 1.26 mm Hg (95% CI: 0.42, 2.09); age 6 years: β = 1.00 mm Hg (95% CI: 0.49, 1.51); age 9 years: β = 0.75 mm Hg (95% CI: 0.17, 1.33)), and formula milk in the first 6 months of life (vs. breast milk only; age 12 years: β = 2.10 mm Hg (95% CI: 0.46, 3.74); age 15 years: β = 3.52 mm Hg (95% CI: 1.40, 5.64); age 18 years: β = 4.94 mm Hg (95% CI: 1.88, 7.99)). Our findings provide evidence of programming of offspring SBP trajectories by gestational diabetes, hypertensive disorders of pregnancy, and formula milk intake and of neonatal BP being a potentially useful marker of childhood BP. These factors could be relevant in identifying children who are at risk of developing elevated BP.
Keywords: blood pressure, blood pressure trajectory, developmental programming, pregnancy, risk factors, systolic blood pressure
High blood pressure (BP) in adulthood is one of the leading risk factors for morbidity and mortality in the world (1) and has been shown to be predicted by childhood high BP (2). Between 1999 and 2012, the prevalence of high BP (defined as systolic or diastolic BP at the 95th percentile or higher) among US youths aged 8–17 years remained relatively unchanged (10.6% in 1999 and 11.0% in 2012) (3). To develop preventive strategies that mitigate adult hypertension as early as possible, a better physiological and epidemiologic understanding of the early determinants of BP is required.
Observational evidence, including our prior work, has shown that intrauterine exposure to adverse conditions, such as older maternal age (4), prepregnancy obesity (5), prenatal smoking (6), hypertensive disorders of pregnancy (HDP) (7), and gestational diabetes mellitus (GDM) (8), could program the development of elevated BP in infancy, childhood, or adolescence. The evidence surrounding associations of birth weight with later BP, however, still remains inconclusive (9). Postnatal experiences and growth, such as rapid infant weight gain, also are associated with BP later in childhood (10, 11). However, these studies did not examine patterns of BP change in relation to these developmental factors. Without sufficient information on dynamic BP patterns throughout the early-life period, the physiological insights into the temporal relationship between early-life risk factors and BP patterns are incomplete. Therefore, identifying developmental factors that are related to BP profiles throughout the early-life period could potentially inform strategies that aim to reduce the level of BP attained later in life.
In order to address these clinically unmet needs, we used data from a Boston, Massachusetts-area prebirth cohort to assess associations of prenatal, perinatal, and postnatal factors with BP trajectories from infancy to adolescence. We hypothesized that the aforementioned factors would be predictive of trajectories of BP from infancy to adolescence.
METHODS
Study population
Children were participants in Project Viva, an ongoing prospective cohort study of prenatal and perinatal influences on maternal, fetal, and child health (12). We recruited eligible pregnant women at clinical visits during the first trimester of pregnancy between April 1999 and November 2002 from 8 obstetrical offices of Atrius Harvard Vanguard Medical Associates, a multisite group medical practice in eastern Massachusetts. We considered participants eligible if pregnant mothers were fluent in English, with less than 22 weeks of gestation at study entry and a singleton pregnancy (12). Of 2,128 live singleton births, we included 1,572 children (73.9%) who had 1 or more systolic BP (SBP) measurements taken between infancy and adolescence. Mothers provided written informed consent at enrollment and each postnatal follow-up visit, and children provided assent at the midchildhood and adolescent visits. The Institutional Review Board of Harvard Pilgrim Health Care approved the project in line with ethical standards established by the Declaration of Helsinki.
Exposures: pre-, peri-, and postnatal factors
Prenatal factors
Mothers reported their age, prepregnancy weight, height, and smoking history via questionnaires and interviews at recruitment. We calculated prepregnancy body mass index (BMI) as self-reported prepregnancy weight (kg) divided by height squared (m2). We obtained results of a 2-stage clinical glycemic screening (a nonfasting 50-g oral glucose challenge test followed by a 100-g, 3-hour oral glucose tolerance test) to categorize women as having normoglycemia, isolated hyperglycemia, impaired glucose tolerance, or GDM, based on previously detailed criteria (13). We also extracted data on clinical blood pressure, urine protein results, and diagnoses from outpatient and hospital medical records, which we used to identify HDP (normal blood pressure, gestational hypertension, preeclampsia, or chronic hypertension) (7).
Perinatal factors
Mothers reported their child’s race/ethnicity, categorized as white, black, Hispanic, Asian, or other. We extracted data on infant sex and birth weight from hospital medical records and calculated length of gestation from a prenatal ultrasonogram or by subtracting the date of the last menstrual period from the date of delivery (14). We calculated birth weight–for–gestational age (BW-for-GA) z scores using national reference data (15). Within 3 days after delivery, we measured neonatal SBP up to 5 times at 1-minute intervals and averaged all measurements for each newborn (4).
Postnatal factors
At the 6-month visit, we grouped infants into 4 categories on the basis of their extent of breastfeeding or formula feeding in the first 6 months of life (i.e., formula only, mixed feeding, weaned (defined as having initiated breastfeeding but having discontinued it completely before 6 months of age), or breast milk only) (16). We used measures of the child’s weight and length at birth and in infancy to calculate BMI and determined age- and sex-specific BMI z scores (zBMIs) using the World Health Organization growth reference chart (17). We used the change in zBMI from birth to infancy as an indicator of infant weight gain.
Outcome: child SBP
At in-person research visits carried out during infancy (median age, 6.3 months (interquartile range, 5.3–9.0)), early childhood (median age, 3.2 years (interquartile range, 2.9–5.9)), midchildhood (median age, 7.7 years (interquartile range, 6.6–10.6)), and early adolescence (median age, 12.9 years (interquartile range, 12.0–16.4)), trained research assistants recorded SBP on the child’s upper arm up to 5 times at 1-minute intervals using biannually calibrated oscillometric automated monitors (in infancy: Dinamap 8100, Pro 100, or Pro 200 (Dinamap, Tampa, Florida); in childhood: Dinamap Pro-100 (Dinamap); in adolescence: Omron HEM-907XL (Omron, Bannockburn, Illinois)) following standardized procedures (11, 18–20). We averaged all 5 SBP measurements at each research visit, since quantifying between-person differences rather than absolute levels improves precision (21). The proportion of missingness for SBP at each research visit ranged from 22.6% to 35.5%; the structure of missingness, however, was completely random (see Web Table 1, available at https://academic.oup.com/aje). In a subset of 378 children, we also obtained additional SBP data ranging from childhood to adolescence (range, 4.2–18.4 years) from medical records, where pediatricians recorded SBP at routine clinic visits (i.e., well-child visits at which health-care providers would administer scheduled immunizations, monitor growth and development, and make recommendations to parents for optimal child health (22)). We used SBP rather than diastolic BP because it is measured more accurately with our automated instrument (6, 8, 10) and because SBP is more predictive of later cardiovascular disease risk than diastolic BP (23). To exclude implausible measurements, we excluded SBP measurements that were more than 5 standard deviations or less than −5 standard deviations from the mean.
