Abstract
Background:
The comparative safety and effectiveness of treatments to prevent thromboembolic complications in atrial fibrillation (AF) remains uncertain.
Purpose:
To compare the available treatment strategies in patients with AF.
Data Sources:
English-language studies in multiple databases from January 1, 2000, to February 14, 2018.
Study Selection:
Two reviewers independently screened citations for studies examining treatments for stroke prevention in patients with AF.
Data Extraction:
Two reviewers independently abstracted data and assessed study quality and applicability.
Data Synthesis:
Data from 220 articles were included. Dabigatran and apixaban were superior while rivaroxaban and edoxaban were similar to warfarin in the prevention of stroke or systemic embolism. Apixaban and edoxaban were superior while rivaroxaban and dabigatran were similar to warfarin in reducing the risk of major bleeding. Treatment effects with dabigatran were similar in patients with renal dysfunction (interaction p>0.05) and patients less than 75 years old had lower rates of bleeding with dabigatran (interaction p<0.001). The benefit of treatment with apixaban was consistent in many subgroups including those with renal impairment, diabetes and prior stroke (interaction p>0.05 for all). The greatest bleeding risk reduction was observed in patients with GFR<50mL/min (p=0.003). Similar treatment effects of rivaroxaban and edoxaban were observed in patients with prior stroke, diabetes, or heart failure (interaction p>0.05 for all).
Limitations:
Heterogeneous study populations, interventions and outcomes.
Conclusion:
The available DOACs are at least as effective and safe when compared to warfarin. Similar benefits of treatment with DOACs were observed across multiple patient subgroups of interest, giving assurance to the safe and efficacious use of DOACs for a wide range of patients with AF.
Primary Funding Source:
Patient-Centered Outcomes Research Institute (PROSPERO #CRD42017069999)
INTRODUCTION
Atrial fibrillation (AF), is characterized by uncoordinated atrial activation with consequent deterioration of mechanical function (1). As the most common sustained cardiac arrhythmia, it is estimated that by the year 2050 there will be 12.1 million Americans with AF (2). Patients with AF have increased risk of embolic stroke, heart failure, and cognitive impairment; reduced quality of life; and higher overall mortality (3–5). Strokes related to AF have been characterized as more severe (6, 7) and translate to a significant economic burden, costing Medicare approximately $8 billion annually (8).
Antithrombotic therapies are the mainstays used to prevent thromboembolic events in patients with AF. Oral anticoagulation with vitamin K antagonists (VKAs) has long been the gold standard therapy for stroke prevention in AF patients. However, the challenges of monitoring VKA therapy and the multiple associated food and drug interactions have fueled the development of novel therapeutic options. Currently, there are four direct-acting oral anticoagulants (DOACs) approved for stroke prevention in patients with nonvalvular AF: dabigatran (a thrombin inhibitor) and apixaban, rivaroxaban, and edoxaban (all factor Xa inhibitors). Additionally, procedural interventions, such as left atrial appendage (LAA) occlusion devices, are an alternative treatment for patients who are at high risk of bleeding on anticoagulation therapy.
Since 2009, four large trials (ROCKET AF, ARISTOTLE, RE-LY, and ENGAGE-AF TIMI-48) that compared treatment with DOACs to warfarin have been completed, creating a combined sample size of over 71,000 subjects (9–12). This systematic review (SR) updates previous reviews (13) and aims to compare the effectiveness of the available medical and procedural therapies in preventing thromboembolic events and bleeding complications in patients with AF.
METHODS
The full report (available at www.efectivehealthcare.ahrq.gov, PROSPERO protocol available at http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42017069999) provides detailed information on the methods used in this SR. This review was commissioned by the Patient-Centered Outcomes Research Institute (PCORI) in partnership with the Agency for Healthcare Research and Quality’s (AHRQ) Evidence-based Practice Center (EPC) Program to explore the data and ongoing uncertainties in the treatment of AF. To inform the review, PCORI held two multi-stakeholder workshops to discuss scoping and prioritization of key questions. Based on feedback from stakeholders, an analytic framework was constructed (Appendix Figure 1) to further explore new evidence related to the Key Questions (KQs) from the original report.
Data Sources, and Searches
Literature was identified by searching PubMed, Embase, and the Cochrane Database of SRs for English language studies published between January 1, 2000, and February 14, 2018. All searches were guided by an experienced search librarian (Appendix Table 1).
Study Selection
All studies were screened based on the inclusion and exclusion criteria (Appendix Table 2). We included studies that examined at least 20 adult patients ≥18 years of age with nonvalvular AF who were treated with anticoagulation, antiplatelet, or procedural therapies. All studies were required to have an active comparator and to assess an outcome of interest. Two independent investigators reviewed each title and abstract for potential relevance. Any abstract included by one investigator was included for full-text review. During full-text review, two investigators independently reviewed the manuscript text and indicated a decision to include or exclude the study. Conflicts were resolved by further review and discussion or a third-party arbitrator.
Data Extraction and Quality Assessment
Pairs of investigators were formed based on methodological and clinical experience to abstract data from the selected studies. One investigator abstracted the data into previously created data abstraction forms and table templates. The second investigator reviewed the abstracted data together with the original article to ensure accuracy and completeness.
We evaluated each study for methodological quality using the Cochrane Risk of Bias tool (for RCTs) and the Risk of Bias in Nonrandomized Studies of Interventions tool (for observational studies). Studies were assigned a rating of good, fair, or poor quality. Quality assessment was outcome-specific, such that a given study that analyzed its primary outcome well but did an incomplete analysis of a secondary outcome could be assigned a different quality grade for each of the two outcomes.
Data Synthesis and Analysis
Key features of each study were reviewed and summarized based on a hierarchy of evidence with the best evidence available being the focus of the synthesis. Treatments were grouped based on drug class to determine whether a quantitative synthesis was feasible. When at least three studies with comparable interventions and populations were available to compare a specified outcome, we used the R statistical package (version 3.1.2) (R Foundation) with the “metafor” meta-analysis library (version 1.9–7) to synthesize the available evidence. We used the random-effects DerSimonian and Laird estimator (14) to generate summary values. In addition, we used the Knapp–Hartung approach (15) to adjust the standard errors of the estimated coefficients. We explored heterogeneity using graphical displays and test statistics (Q and an statistics). For observational studies we used adjusted results from the individual studies for pooled analyses. When an individual study included findings from more than one dose for a specific drug, we chose the dose most typical for use within the United States. We considered the use of a network meta-analysis to synthesize findings from the observational studies although given the heterogeneity of populations, treatment doses, treatment protocols, outcome definitions, and study quality did not include such analyses in our review.
We rated strength of evidence (SOE) using the approach described in the Methods Guide (16, 17). We graded the SOE for each outcome individually; thus, the SOE for two separate outcomes in a given study may be graded differently. Briefly, the approach requires assessment of five domains: study limitations, consistency, directness, precision, and reporting bias, which includes publication bias, outcome reporting, and analysis reporting bias. The five domains were considered qualitatively, and a summary rating of “high,” “moderate,” “low,” or “insufficient” SOE was assigned after discussion by two reviewers. When outcomes were assessed by RCTs and observational studies, we focused our SOE rating on the findings from the RCTs and then increased or decreased the SOE rating depending on whether findings from the observational studies were consistent or inconsistent with those from the RCTs. We provided greatest weight to findings from large RCTs.