Other covariates
Mothers reported their highest level of education and the fathers’ highest level of education via questionnaires and interviews at recruitment, which we categorized as university-educated or not university-educated. We extracted data on parity from hospital medical records. We used the child’s weight and length/height obtained at research visits and from medical records (14, 18) (which were matched in time with BP recorded at routine clinic visits) to calculate BMI and determined age- and sex-specific height z scores and zBMIs using the World Health Organization growth standards (17).
Statistical analysis
We used linear spline mixed-effects models to examine associations of the aforementioned pre-, peri-, and postnatal factors with SBP from infancy to adolescence. We found that 2 knot points in the early childhood (age 3 years) and midchildhood (age 9 years) periods sufficiently described the trajectory of SBP change, resulting in 3 linear slopes representing the “infancy to early childhood,” “early to midchildhood,” and “midchildhood to adolescence” periods, respectively (see Web Appendix 1, Web Table 2, and Web Figures 1 and 2 for details). We used actual values instead of z scores because there are currently no age-, sex-, and height-specific national reference values available for children below 2 years of age.
We included each pre-, peri-, and postnatal factor as a fixed effect and as an interaction with each of the 3 linear slopes in the linear spline models. We also included a random intercept and random slopes to account for repeated SBP observations in each child and to reflect the heterogeneity in the data. Because child length/height is a major determinant of SBP, we included length/height z score in all models. In sensitivity analyses, we further adjusted for child zBMI. We additionally adjusted for maternal and paternal educational level, maternal height, parity, and type of study visit (research or clinic visit) in all models. Details on the construction of these models are provided in Web Appendix 2.
For each pre-, peri-, and postnatal factor, we used the linear spline model to predict SBP from age 6 months to age 18 years and plot its corresponding trajectory, while holding other covariates constant at their mean values. We estimated adjusted differences (and 95% confidence intervals) in predicted SBP between categories of pre-, peri-, and postnatal factors at 3-year intervals using the margins command in Stata (StataCorp LLC, College Station, Texas). We also analyzed terms for the interaction of each factor with each of the 3 linear SBP slopes (in mm Hg/year); these estimates describe whether the association of each pre-, peri-, or postnatal factor with SBP becomes stronger/weaker with increasing age. We restricted all analyses to children without missing data on pre- (n = 1,370), peri- (n = 825), and postnatal factors (n = 662). In additional sensitivity analyses, we further restricted our analyses to 1) children with SBP measured at research visits only and 2) children with complete data for all pre-, peri-, and postnatal factors (n = 662). We performed all analyses using Stata 15.1.
RESULTS
Cohort description
Table 1 shows the characteristics of children in the study. Compared with those excluded, children included in the study were more likely to be white (68% vs. 54%) and exclusively fed breast milk in the first 6 months of life (28% vs. 18%), and they had mothers who were older (32.6 years vs. 30.4 years), had lower prepregnancy BMI (24.6 vs. 25.4), were more likely to be university-educated (71% vs. 52%), and were less likely to have smoked during pregnancy (10% vs. 18%) (Web Table 3). At successive research visits, the mean SBP was 90.2 (standard deviation (SD), 13.4) mm Hg in infancy, 92.3 (SD, 10.9) mm Hg in early childhood, 94.4 (SD, 8.5) mm Hg in midchildhood, and 107.2 (SD, 9.2) mm Hg in early adolescence (Web Table 1). No differences in SBP were observed between research and clinic visits across age categories, except at ages 7–8 years and 8–9 years, where SBP was higher at clinic visits than at research visits (Web Figures 3 and 4). The predicted mean linear SBP velocities were 0.4 (SD, 3.4) mm Hg/year for the infancy–early childhood period, 1.1 (SD, 0.5) mm Hg/year for early childhood–midchildhood, and 2.1 (SD, 0.4) mm Hg/year for midchildhood–adolescence.
Table 1.
Characteristics of Study Participants Recruited for Project Viva in 1999–2002, Boston, Massachusetts
| Maternal or Child Factor | No. of Subjects | Mean (SD) | % |
|---|---|---|---|
| Maternal prenatal factors | |||
| University educational level | 979 | 71 | |
| Nulliparous | 651 | 48 | |
| Age, years | 1,370 | 32.6 (4.7) | |
| Height, m | 1,370 | 1.7 (0.1) | |
| Prepregnancy BMIa | 1,370 | 24.6 (5.2) | |
| Smoking history | |||
| Never smoked | 949 | 69 | |
| Smoked before pregnancy | 286 | 21 | |
| Smoked during pregnancy | 135 | 10 | |
| Gestational glucose tolerance status | |||
| Normoglycemia | 1,138 | 83 | |
| Isolated hyperglycemia | 114 | 8 | |
| Impaired glucose tolerance | 48 | 4 | |
| Gestational diabetes | 70 | 5 | |
| Hypertensive disorders of pregnancy | |||
| Normal blood pressure | 1,212 | 89 | |
| Gestational hypertension or preeclampsia | 138 | 10 | |
| Chronic hypertension | 20 | 1 | |
| Father university-educated | 911 | 66 | |
| Child factors | |||
| Sex | |||
| Female | 667 | 49 | |
| Male | 703 | 51 | |
| Race/ethnicity | |||
| White | 943 | 68 | |
| Black | 178 | 13 | |
| Hispanic | 60 | 4 | |
| Asian | 53 | 4 | |
| Other | 136 | 10 | |
| Infant perinatal factors | |||
| Birth weight-for-gestational age z score (in SD units) | 825 | 0.2 (0.9) | |
| Neonatal systolic blood pressure | 825 | 72.4 (8.8) | |
| Postnatal factors | |||
| Infant feeding type in first 6 months of life | |||
| Breast milk only | 186 | 28 | |
| Mixed feeding | 176 | 27 | |
| Weaned | 225 | 34 | |
| Formula only | 75 | 11 | |
| Infancy zBMI gain (in SD units) | 662 | 0.03 (1.29) |
Abbreviations: BMI, body mass index; SD, standard deviation; zBMI, age- and sex-specific BMI z score.
a Weight (kg)/height (m)2.
Associations of prenatal factors with child SBP trajectories
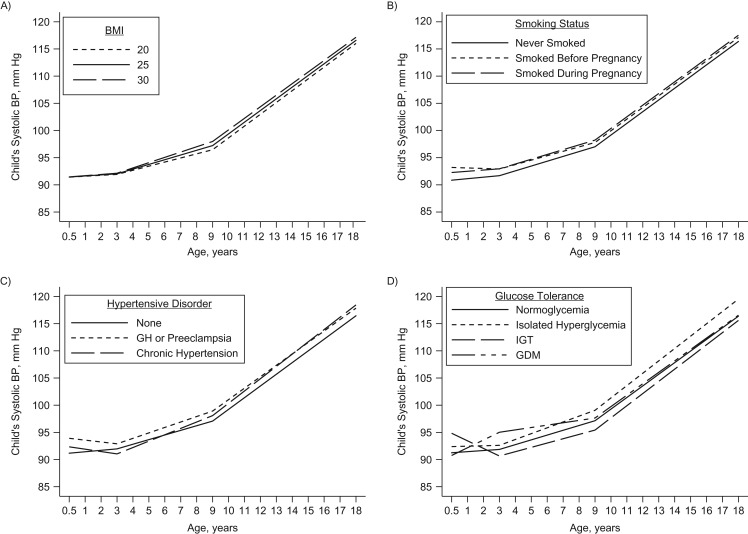
Exposure to higher prepregnancy BMI was associated with higher SBP trajectories that were significant at ages 6, 9, 12, and 15 years (Figure 1A and Table 2). Maternal age and smoking history were not associated with child SBP from infancy to adolescence, although children exposed to intrauterine smoking before or during pregnancy appeared to have higher SBP trajectories (Figure 1B). Exposure to gestational hypertension or preeclampsia in utero (vs. normal blood pressure) (Figure 1C) was similarly associated with higher SBP trajectories that were significant at ages 6 years (β = 1.39 mm Hg, 95% confidence interval (CI): 0.10, 2.67), 9 years (β = 1.84 mm Hg, 95% CI: 0.34, 3.34), and 12 years (β = 1.70 mm Hg, 95% CI: 0.48, 2.92). Maternal glucose intolerance (vs. normoglycemia) was associated with higher SBP trajectories (Figure 1D), which were significant at ages 3–6 years for exposure to GDM and at ages 6–18 years for exposure to isolated hyperglycemia (Table 2). No prenatal factors were associated with rate of SBP change across the different periods (Table 3).
Figure 1.
Predicted trajectories of child systolic blood pressure (BP) from ages 6 months to 18 years according to maternal prepregnancy body mass index (BMI; weight (kg)/height (m)2) (A), smoking history (B), hypertensive disorders of pregnancy (C), and glucose tolerance status (D) among participants recruited into Project Viva in 1999–2002, Boston, Massachusetts. The models adjusted for type of study visit, all prenatal factors, and child height z score. GDM, gestational diabetes mellitus; GH, gestational hypertension; IGT, impaired glucose tolerance.
Table 2.
Associations of Prenatal, Perinatal, and Postnatal Factors With Predicted Systolic Blood Pressure in Infancy, Childhood, and Adolescence Among Participants Recruited Into Project Viva in 1999–2002, Boston, Massachusetts
| Maternal or Child Factor | Child’s Age and Mean Difference in Predicted Systolic BP, mm Hg | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Subjects | 6 Months | 3 Years | 6 Years | 9 Years | 12 Years | 15 Years | 18 Years | ||||||||
| β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | β | 95% CI | ||
| Maternal prenatal factorsa | |||||||||||||||
| Age, years (per 5-year increase) | 1,370 | 0.10 | −1.13, 1.33 | 0.54 | −0.17, 1.24 | 0.19 | −0.24, 0.62 | −0.16 | −0.64, 0.32 | −0.05 | −0.44, 0.35 | 0.07 | −0.47, 0.61 | 0.18 | −0.62, 0.99 |
| Prepregnancy BMIb (per 5-unit increase) | 1,370 | 0.02 | −0.99, 1.03 | 0.11 | −0.52, 0.74 | 0.43 | 0.05, 0.82 | 0.76 | 0.32, 1.19 | 0.69 | 0.34, 1.05 | 0.63 | 0.14, 1.11 | 0.56 | −0.15, 1.28 |
| Smoking history (vs. having never smoked) | 1,370 | ||||||||||||||
| Smoked before pregnancy | 2.35 | −0.16, 4.86 | 1.23 | −0.25, 2.70 | 1.01 | 0.10, 1.92 | 0.79 | −0.25, 1.83 | 0.77 | −0.08, 1.63 | 0.76 | −0.41, 1.92 | 0.74 | −0.99, 2.47 | |
| Smoked during pregnancy | 1.41 | −2.26, 5.08 | 1.26 | −0.89, 3.42 | 1.24 | −0.10, 2.59 | 1.22 | −0.40, 2.85 | 1.20 | −0.12, 2.51 | 1.17 | −0.68, 3.03 | 1.15 | −1.65, 3.94 | |
| Hypertensive disorders of pregnancy (vs. normal blood pressure) | 1,370 | ||||||||||||||
| Gestational hypertension or preeclampsia | 2.74 | −0.53, 6.01 | 0.93 | −1.14, 3.00 | 1.39 | 0.10, 2.67 | 1.84 | 0.34, 3.34 | 1.70 | 0.48, 2.92 | 1.55 | −0.12, 3.23 | 1.41 | −1.09, 3.90 | |