Role of the Funding Source
This topic was nominated and funded by the Patient-Centered Outcomes Research Institute (PCORI) for SR by an EPC in partnership with the Agency for Healthcare Research and Quality (AHRQ). A representative from AHRQ served as a Contracting Officer’s Representative (COR) and provided technical assistance during the conduct of the full evidence report. The AHRQ COR and PCORI program officers provided comments on draft versions of the protocol and full evidence report. PCORI and AHRQ did not directly participate in the literature search; determination of study eligibility criteria; data analysis or interpretation; or preparation, review, or approval of the manuscript for publication.
RESULTS
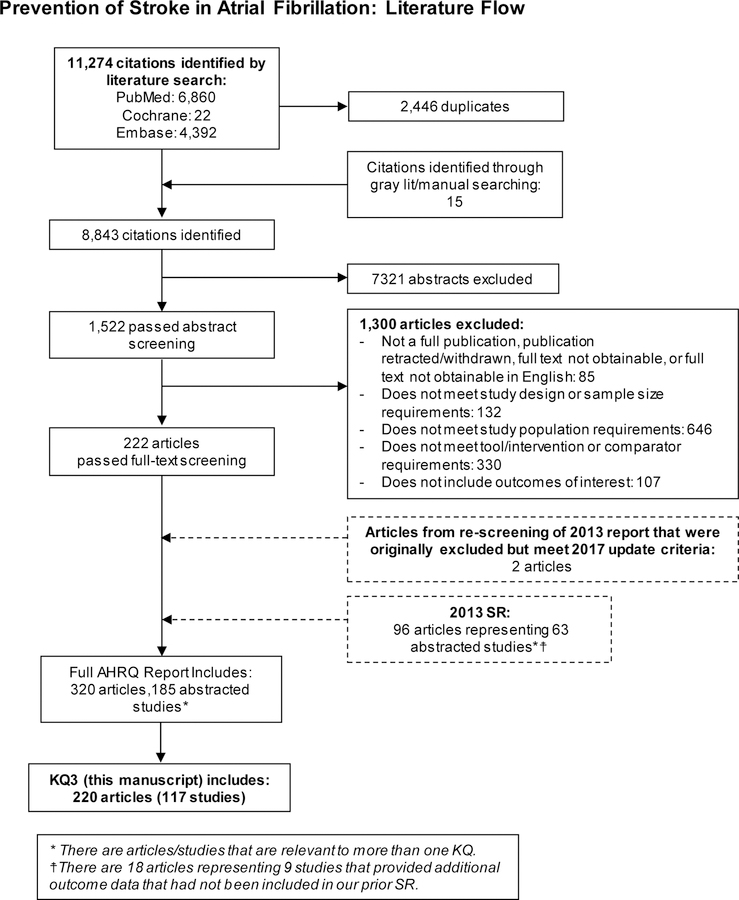
For this specific KQ, our 2018 update and articles from our original review resulted in 220 included articles, which represented 117 unique studies involving 3,934,374 patients (Figure 1). Appendix Table 3 provides specific details about each study including patient population and outcomes analyzed.
Figure 1. Literature flow diagram.
KQ=Key Question; SR=systematic review
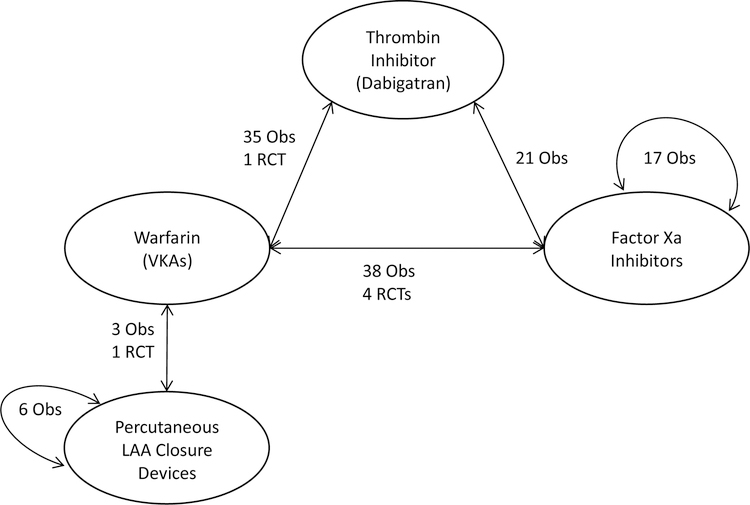
Given the large number of studies and comparisons, we focus in this paper on the most applicable clinical comparative effectiveness comparisons. Figure 2 graphically displays the number of studies for each direct comparison available and the specific study designs. Additional studies that evaluated multiple different treatment comparisons including VKA versus aspirin, clopidogrel versus warfarin, dabigatran with or without aspirin versus warfarin, and factor Xa inhibitors versus aspirin are detailed in the full report available online.
Figure 2. Treatment comparisons.
Obs=observational; RCT=randomized controlled trials; LAA= Left Atrial Appendage; VKA=vitamin K antagonist
Warfarin Compared with Thrombin Inhibitor (Dabigatran)
The RE-LY trial (9, 18) showed that treatment with dabigatran, dosed at 150mg, is superior to warfarin in reducing the incidence of the composite outcome of stroke or systemic embolism. There was no statistically significant difference between the two treatment modalities with regard to major bleeding, myocardial infarction or all-cause mortality. A prespecified secondary analysis examined outcomes related to renal function. The rates of stroke or systemic embolism were lower with dabigatran 150mg and similar with 110mg twice daily compared with warfarin, without significant heterogeneity in subgroups defined by renal function (interaction p>0.1 for all) (19). Similarly, there was no interaction between treatment with dabigatran 150mg and the presence of left ventricular hypertrophy on the outcomes of stroke or systemic embolism (p=0.24) or major bleeding (p=0.89) (20). Conversely, there was a statistically significant treatment-by-age interaction in that dabigatran 150mg twice a day compared with warfarin was associated with a lower risk of major bleeding in patients <75 years of age, with a trend toward a higher risk of major bleeding in those ≥75 years of age (p<0.001) (9).
As an extension of the RCT, the RELY-ABLE observational study (21) extended follow-up of patients on dabigatran to provide additional long-term information of dabigatran 150mg versus 110mg. The RELY-ABLE study showed no difference in the rate of stroke or systemic embolism (HR 0.91; 95% CI 0.69–1.20) or all-cause mortality (HR 0.97; 95% CI 0.80–1.19) between the groups. However, patients treated with the 150mg dose had higher rates of major hemorrhage (HR 1.26; 95% CI 1.04–1.53). Of note, the currently approved doses of dabigatran for stroke prevention in AF in the United States are 150mg and 75mg (for patients with creatinine clearance 15–30ml/min) (22).