| Chronic hypertension | 1.17 | −6.71, 9.04 | −0.92 | −5.78, 3.94 | 0.04 | −2.90, 2.98 | 1.01 | −2.22, 4.23 | 1.34 | −1.42, 4.09 | 1.67 | −2.11, 5.45 | 2.00 | −3.53, 7.53 | |
| Glucose tolerance status (vs. normoglycemia) | 1,370 | ||||||||||||||
| Isolated hyperglycemia | 1.13 | −2.58, 4.84 | 0.75 | −1.34, 2.85 | 1.34 | 0.04, 2.63 | 1.92 | 0.40, 3.44 | 2.33 | 1.08, 3.58 | 2.74 | 1.01, 4.46 | 3.15 | 0.59, 5.71 | |
| Impaired glucose tolerance | 3.59 | −1.95, 9.12 | −1.21 | −4.49, 2.07 | −1.47 | −3.48, 0.54 | −1.72 | −3.98, 0.54 | −1.41 | −3.27, 0.45 | −1.09 | −3.64, 1.45 | −0.78 | −4.54, 2.98 | |
| Gestational diabetes | −0.50 | −5.22, 4.22 | 3.16 | 0.28, 6.04 | 1.83 | 0.06, 3.60 | 0.50 | −1.50, 2.50 | 0.39 | −1.24, 2.02 | 0.28 | −1.91, 2.48 | 0.18 | −3.07, 3.42 | |
| Child factorsa | |||||||||||||||
| Female sex (vs. male) | 1,370 | −2.66 | −4.67, −0.66 | −0.85 | −2.02, 0.32 | −0.29 | −1.01, 0.43 | 0.27 | −0.56, 1.10 | −1.72 | −2.40, −1.04 | −3.71 | −4.65, −2.78 | −5.70 | −7.09, −4.32 |
| Race/ethnicity (vs. white) | 1,370 | ||||||||||||||
| Black | 0.71 | −2.68, 4.11 | 0.61 | −1.44, 2.66 | 0.54 | −0.68, 1.77 | 0.47 | −0.83, 1.78 | −0.42 | −1.50, 0.66 | −1.32 | −2.78, 0.14 | −2.22 | −4.36, −0.07 | |
| Hispanic | −0.17 | −5.61, 5.27 | −0.51 | −3.73, 2.71 | −0.37 | −2.29, 1.55 | −0.23 | −2.35, 1.90 | −0.81 | −2.57, 0.95 | −1.39 | −3.77, 0.99 | −1.97 | −5.47, 1.53 | |
| Asian | −3.79 | −8.96, 1.37 | 0.40 | −2.91, 3.71 | −0.49 | −2.52, 1.55 | −1.38 | −3.70, 0.95 | 0.76 | −1.17, 2.69 | 2.90 | 0.15, 5.64 | 5.03 | 0.93, 9.14 | |
| Other | 1.14 | −2.19, 4.47 | −0.51 | −2.47, 1.44 | 0.35 | −0.85, 1.55 | 1.21 | −0.15, 2.57 | 0.77 | −0.34, 1.88 | 0.33 | −1.17, 1.83 | −0.11 | −2.33, 2.10 | |
| Infant perinatal factorsc | |||||||||||||||
| BW-for-GA (per 1-SD increase) | 825 | 0.34 | −1.19, 1.88 | −0.45 | −1.30, 0.41 | −0.65 | −1.16, −0.13 | −0.85 | −1.45, −0.25 | −0.51 | −1.00, −0.02 | −0.17 | −0.82, 0.49 | 0.17 | −0.79, 1.13 |
| Neonatal systolic BP (per 10-mm Hg increase) | 825 | 0.71 | −0.71, 2.13 | 1.26 | 0.42, 2.09 | 1.00 | 0.49, 1.51 | 0.75 | 0.17, 1.33 | 0.36 | −0.12, 0.85 | −0.02 | −0.67, 0.63 | −0.40 | −1.35, 0.55 |
| Postnatal factorsd | |||||||||||||||
| Infant feeding type in first 6 months of life (vs. breast milk only) | 662 | ||||||||||||||
| Mixed feeding | −2.18 | −5.68, 1.33 | 1.32 | −0.73, 3.37 | 0.84 | −0.44, 2.11 | 0.35 | −1.21, 1.91 | 0.51 | −0.77, 1.80 | 0.67 | −0.99, 2.34 | 0.83 | −1.58, 3.25 | |
| Weaned | −1.96 | −5.47, 1.55 | −0.63 | −2.67, 1.41 | −0.31 | −1.57, 0.94 | 0.01 | −1.48, 1.49 | 0.39 | −0.84, 1.62 | 0.78 | −0.80, 2.37 | 1.17 | −1.11, 3.45 | |
| Formula only | −2.51 | −7.42, 2.41 | 1.66 | −1.12, 4.43 | 1.17 | −0.52, 2.85 | 0.68 | −1.30, 2.65 | 2.10 | 0.46, 3.74 | 3.52 | 1.40, 5.64 | 4.94 | 1.88, 7.99 | |
| Infancy zBMI gain (per 1-SD increase) | 662 | 1.33 | 0.12, 2.54 | 0.95 | 0.24, 1.67 | 0.67 | 0.23, 1.11 | 0.39 | −0.16, 0.95 | 0.21 | −0.24, 0.66 | 0.03 | −0.57, 0.63 | −0.15 | −1.03, 0.73 |
Abbreviations: BMI, body mass index; BP, blood pressure; BW-for-GA, birth weight for gestational age; CI, confidence interval; SD, standard deviation; zBMI, age- and sex-specific BMI z score.
a Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, and child height z score.
b Weight (kg)/height (m)2.
c Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, all prenatal factors, child factors, and height z score.
d Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, all prenatal and perinatal factors, child factors, and height z score.
Table 3.
Associations of Prenatal, Perinatal, and Postnatal Factors With Rate of Systolic Blood Pressure Change From Infancy to Early Childhood, Early Childhood to Midchildhood, and Midchildhood to Adolescence Among Participants Recruited Into Project Viva in 1999–2002, Boston, Massachusetts
| Maternal or Child Factor | Average Rate of Systolic BP Change, mm Hg/year | ||||||
|---|---|---|---|---|---|---|---|
| No. of Subjects | From Infancy to Early Childhood | From Early Childhood to Midchildhood | From Midchildhood to Adolescence | ||||
| β | 95% CI | β | 95% CI | β | 95% CI | ||
| Maternal prenatal factorsa | |||||||
| Age, years (per 5-year increase) | 1,370 | 0.15 | −0.34, 0.64 | −0.12 | −0.26, 0.02 | 0.04 | −0.07, 0.15 |
| Prepregnancy BMIb (per 5-unit increase) | 1,370 | 0.03 | −0.38, 0.44 | 0.11 | −0.02, 0.23 | −0.02 | −0.12, 0.08 |
| Smoking history (vs. having never smoked) | 1,370 | ||||||
| Smoked before pregnancy | −0.37 | −1.38, 0.63 | −0.07 | −0.37, 0.23 | −0.01 | −0.24, 0.23 | |
| Smoked during pregnancy | −0.05 | −1.52, 1.42 | −0.01 | −0.46, 0.44 | −0.01 | −0.39, 0.37 | |
| Hypertensive disorders of pregnancy (vs. normal blood pressure) | 1,370 | ||||||
| Gestational hypertension or preeclampsia | −0.60 | −1.95, 0.74 | 0.15 | −0.27, 0.57 | −0.05 | −0.39, 0.29 | |
| Chronic hypertension | −0.70 | −3.90, 2.51 | 0.32 | −0.64, 1.29 | 0.11 | −0.62, 0.84 | |
| Glucose tolerance status (vs. normoglycemia) | 1,370 | ||||||
| Isolated hyperglycemia | −0.13 | −1.59, 1.34 | 0.19 | −0.23, 0.62 | 0.14 | −0.21, 0.48 | |
| Impaired glucose tolerance | −1.60 | −3.83, 0.63 | −0.09 | −0.74, 0.57 | 0.10 | −0.40, 0.61 | |
| Gestational diabetes | 1.22 | −0.70, 3.13 | −0.44 | −1.02, 0.14 | −0.04 | −0.48, 0.41 | |
| Child factorsa | |||||||
| Female sex (vs. male) | 1,370 | 0.61 | −0.19, 1.41 | 0.19 | −0.05, 0.42 | −0.66 | −0.85, −0.48 |
| Race/ethnicity (vs. white) | 1,370 | ||||||
| Black | −0.03 | −1.41, 1.34 | −0.02 | −0.43, 0.38 | −0.30 | −0.59, −0.01 | |
| Hispanic | −0.11 | −2.30, 2.08 | 0.05 | −0.60, 0.69 | −0.19 | −0.67, 0.28 | |
| Asian | 1.40 | −0.73, 3.53 | −0.30 | −0.97, 0.37 | 0.71 | 0.16, 1.27 | |
| Other | −0.55 | −1.88, 0.78 | 0.29 | −0.11, 0.68 | −0.15 | −0.45, 0.15 | |
| Infant perinatal factorsc | |||||||
| BW-for-GA (per 1-SD increase) | 825 | −0.26 | −0.87, 0.35 | −0.07 | −0.24, 0.11 | 0.11 | −0.02, 0.24 |
| Neonatal systolic BP (per 10-mm Hg increase) | 825 | 0.18 | −0.39, 0.76 | −0.09 | −0.26, 0.08 | −0.13 | −0.25, 0.00 |
| Postnatal factorsd | |||||||
| Infant feeding type in first 6 months of life (vs. breast milk only) | 662 | ||||||
| Mixed feeding | 1.17 | −0.24, 2.57 | −0.16 | −0.60, 0.27 | 0.05 | −0.27, 0.38 | |
| Weaned | 0.44 | −0.96, 1.84 | 0.11 | −0.32, 0.53 | 0.13 | −0.18, 0.44 | |
| Formula only | 1.39 | −0.56, 3.33 | −0.16 | −0.74, 0.41 | 0.47 | 0.06, 0.88 | |
| Infancy zBMI gain (per 1-SD increase) | 662 | −0.13 | −0.61, 0.36 | −0.09 | −0.25, 0.06 | −0.06 | −0.18, 0.06 |
Abbreviations: BMI, body mass index; BP, blood pressure; BW-for-GA, birth weight for gestational age; CI, confidence interval; SD, standard deviation; zBMI, age- and sex-specific BMI z score.
a Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, and child height z score.
b Weight (kg)/height (m)2.
c Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, all prenatal factors, child factors, and height z score.
d Adjusted for type of study visit, maternal and paternal educational level, maternal height, parity, all prenatal and perinatal factors, child factors, and height z score.
Associations of perinatal factors with child SBP trajectories
Compared with males, females had lower SBP trajectories which were significant in infancy (β = −2.66 mm Hg, 95% CI: −4.67, −0.66) and adolescence (age 12 years: β = −1.72 mm Hg (95% CI: −2.40, −1.04); age 15 years: β = −3.71 mm Hg (95% CI: −4.65, −2.78); age 18 years: β = −5.70 mm Hg (95% CI: −7.09, −4.32)) (Table 2) and had a slower rate of SBP change from midchildhood to adolescence (β = −0.66 mm Hg/year, 95% CI: −0.85, −0.48) (Table 3). We observed no differences in SBP trajectories by child’s race/ethnicity, except in adolescence, where Asian children (vs. white children) had higher adolescent SBP and a faster rate of SBP change from midchildhood to adolescence (Tables 2 and 3).
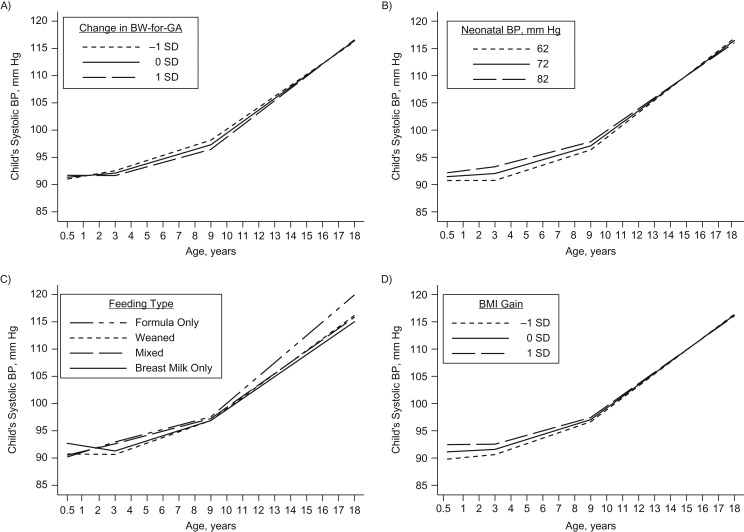
Children with higher BW-for-GA had lower SBP trajectories during childhood (Figure 2A), significant at ages 6 years (β = −0.65 mm Hg, 95% CI: −1.16, −0.13), 9 years (β = −0.85 mm Hg, 95% CI: −1.45, −0.25), and 12 years (β = −0.51 mm Hg, 95% CI: −1.00, −0.02) (Table 2). Children with higher neonatal SBP had higher SBP trajectories throughout childhood (Figure 2B) (age 3 years: β = 1.26 mm Hg (95% CI: 0.42, 2.09); age 6 years: β = 1.00 mm Hg (95% CI: 0.49, 1.51); age 9 years: β = 0.75 mm Hg (95% CI: 0.17, 1.33)) for every 10-mm Hg increase in neonatal SBP (Table 2), which gradually weakened in adolescence.