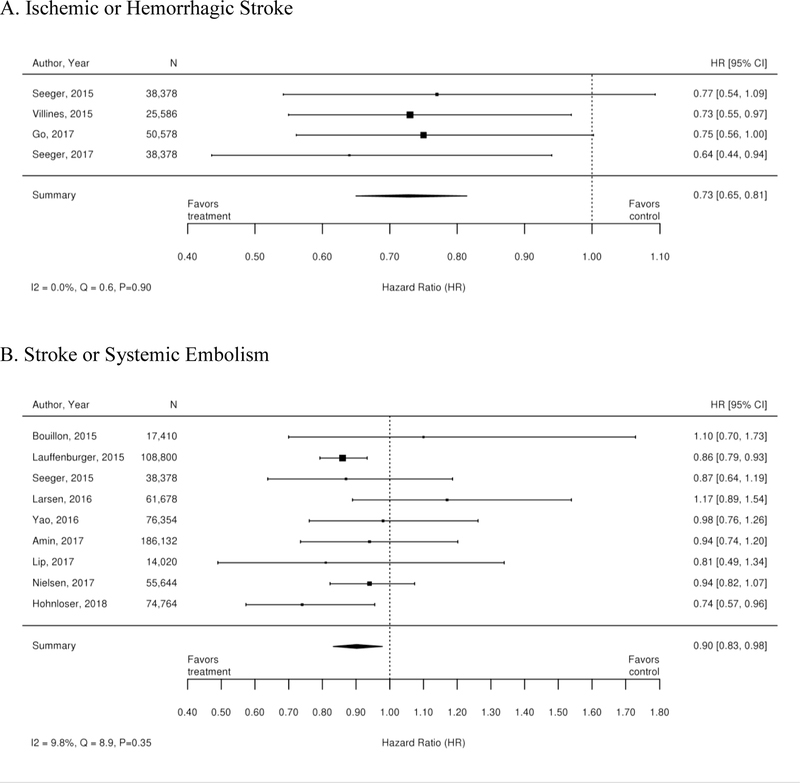
Additionally, 35 observational studies compared warfarin with dabigatran. A meta-analysis of these studies demonstrated a reduction in the risk of stroke, stroke or systemic embolism, hemorrhagic stroke, intracranial bleeding, minor bleeding, GI bleeding and all-cause mortality for patients treated with dabigatran compared with warfarin. There was no statistically significant difference between treatments in rates of ischemic stroke or myocardial infarction. Forest plots for each outcome are available in Appendix Figure 2. The observational studies demonstrated a benefit in all-cause mortality for patients on dabigatran compared with warfarin. RCT evidence, however did not demonstrate a difference.
Warfarin Compared with Factor Xa Inhibitors (Apixaban, Rivaroxaban, and Edoxaban)
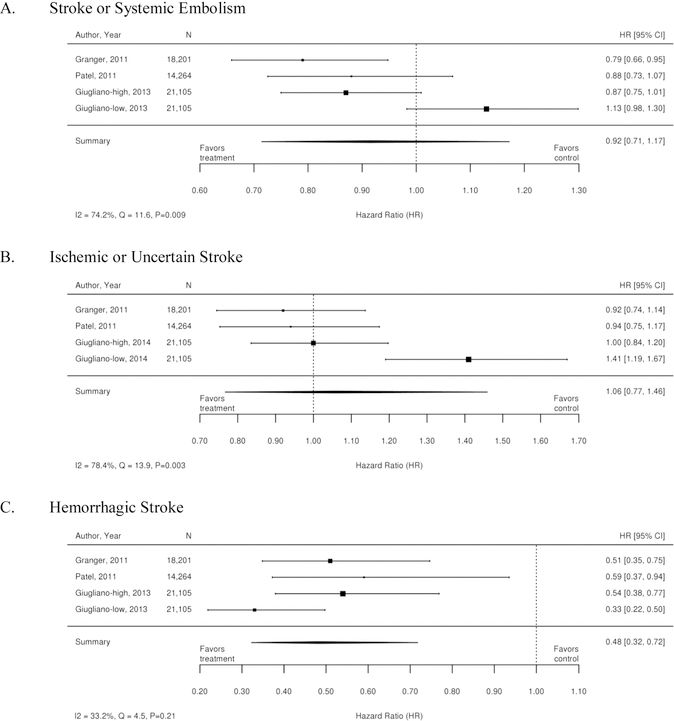
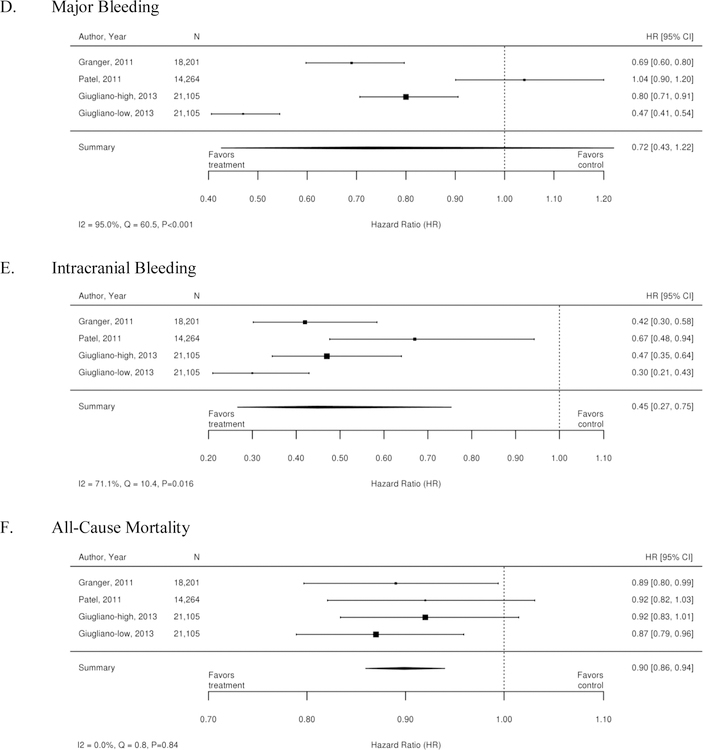
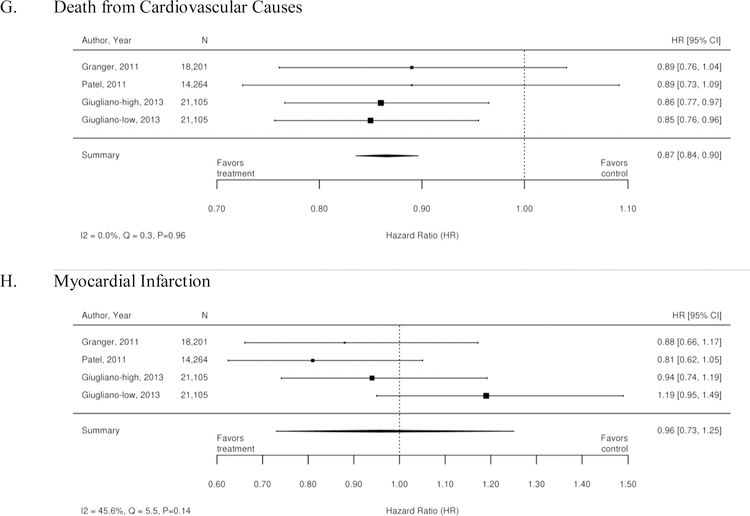
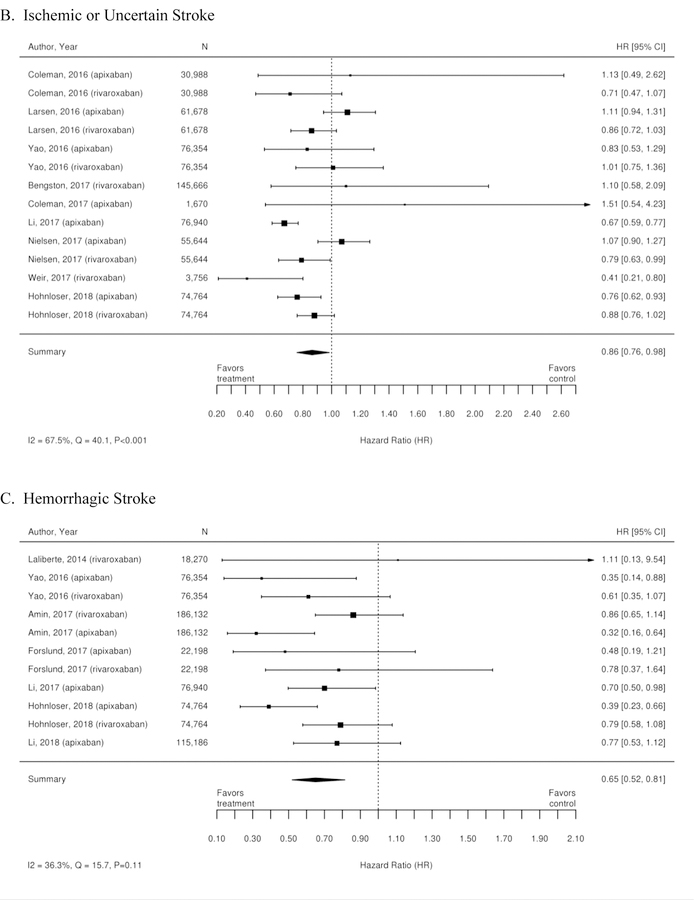
The three RCTs detailed below compared warfarin with factor Xa inhibitors. Table 1 summarizes the results of these trials. A meta-analysis of these 3 trials was conducted, and the results are summarized as forest plots in Figure 3. Overall, there was no difference between treatment with a Xa inhibitor or warfarin in the outcomes of stroke or systemic embolism, ischemic or uncertain stroke, major bleeding, and myocardial infarction. However, there was overall benefit from treatment with an Xa inhibitor with regard to hemorrhagic stroke, intracranial bleeding, all-cause mortality, and death from cardiovascular causes.