Figure 2.
Predicted trajectories of child systolic blood pressure (BP) from ages 6 months to 18 years according to child birth weight for gestational age (BW-for-GA) (A), neonatal BP (B), infant feeding type in the first 6 months of life (C), and infancy gain in age- and sex-specific body mass index (BMI; weight (kg)/height (m)2) z score (zBMI) (D) among participants recruited into Project Viva in 1999–2002, Boston, Massachusetts. Models for BW-for-GA and neonatal BP adjusted for prenatal factors; models for infant feeding type and infancy zBMI gain adjusted for prenatal and perinatal factors. All models additionally adjusted for type of study visit and child height z score. SD, standard deviation.
Associations of early postnatal factors with child SBP trajectories
Children who were fed formula milk only (vs. breast milk only) in the first 6 months of life had higher SBP trajectories in adolescence (Figure 2C) (age 12 years: β = 2.10 mm Hg (95% CI: 0.46, 3.74); age 15 years: β = 3.52 mm Hg (95% CI: 1.40, 5.64); age 18 years: β = 4.94 mm Hg (95% CI: 1.88, 7.99)) (Table 2) and a faster rate of SBP change from midchildhood to adolescence (β = 0.47 mm Hg/year, 95% CI: 0.06, 0.88) (Table 3). Children who were weaned or were on mixed feeding in the first 6 months of life showed no associations with SBP trajectories. Children with greater zBMI gain during infancy had higher SBP trajectories in early childhood that were significant between 6 months and 6 years of age (Table 2) but gradually weakened into adolescence (Figure 2D).
Sensitivity analyses
Additional adjustment for child zBMI did not appreciably change the associations of isolated hyperglycemia/GDM, HDP, BW-for-GA, neonatal SBP, or infant feeding type with child SBP trajectories (Web Figures 5 and 6). The observed associations for prepregnancy BMI and infancy zBMI change with child SBP, however, were attenuated to the null (Web Tables 4 and 5). Furthermore, similar results were observed when the analyses were restricted to children with SBP measured at research visits only (Web Tables 6 and 7) or to those with complete data for all pre-, peri-, and postnatal factors (Web Tables 8 and 9)—albeit with wider 95% confidence intervals due to the smaller number of SBP measurements.
DISCUSSION
In this study, we characterized SBP trajectories in 3 distinct periods, namely infancy to early childhood, early childhood to midchildhood, and midchildhood to adolescence, and demonstrated its associations with pre-, peri-, and postnatal factors. In general, children with intrauterine exposure to isolated hyperglycemia/GDM or HDP had higher SBP trajectories than unexposed children. Children with higher neonatal SBP exhibited higher SBP trajectories in childhood, while those with higher BW-for-GA exhibited lower SBP trajectories in childhood. Additionally, children who were fed formula milk only in the first 6 months of life exhibited higher SBP trajectories in adolescence.
Few longitudinal studies have assessed follow-up measures of BP from infancy to later childhood or adolescence. We assessed BP trajectories from infancy onward rather than from birth onward, since other investigators had reported poor tracking of BP from birth to infancy (24). Zinner et al. (25) previously reported that BP measured in the first days of life was weakly correlated with BP at 6–24 months. Levine et al. (26) also noted that BP measured 2 days after birth was not significantly correlated with BP at 1, 3, 6, or 12 months, which is consistent with our findings of a null association between SBP at birth and in infancy. However, we found that higher neonatal SBP was persistently associated with higher SBP during childhood; thus, neonatal BP might be a potentially useful marker for identification of children who are likely to experience elevated BP trajectories throughout childhood.
Consistent with previous findings, we found that maternal prepregnancy BMI (27) and infant zBMI gain (10, 11, 28) were significantly associated with higher SBP during childhood and adolescence. These associations diminished to the null, however, after adjustment for child zBMI, which could reflect that children of mothers with high prepregnancy BMI or children who experienced rapid infant growth were likely to have high BMI themselves, and thus have higher BP. The observed sex differences in BP are consistent with previous studies and are probably explained by sex differences in body composition and the relationships of individual body compartments to BP (29). The observed ethnic differences in BP are also consistent with a recent study showing Asians to have a higher prevalence of hypertension than whites in similar weight and socioeconomic status categories (30), suggesting that there may be other environmental or genetic factors driving the racial/ethnic disparities in BP.
Our results corroborate previous findings that have shown associations of intrauterine exposure to GDM or HDP with childhood BP (31–34), although BP was measured only on a single occasion in those prior studies. Our study extends those findings by showing that the associations may persist as the child ages. These associations could be driven by shared genetic factors between the mother and child (35). For example, Howe et al. (36) reported that genetic variants of adult BP were associated with child BP at age 6 years, with the genetic effect on BP remaining similar from age 6 years to age 17 years. We accounted for potential shared environmental factors such as parental socioeconomic status (37) and other drivers of child BP (i.e., height and BMI), but it is possible that unmeasured factors could have contributed to the findings. The physiological mechanisms by which these prenatal factors might influence child BP are still unclear. We speculate that exposure to HDP or GDM may have an indirect effect through changes in maternal vasculature, thereby affecting placenta formation and blood flow (38–40). Animal and human studies also suggest a direct influence of exposure to hyperglycemia in utero on the kidneys of offspring, leading to altered BP regulation (41, 42).
Our observations of infant feeding type in the first 6 months of life also corroborate previous findings, where infants who were never breastfed were shown to have higher SBP in childhood and adolescence than those who were exclusively breastfed (43, 44). It is possible that other factors, such as being born small for gestational age or having accelerated infant growth, could explain these findings; that is, infants who are growing rapidly due to a period of intrauterine growth restraint may be formula-fed because of a higher demand for milk (45). However, the association between infant feeding type and SBP was not altered in our study after we accounted for birth weight and infant weight gain. We speculate that these findings could be explained by the high protein content in formula milk, which might further stimulate insulin-like growth factor 1 secretion and, in turn, raise BP through a posterior adrenergic response in the sympathetic nervous system (46).