Table 1.
Outcomes of interest within RCTs evaluating factor Xa inhibitors: apixaban, rivaroxaban, or edoxaban vs. warfarin
| Outcome of Interest | Randomized Controlled Trial | |||
|---|---|---|---|---|
| ARISTOTLE (N=18,201) (Apixaban vs. Warfarin) |
ROCKET AF (N=14,264) (Rivaroxaban vs. Warfarin) |
ENGAGE AF (High Dose Edoxaban vs. Warfarin) (N=14,071) |
ENGAGE AF (Low Dose Edoxaban vs. Warfarin) (N=14,070) |
|
| Stroke or Systemic Embolism | In the ITT population: 1.27% of patients per year with apixaban, 1.60% of patients per year in the warfarin group (HR 0.79; 95% CI 0.66 to 0.95, p=0.01) NNT = 167/2 years |
In the ITT population: 2.1% of patients per year in the rivaroxaban group and 2.4% of patients per year with warfarin (HR 0.88; 95% CI 0.7 to 1.03; p<0.001 for noninferiority; p=0.12 for superiority) In the per-protocol population,: 1.7% per year in the rivaroxaban group and 2.2% with warfarin (noninferiority HR 0.79; 95% CI 0.66 to 0.96; p<0.001; superiority HR 0.79; 95% CI 0.65 to 0.95; p=0.01) |
In the ITT analysis in the overall study period, event rates were higher in all groups and there were no statistically significant differences (1.80% per year for warfarin, 1.57%/year for high dose edoxaban [HR 0.87; 97.5% CI 0.73–1.04 as compared to warfarin]) In the modified ITT: 1.5% of patients per year with warfarin, 1.18% of patients per year in the high-dose edoxaban group (HR 0.79; 97.5% CI 0.63–0.99, p < 0.001 for noninferiority and p=0.02 for superiority) |
In the ITT analysis in the overall study period, event rates were higher in all groups and there were no statistically significant differences (2.04%/year for low dose edoxaban [HR 1.13; 97.5% CI 0.96 to 1.34 as compared to warfarin]) In the modified ITT: 1.61% of patients per year in the low-dose edoxaban group (HR1.07; 97.5% CI 0.87 to 1.31, p=0.005 for noninferiority and p=0.44 for superiority). |
| Ischemic or Uncertain Stroke | 0.97% per year for apixaban and 1.05% per year for warfarin HR 0.92; 95% CI 0.74 to 1.13; p=0.42 |
1.34% per year for rivaroxaban and 1.42% per year for warfarin HR 0.94; 95% CI 0.75 to 1.17; p=0.58 |
1.25% per year for warfarin and 1.25% per year for edoxaban, HR1.00; 95% CI 0.83 to 1.19 | 1.77% per year for edoxaban and 1.25% per year for warfarin, HR 1.41; 95% CI 1.19 to 1.67 |
| Hemorrhagic Stroke | 0.24% per year for apixaban and 0.47% per year for warfarin HR 0.51; 95% CI 0.35 to 0.75; p<0.001 |
0.26% per year for rivaroxaban and 0.44% per year for warfarin HR 0.59; 95% CI 0.37 to 0.93; p=0.024 |
0.26% of patients per year, HR 0.54; 95% CI 0.38 to 0.77 0.47% patients per year for warfarin |
0.16% of patients per year, HR 0.33; 95% CI 0.22 to 0.50 0.47% patients per year for warfarin |
| Any Stroke or TIA | NA | NA |
Any stroke 1.49% of patients per year, HR 0.88; 95% CI 0.75 to 1.03 1.69% patients per year for warfarin |
Any stroke 1.91% of patients per year, HR 1.13; 95% CI 0.97 to 1.31 1.69% patients per year for warfarin |
| Systemic Embolism | 0.09% per year for apixaban and 0.10% per year for warfarin HR 0.87; 95% CI 0.44 to 1.75; p=0.70 |
0.04% per year for rivaroxaban and 0.19% per year for warfarin HR 0.23; 95% CI 0.09 to 0.61; p=0.003 |
0.08% of patients per year, HR 0.65; 95% CI 0.34 to 1.20 0.12% of patients per year for warfarin Subanalysis: Geller, 2015 (23): there was no difference in nonfatal systemic embolic or fatal events between high dose edoxaban compared with warfarin. |
0.15% of patients per year, HR 1.24; 95% CI 0.72 to 2.15 0.12% of patients per year for warfarin Subanalysis: Geller, 2015 (23): there was no difference in nonfatal systemic embolic or fatal events between low dose edoxaban compared with warfarin. |
| Major Bleeding | 2.13% per year for apixaban and 3.09% per year for warfarin HR 0.69; 95% CI 0.60 to 0.80; p<0.001 |
3.6% per year for rivaroxaban and 3.4% per year for warfarin HR 1.04; 95% CI 0.90 to 1.20; p=0.58 |
2.75% of patients per year, HR 0.80; 95% CI 0.71 to 0.91 3.43% patients per year for warfarin |
1.61% of patients per year, HR 0.47; 95% CI 0.41 to 0.55 3.43% patients per year for warfarin |
| Major, NMCR and Minor Bleeding |
Major or NMCR bleeding: 4.07% per year in the apixaban group and 6.01% per year in the warfarin group HR 0.68; 95% CI 0.61 to 0.75 Non-major bleeding: 6.4% per year for apixaban, 9.4 %per year for warfarin HR 0.69; 95% CI 0.63 to 0.75 |
Major or NMCR bleeding: 14.9% per year in the rivaroxaban group and 14.5% per year years for warfarin HR 1.03; 95% CI 0.96 to 1.11 NMCR bleeding: 11.8% per year in the rivaroxaban group and 11.4% per year for warfarin HR 1.04; 95% CI 0.96 to 1.13 |