Taken together, our findings suggest evidence of programming of offspring SBP trajectories by isolated hyperglycemia/GDM, HDP, and exclusive formula feeding. These factors could be informative for identification of children who are at risk of developing high BP between infancy and adolescence. Additionally, the higher SBP conferred through exposure to these adverse conditions may have cardiovascular implications in adulthood, as evident in recent studies that showed associations between higher childhood SBP and markers of adult preclinical cardiovascular disease (47, 48). Ongoing efforts to tailor effective interventions in changing dynamic BP patterns among at-risk children are warranted to reduce future cardiovascular disease risk.
Strengths of our study include its relatively large sample size, its prospective design, multiple measures of child BP, and the availability of data on a wide range of maternal and child factors obtained by highly trained staff using standardized protocols. One limitation of the study is that we estimated SBP trajectories using linear-spline models, which assumes a piecewise linear relationship between BP and age. This assumption may be biologically implausible, but results derived under it are often more interpretable than those from other types of models such as fractional polynomials (49). Despite the implausible linearity assumption, our 2-knot linear spline model appeared to fit the BP data equally well to a 2° fractional polynomial model, as evidenced through the residual plots (see Web Figure 2). Another limitation was the different methods of BP measurement employed during research and clinic visits; we used automated oscillometric devices to measure child BP at research visits, while pediatricians were likely to have used manual auscultative methods to measure BP at clinic visits. However, our analytical results were additionally adjusted for type of study visit—a potential proxy for method of BP measurement—which probably would have accounted for any variability that may have arisen from the use of different measurement methods at research and clinic visits. Finally, differences between children with and without follow-up measures of SBP might conceivably have led to selection bias. In addition, our study findings may not be generalizable to other populations from different settings, since a majority of our participants were white and university-educated.
In summary, this study characterized the trajectories of systolic BP from infancy to adolescence. Our findings provide evidence of programming of offspring SBP trajectories by important pregnancy complications (isolated hyperglycemia, GDM, HDP) and early-life nutrition (exclusive formula feeding) and of neonatal BP’s being a potentially useful marker of childhood BP. These factors could be relevant for identifying children who are at risk of high BP. Further studies are warranted to identify interventions that are effective in changing BP in these children and, if so, to determine whether such interventions can reduce the risk of future cardiovascular disease.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Division of Chronic Disease Research Across the Lifecourse, Department of Population Medicine, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts (Izzuddin M. Aris, Sheryl L. Rifas-Shiman, Ling-Jun Li, Marie-France Hivert, Emily Oken); Department of Obstetrics and Gynecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore (Izzuddin M. Aris); Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research, Singapore (Izzuddin M. Aris); Division of Obstetrics and Gynecology, KK Women’s and Children’s Hospital, Singapore (Ling-Jun Li); Obstetrics and Gynecology Academic Clinical Program, Duke-National University of Singapore Graduate Medical School, Singapore (Ling-Jun Li); Department of Pediatric Newborn Medicine, Brigham and Women’s Hospital, Boston, Massachusetts (Mandy B. Belfort); Diabetes Unit, Massachusetts General Hospital, Boston, Massachusetts (Marie-France Hivert); and Department of Nutrition, T.H. Chan School of Public Health, Harvard University, Boston, Massachusetts (Emily Oken).
All authors contributed equally to this work.
This work was supported by the National Institutes of Health (grants R01 HD034568 and UH3 OD023286). I.M.A. was additionally supported by a National University of Singapore Overseas Postdoctoral Fellowship (grant NUS OPF/2017). L.-J.L. was additionally supported by a Singapore National Medical Council Transition Award (NMRC TA/0027/2014).
We are very grateful to the participants and staff of Project Viva.
Conflict of interest: none declared.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- BW-for-GA
birth weight for gestational age
- CI
confidence interval
- GDM
gestational diabetes mellitus
- HDP
hypertensive disorders of pregnancy
- SBP
systolic blood pressure
- SD
standard deviation
- zBMI
age- and sex-specific BMI z score
REFERENCES
- 1. NCD Risk Factor Collaboration Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389(10064):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Theodore RF, Broadbent J, Nagin D, et al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66(6):1108–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kit BK, Kuklina E, Carroll MD, et al. Prevalence of and trends in dyslipidemia and blood pressure among US children and adolescents, 1999–2012. JAMA Pediatr. 2015;169(3):272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, et al. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144(2):240–245. [DOI] [PubMed] [Google Scholar]
- 5. Gademan MG, van Eijsden M, Roseboom TJ, et al. Maternal prepregnancy body mass index and their children’s blood pressure and resting cardiac autonomic balance at age 5–6 years. Hypertension. 2013;62(3):641–647. [DOI] [PubMed] [Google Scholar]
- 6. Oken E, Huh SY, Taveras EM, et al. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tripathi RR, Rifas-Shiman SL, Hawley N, et al. Hypertensive disorders of pregnancy and offspring cardiometabolic health at midchildhood: Project Viva findings. J Am Heart Assoc. 2018;7(3):pii:e007426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright CS, Rifas-Shiman SL, Rich-Edwards JW, et al. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens. 2009;22(2):215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edvardsson VO, Steinthorsdottir SD, Eliasdottir SB, et al. Birth weight and childhood blood pressure. Curr Hypertens Rep. 2012;14(6):596–602. [DOI] [PubMed] [Google Scholar]