Major or NMCR bleeding: 11.1% of patients per year, HR 0.86; 95% CI 0.80 to 0.92 13.02% patients per year for warfarin Minor bleeding:4.12% of patients per year, HR 0.84; 95% CI 0.76 to 0.94 4.89% patients per year for warfarin |
Major or NMCR bleeding: 7.97% of patients per year, HR 0.62; 95% CI 0.57 to 0.67 13.02% patients per year for warfarin Minor bleeding:3.52% of patients per year, HR 0.72; 95% CI 0.65 to 0.81 4.89% patients per year for warfarin |
| Gastrointestinal Bleeding | NA | 3.61% per year for rivaroxaban and 2.60% per year; HR 1.42; 95% CI 1.22 to 1.66 | NA | NA |
| Intracranial Bleeding | Lower intracranial bleeding in patients treated with apixaban compared to warfarin HR 0.42; 95% CI 0.30 to 0.58; p<0.001 |
Overall event rate of 0.67% per year Lower intracranial bleeding in patients treated with rivaroxaban compared to warfarin HR 0.67; 95% CI 0.47 to 0.93; p=0.023 |
0.39% of patients per year, HR 0.47; 95% CI 0.34 to 0.63 0.85% patients per year for warfarin |
0.26% of patients per year, HR 0.30; 95% CI 0.21 to 0.43 0.85% patients per year for warfarin |
| All-Cause Mortality | 3.52% per year for apixaban and 3.94% per year in the warfarin group HR 0.89; 95% CI 0.80 to 0.998; p=0.047 |
In the ITT analysis: 4.5% per year in the rivaroxaban group and 4.9% per year for warfarin HR 0.92; 95% CI 0.82 to 1.03; p=0.15 In treatment per protocol analysis: 1.9% per year for rivaroxaban and 2.2% per year for warfarin HR 0.85; 95% CI 0.70 to 1.02; p=0.07 |
3.99% of patients per year, HR 0.92; 95% CI 0.83 to 1.01 4.35% patients per year for warfarin |
3.80% of patients per year, HR 0.87; 95% CI 0.79 to 0.96 4.35% patients per year for warfarin |
| Death from Cardiovascular Causes | 1.8% per year for apixaban and 2.02% per year for the warfarin group HR 0.89; 95% CI 0.76 to 1.04 |
1.53% per year for the rivaroxaban group and 1.71 %per year for warfarin HR 0.89; 95% CI 0.73 to 1.10; p=0.289 |
2.74 % patients per year, HR 0.86; 95% CI 0.77 to 0.97 3.17% patients per year for warfarin |
2.71% patients per year, HR 0.85; 95% CI 0.76 to 0.96 3.17% patients per year for warfarin |
| Myocardial Infarction | 0.53% per year for apixaban and 0.61% per year for warfarin HR 0.88; 95% CI 0.66 to 1.17; p=0.37 |
0.9% per year for rivaroxaban and 1.1% per year in the warfarin group HR 0.81; 95% CI 0.63 to 1.06; p=0.12 |
0.70% of patients per year, HR 0.94; 95% CI 0.74 to 1.19 0.75% patients per year for warfarin |
0.89% of patients per year, HR 1.19; 95% CI 0.95 to 1.49 0.75% patients per year for warfarin |
| Specific Subgroups of Interest | ||||
| Prior Stroke | No statistically significant interaction was found between prior stroke/TIA and treatment for stroke or systemic embolism, cardiovascular death, disabling or fatal stroke, all-cause mortality, major bleeding (24). | No statistically significant interaction was found between prior stroke/TIA and treatment for stroke or systemic embolism, major or non-major clinically relevant bleeding (25). | No statistically significant interaction was found between prior stroke/TIA and treatment (high dose edoxaban vs. warfarin) for stroke or systemic embolic event, any stroke, hemorrhagic stroke, ischemic stroke, any cause death, or cardiovascular death (26). | NA |
| Aspirin Treatment | No statistically significant interactions between treatment and use of aspirin vs. none on stroke or systemic embolism, ischemic stroke, MI, death, major bleeding, hemorrhagic stroke, major or clinically-relevant non-major bleeding or any bleeding (27). | No statistically significant interactions between treatment and use of aspirin versus none on stroke or systemic embolism, major bleeding or all-cause death (28). | No statistically significant interactions between treatment and use of single antiplatelet drug vs. none on stroke or systemic embolic events, ischemic stroke, hemorrhagic stroke, MI, cardiovascular death, major bleeding, intracranial bleeding, or any bleeding (29). | No statistically significant interactions between treatment and use of single antiplatelet drug vs. none on stroke or systemic embolic events, ischemic stroke, hemorrhagic stroke, MI, cardiovascular death, major bleeding, intracranial bleeding, or any bleeding (29). |
Abbreviations: ARISTOTLE=Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation trial; CI=confidence interval; ENGAGE AF-TIMI 48=Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 trial; HR=hazard ratio; ITT=intent to treat; MI=myocardial infarction; NA=not applicable; NMCR=nonmajor clinically relevant; RE-LY=Randomized Evaluation of Long-term Anticoagulation Therapy trial; ROCKET AF=Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation; TIA=transient ischemic attack
Figure 3. Forest plots for comparison of Xa inhibitors versus warfarin (RCTs).
A. Stroke or Systemic Embolism; B. Ischemic or Uncertain Stroke; C. Hemorrhagic Stroke; D. Major bleeding; E. Intracranial Bleeding; F. All-Cause Mortality’ G. Death from Cardiovascular Causes; H. Myocardial Infarction
CI=confidence interval; RCTs=randomized controlled trials
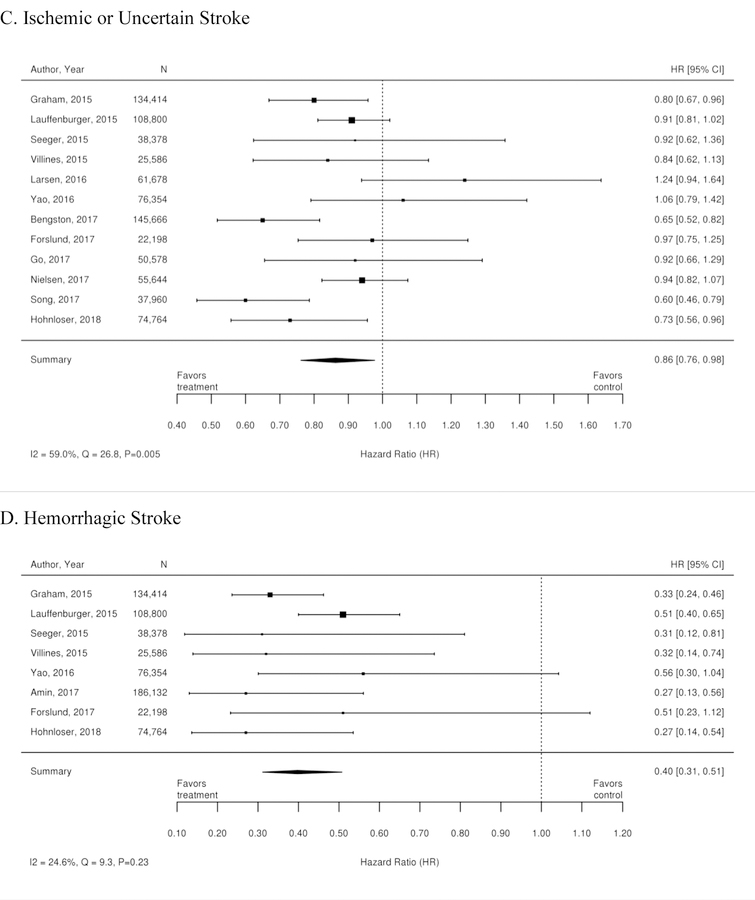
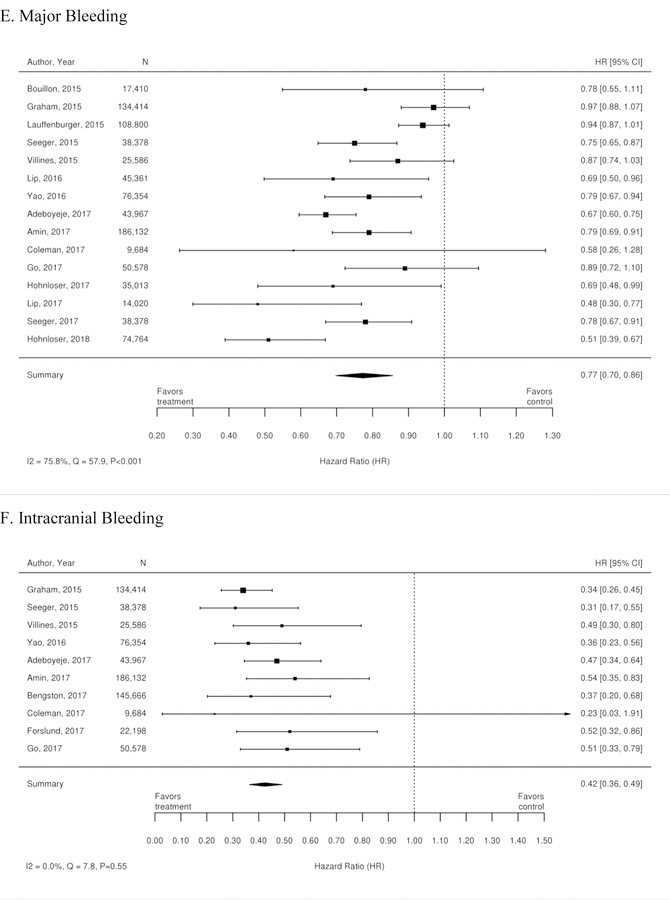
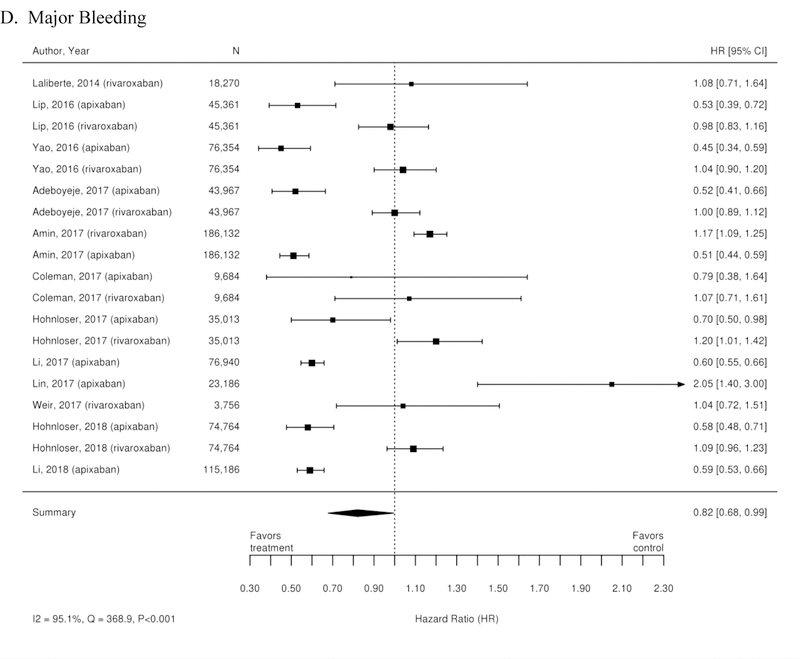
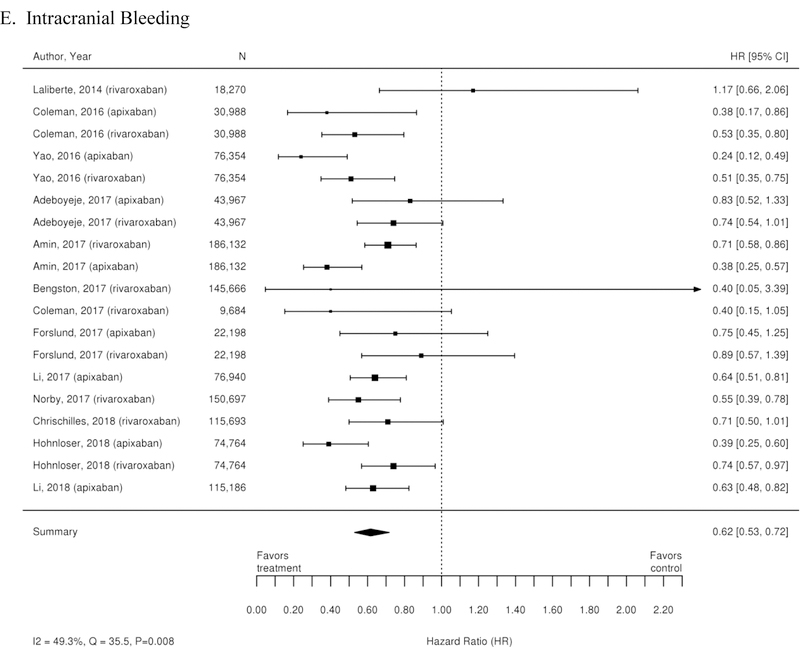
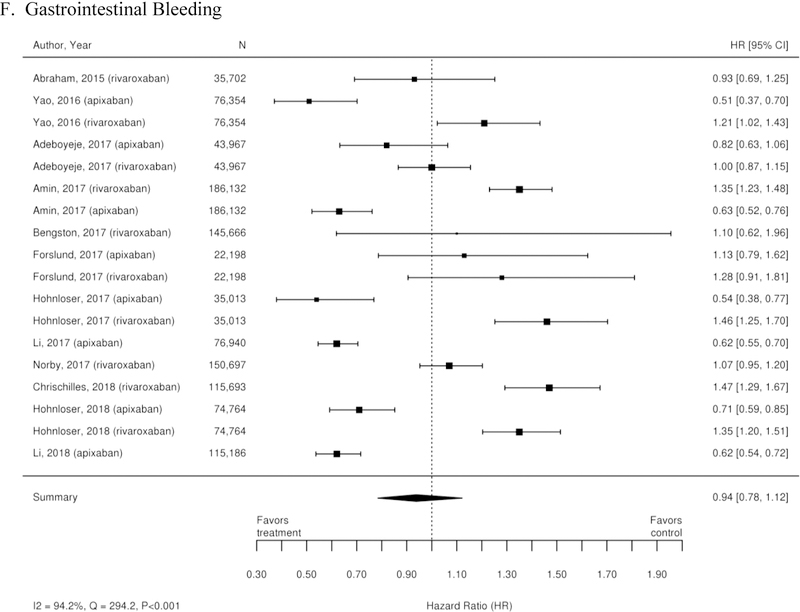
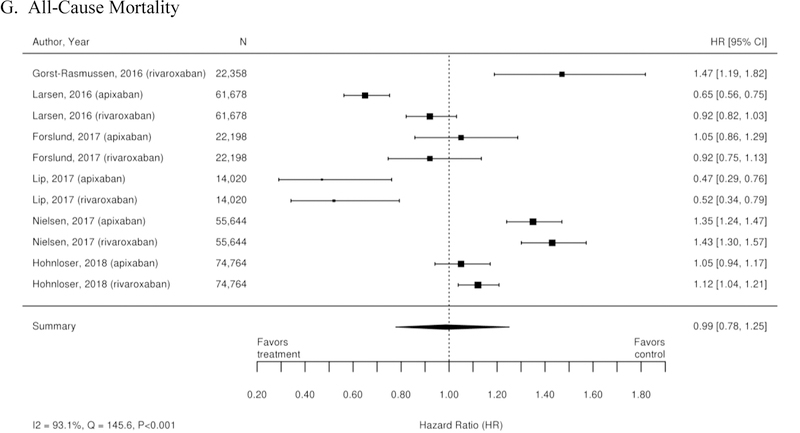
Additionally, 38 observational studies compared warfarin with factor Xa inhibitors. Similar analyses were completed for the observational trial data and are available in Appendix Figure 3. The observational studies did not show a reduction in all-cause mortality across Xa inhibitors, whereas RCTs showed reduction in all-cause mortality across Xa inhibitors. In addition, inconsistent with the RCT findings, observational studies exploring rivaroxaban versus warfarin supported a reduction in stroke or systemic embolism and a trend towards a reduction in ischemic or uncertain stroke, while also providing evidence of a small increase in the risk of major bleeding. Other RCT findings were supported by existing observational studies.
Warfarin versus Apixaban
The ARISTOTLE trial (12) showed that, compared with warfarin, treatment with apixaban 5mg twice daily (or a reduced dose of 2.5mg twice daily in patients meeting at least two of the following criteria: age ≥80 years, weight ≤60 kg, or serum creatinine concentration ≥1.5mg/dl) reduces the risk of stroke, systemic embolism, major bleeding, and all-cause mortality. Multiple substudies of the ARISTOTLE trial have been completed. Studies evaluating bleeding outcomes showed that patients treated with apixaban versus warfarin were less likely to die within 30 days of a major hemorrhagic event (HR 0.50; 95% CI 0.33–0.74) (30); were less likely to experience nonmajor bleeding (HR 0.69; 95% CI 0.63–0.75) (31); and had lower rates of intracranial hemorrhage (intracranial HR 0.42; 95% CI 0.30–0.58; intracerebral HR 0.45; 95% CI 0.30–0.68; subdural HR 0.33; 95% CI 0.17–0.65) (32). However, there was no statistically significant difference in the number of hospital admissions between the apixaban and warfarin treatment arms (26.6% versus 28.1%; p=0.31) (33).
Additional substudies of the ARISTOTLE trial evaluated outcomes in specific patient groups of interest. Overall, with regard to the primary ischemic outcome of stroke or systemic embolism, there was no statistically significant interaction between treatment and level of renal impairment (p=0.71) (34); paroxysmal versus persistent AF (p =0.71) (35); timing of AF diagnosis (p=0.94) (36); history of prior stroke (p=0.71) (24); age (p=0.11) (37); history of peripheral arterial disease (p=0.52) (38); underlying anemia (p=0.17) (39); history of chronic obstructive pulmonary disease (p=0.62) (40); sex (p=0.45) (41); diabetes (p=0.71) (42); concomitant aspirin therapy (p=0.10) (27); or history of falls (43). With regard to major bleeding, there was no statistically significant interaction between treatment and paroxysmal versus persistent AF (p=0.75) (35); timing of AF diagnosis (p=0.78) (36); history of prior stroke (p=0.69) (24); age (p=0.63) (37); history of peripheral arterial disease (p=0.58) (38); underlying anemia (39); history of chronic obstructive pulmonary disease (p=0.42) (40); sex (p=0.06) (41); concomitant aspirin therapy (p=0.29) (27); or history of falls (43). While apixaban was associated with fewer bleeding events across renal functions, the relative risk reduction was greatest in patients with GFR<50 mL/min (p=0.003) (34). Additionally, patients without diabetes had lower rates of International Society on Thrombosis and Haemostasis (ISTH) major bleeding with apixaban, a treatment effect that was not seen in diabetic patients (p=0.003) (42).
Warfarin versus Rivaroxaban
The ROCKET-AF trial (11) showed that treatment with rivaroxaban 20mg daily was noninferior to warfarin with regard to prevention of stroke or systemic embolism. There were also similar rates of major bleeding and all-cause mortality between groups.
There have been multiple secondary analyses of the ROCKET-AF trial evaluating treatment with rivaroxaban compared with warfarin. One such study (44) showed higher rates of gastrointestinal bleeding (HR 1.42; 95% CI 1.22–1.66) in patients treated with rivaroxaban compared with warfarin. Conversely, there was a trend toward lower rates of ischemic cardiovascular outcomes (HR 0.86; 95% CI 0.73–1.00) and all-cause mortality (HR 0.85; 95% CI 0.70–1.02) in patients treated with rivaroxaban (45). There was no statistically significant difference between treatment groups with regard to rates of hospitalization (p=0.45) (46).
Subsequent substudies of the ROCKET-AF trial have evaluated treatment in specific patient groups of interest. In patients with renal impairment (creatinine clearance 30–49 mL/min), there was no difference in stroke or systemic embolism (HR 0.86; 95% CI 0.63–1.17) or major and nonmajor clinically relevant bleeding (p=0.76) between rivaroxaban and warfarin (47). Overall, with regard to the primary outcome of stroke or systemic embolism, there was no statistically significant interaction between treatment and history of stroke/TIA (p=0.23) (25); age (p=0.31) (48); diabetes (p=0.53) (49); concomitant aspirin therapy (p=0.95) (28); hypertension (p=0.06) (50); or history of heart failure (p=0.51) (51). With regard to major and nonmajor clinically relevant bleeding events, there was no statistically significant interaction between treatment and history of stroke/TIA (p=0.08) (25); age (p=0.34) (48); diabetes (p=0.17) (49); concomitant aspirin therapy (p=0.76) (28); hypertension (p=0.30) (50); or history of heart failure (p=0.99) (51).