- 10. Belfort MB, Rifas-Shiman SL, Rich-Edwards J, et al. Size at birth, infant growth, and blood pressure at three years of age. J Pediatr. 2007;151(6):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Perng W, Rifas-Shiman SL, Kramer MS, et al. Early weight gain, linear growth, and mid-childhood blood pressure: a prospective study in Project Viva. Hypertension. 2016;67(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. Int J Epidemiol. 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regnault N, Gillman MW, Rifas-Shiman SL, et al. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013;36(10):3045–3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aris IM, Rifas-Shiman SL, Li LJ, et al. Pre-, perinatal, and parental predictors of body mass index trajectory milestones. J Pediatr. 2018;201:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oken E, Kleinman KP, Rich-Edwards J, et al. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:Article 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taveras EM, Scanlon KS, Birch L, et al. Association of breastfeeding with maternal control of infant feeding at age 1 year. Pediatrics. 2004;114(5):e577–e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization WHO Child Growth Standards: Methods and Development. Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 18. Aris IM, Rifas-Shiman SL, Li LJ, et al. Patterns of body mass index milestones in early life and cardiometabolic risk in early adolescence. Int J Epidemiol. 2019;48(1):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huh SY, Rifas-Shiman SL, Kleinman KP, et al. Maternal protein intake is not associated with infant blood pressure. Int J Epidemiol. 2005;34(2):378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li LJ, Rifas-Shiman SL, Aris IM, et al. Leptin trajectories from birth to mid-childhood and cardio-metabolic health in early adolescence. Metabolism. 2019;91:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92(4):1049–1057. [DOI] [PubMed] [Google Scholar]
- 22. Committee on Practice and Ambulatory Medicine; Bright Futures Periodicity Schedule Workgroup 2015 recommendations for preventive pediatric health care: Committee on Practice and Ambulatory Medicine and Bright Futures Periodicity Schedule Workgroup. Pediatrics. 2015;136(3):e727. [DOI] [PubMed] [Google Scholar]
- 23. Sun SS, Grave GD, Siervogel RM, et al. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–246. [DOI] [PubMed] [Google Scholar]
- 24. Schachter J, Kuller LH, Perfetti C. Blood pressure during the first five years of life: relation to ethnic group (black or white) and to parental hypertension. Am J Epidemiol. 1984;119(4):541–553. [DOI] [PubMed] [Google Scholar]
- 25. Zinner SH, Rosner B, Oh W, et al. Significance of blood pressure in infancy. Familial aggregation and predictive effect on later blood pressure. Hypertension. 1985;7(3):411–416. [PubMed] [Google Scholar]
- 26. Levine RS, Hennekens CH, Klein B, et al. Tracking correlations of blood pressure levels in infancy. Pediatrics. 1978;61(1):121–125. [PubMed] [Google Scholar]
- 27. Wen X, Triche EW, Hogan JW, et al. Prenatal factors for childhood blood pressure mediated by intrauterine and/or childhood growth? Pediatrics. 2011;127(3):e713–e721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowson CA, Crozier SR, Robinson SM, et al. Association of early childhood abdominal circumference and weight gain with blood pressure at 36 months of age: secondary analysis of data from a prospective cohort study. BMJ Open. 2014;4(7):e005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Syme C, Abrahamowicz M, Leonard GT, et al. Sex differences in blood pressure and its relationship to body composition and metabolism in adolescence. Arch Pediatr Adolesc Med. 2009;163(9):818–825. [DOI] [PubMed] [Google Scholar]
- 30. Young DR, Fischer H, Arterburn D, et al. Associations of overweight/obesity and socioeconomic status with hypertension prevalence across racial and ethnic groups. J Clin Hypertens (Greenwich). 2018;20(3):532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A1c and systolic blood pressure in Pima Indian children. J Clin Endocrinol Metab. 2005;90(6):3225–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cho NH, Silverman BL, Rizzo TA, et al. Correlations between the intrauterine metabolic environment and blood pressure in adolescent offspring of diabetic mothers. J Pediatr. 2000;136(5):587–592. [DOI] [PubMed] [Google Scholar]
- 33. Geelhoed JJ, Fraser A, Tilling K, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122(12):1192–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lawlor DA, Macdonald-Wallis C, Fraser A, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33(3):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li LJ, Liao J, Fan Q, et al. Familial correlation of retinal vascular caliber in Singapore Chinese. Invest Ophthalmol Vis Sci. 2013;54(8):5638–5642. [DOI] [PubMed] [Google Scholar]
- 36. Howe LD, Parmar PG, Paternoster L, et al. Genetic influences on trajectories of systolic blood pressure across childhood and adolescence. Circ Cardiovasc Genet. 2013;6(6):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kagura J, Adair LS, Pisa PT, et al. Association of socioeconomic status change between infancy and adolescence, and blood pressure, in South African young adults: Birth to Twenty Cohort. BMJ Open. 2016;6(3):e008805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pausová Z, Paus T, Sedová L, et al. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64(3):829–835. [DOI] [PubMed] [Google Scholar]
- 39. Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376(9741):631–644. [DOI] [PubMed] [Google Scholar]
- 40. Li HP, Chen X, Li MQ. Gestational diabetes induces chronic hypoxia stress and excessive inflammatory response in murine placenta. Int J Clin Exp Pathol. 2013;6(4):650–659. [PMC free article] [PubMed] [Google Scholar]
- 41. Nelson RG, Morgenstern H, Bennett PH. Intrauterine diabetes exposure and the risk of renal disease in diabetic Pima Indians. Diabetes. 1998;47(9):1489–1493. [DOI] [PubMed] [Google Scholar]
- 42. Amri K, Freund N, Vilar J, et al. Adverse effects of hyperglycemia on kidney development in rats: in vivo and in vitro studies. Diabetes. 1999;48(11):2240–2245. [DOI] [PubMed] [Google Scholar]
- 43. Martin RM, Ness AR, Gunnell D, et al. Does breast-feeding in infancy lower blood pressure in childhood? The Avon Longitudinal Study of Parents and Children (ALSPAC). Circulation. 2004;109(10):1259–1266. [DOI] [PubMed] [Google Scholar]
- 44. Lawlor DA, Najman JM, Sterne J, et al. Associations of parental, birth, and early life characteristics with systolic blood pressure at 5 years of age: findings from the Mater-University Study of Pregnancy and Its Outcomes. Circulation. 2004;110(16):2417–2423. [DOI] [PubMed] [Google Scholar]
- 45. Kramer MS, Moodie EE, Dahhou M, et al. Breastfeeding and infant size: evidence of reverse causality. Am J Epidemiol. 2011;173(9):978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Collell R, Closa-Monasterolo R, Ferré N, et al. Higher protein intake increases cardiac function parameters in healthy children: metabolic programming by infant nutrition—secondary analysis from a clinical trial. Pediatr Res. 2016;79(6):880–888. [DOI] [PubMed] [Google Scholar]
- 47. Juonala M, Järvisalo MJ, Maki-Torkko N, et al. Risk factors identified in childhood and decreased carotid artery elasticity in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2005;112(10):1486–1493. [DOI] [PubMed] [Google Scholar]
- 48. Li S, Chen W, Yun M, et al. Sex and race (black-white) differences in the relationship of childhood risk factors to adulthood arterial stiffness: the Bogalusa Heart Study. Am J Med Sci. 2014;348(2):101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tilling K, Macdonald-Wallis C, Lawlor DA, et al. Modelling childhood growth using fractional polynomials and linear splines. Ann Nutr Metab. 2014;65(2-3):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.