Warfarin versus Edoxaban
The ENGAGE-AF trial (10) showed that both edoxaban 30mg daily and 60mg daily were noninferior to warfarin for the prevention of stroke or systemic embolism. Additionally, both doses of edoxaban reduced the risk of hemorrhagic stroke and the rates of major bleeding compared with warfarin. There was no difference in the risk of stroke, myocardial infarction, or all-cause mortality between groups. In a prespecified additional analysis (23), there was no statistically significant difference in nonfatal systemic embolic or fatal events between high- or low-dose edoxaban compared with warfarin. Overall, with regard to stroke or systemic embolism, there was no statistically significant interaction between treatment and renal function (p=0.94) (10); history of prior stroke (26); concomitant aspirin use (p=0.42) (29); history of heart failure (p=0.97) (52); or history of coronary artery disease (53). With regard to major bleeding, there was no statistically significant interaction between treatment and renal function (p=0.62) (10); concomitant aspirin use (p=0.59) (29); history of heart failure (p=0.96) (52); or history of coronary artery disease (53).
Factor Xa Inhibitors Compared with Dabigatran
No RCTs have directly compared Xa inhibitors with dabigatran although multiple observational studies have attempted to do so. Compared with treatment with dabigatran, patients treated with rivaroxaban or apixaban had a similar risk of stroke or systemic embolism (HR 1.00; 95% CI 0.75–1.32 for rivaroxaban vs. dabigatran; HR 0.82; 95% CI 0.51–1.31 for apixaban vs. dabigatran) (54); thromboembolic stroke (AHR 0.81; 95% CI 0.65–1.01) (55); ischemic stroke (HR 0.91; 95% CI 0.66–1.27 for rivaroxaban vs. dabigatran; HR 0.93; 95% CI 0.55–1.57 for apixaban vs. dabigatran) (54); or hemorrhagic stroke (HR 1.70; 95% CI 0.84–3.43 for rivaroxaban vs. dabigatran; HR 0.72; 95% CI 0.18–2.86 for apixaban vs. dabigatran).
Intracranial hemorrhage was evaluated in three observational studies which showed an increased risk of intracranial hemorrhage with Xa inhibitors as compared to dabigatran (HR 1.63, 95% CI 1.14–2.34). This finding was also found in the three studies that targeted rivaroxaban versus dabigatran (HR 1.75, 95% CI 1.34–2.28) (SOE low). Seven observational studies evaluated major bleeding for Xa inhibitors as compared to dabigatran which did not demonstrate a difference in major bleeding (HR 0.91, 95% CI 0.66–1.24) across all Xa inhibitors as compared to dabigatran. They did however demonstrate a reduction in major bleeding for apixaban (HR 0.67, 95% CI 0.47–0.94) as compared to dabigatran, while demonstrating an increase in major bleeding risk with rivaroxaban compared with dabigatran (HR 1.32, 95% CI 1.02–1.70) (SOE low for all comparisons). In regard to GI bleeding for Xa inhibitors as compared to dabigatran, there was no difference in GI bleeding seen (HR 0.84, 95% CI 0.47–1.49) across all Xa inhibitors as compared to dabigatran, nor for the three studies which focused on the comparison of rivaroxaban versus dabigatran (HR 1.09, 95% CI 0.63–1.88) (SOE low).
Factor Xa Inhibitors Compared with Another Factor Xa Inhibitor
No RCTs have compared the available factor Xa inhibitors. Several observational studies compared apixaban with rivaroxaban. There was no statistically significant difference between apixaban and rivaroxaban in the rates of stroke or systemic embolism (HR 1.05; 95% CI 0.64–1.72), ischemic stroke (HR 1.27; 95% CI 0.73–2.23) or hemorrhagic stroke (HR 0.66, 95% CI 0.16–2.78) (54). However, given the wide range in the confidence intervals available, a difference between the two treatments cannot be definitively excluded. With regard to bleeding outcomes, studies (54, 56–59) demonstrated a reduction in major bleeding with apixaban as compared to rivaroxaban (HR 0.51, 95% CI 0.38–0.68).
Left Atrial Appendage Closure Devices
Two RCTs, PROTECT AF (60) and PREVAIL (61), compared LAA closure with warfarin therapy. Overall, LAA closure showed a decrease in the rate of major bleeding compared to treatment with warfarin (3.5% vs. 4.1%) and was noninferior to warfarin with regard to stroke (RR 0.71; 95% CI 0.35–1.64) and all-cause mortality (RR 0.62; 95% CI 0.34–1.24). In the PREVAIL trial, there was no difference in the composite endpoint of stroke, systemic embolism, or cardiovascular/unexplained death (rate ratio 1.07 [95% credible interval (CrI) 0.57 to 1.89]) with LAA closure compared with warfarin. While LAA closure was associated with higher rates of adverse safety events overall (e.g., serious pericardial effusion, major bleeding, device embolization), there were fewer safety events in the PREVAIL trial compared with the earlier PROTECT AF study (4.2% vs. 8.7%; p=0.004).
The SOE for all comparisons and outcomes where the SOE was rated as low, moderate, or high are summarized graphically in Table 2; a detailed table of findings is provided Appendix Table 4.
Table 2.
Summary strength of evidence (SOE) ratings of interventions compared to warfarin
| Outcome | Comparator – Increase (↑) / Decrease (↓) / Similar (≈) Risk – compared to Warfarin | Strength of Evidence (SOE) |
|---|---|---|
| Factor IIa Inhibitor (Dabigatran 150 mg) vs. Warfarin | ||
| Stroke or systemic embolism | ↓ | SOE=High |
| Ischemic or uncertain stroke | ↓ | SOE=Low |
| Hemorrhagic stroke | ↓ | SOE=High |
| Major bleeding | ≈ | SOE=High |
| Minor bleeding | ↓ | SOE=Moderate |
| Intracranial bleeding | ↓ | SOE=High |
| GI bleeding | ↓ | SOE-Low |
| All-cause mortality | ≈ | SOE=Low |
| Death from vascular causes | ↓ | SOE=Moderate |
| Myocardial infarction | ≈ | SOE=Low |
| Hospitalization | ≈ | SOE=Moderate |
| Adverse events | Dyspepsia: ↑ Other adverse events: ≈ |
SOE=Moderate |
| Factor IIa Inhibitor (Dabigatran 110 mg) vs. Warfarin | ||
| Stroke or systemic embolism | ≈ | SOE=Moderate |
| Ischemic or uncertain stroke | ≈ | SOE=High |
| Hemorrhagic stroke | ↓ | SOE=High |
| Major bleeding | ↓ | SOE=High |
| Minor bleeding | ↓ | SOE=Moderate |
| Intracranial bleeding | ↓ | SOE=High |
| GI bleeding | ↓ | SOE=Low |
| All-cause mortality | ≈ | SOE=Low |
| Death from vascular causes | ≈ | SOE=Moderate |
| Myocardial infarction | ≈ | SOE=Low |
| Hospitalization | ↓ | SOE=High |
| Adverse events | Dyspepsia: ↑ Other adverse events: ≈ |
SOE=Moderate |
| Xa Inhibitor (Apixaban) vs. Warfarin | ||
| Stroke or systemic embolism | ↓ | SOE=High |
| Ischemic stroke | ≈ | SOE=High |
| Hemorrhagic stroke | ↓ | SOE=High |
| Systemic embolism | ≈ | SOE=Moderate |
| Major bleeding | ↓ | SOE=High |
| Intracranial bleeding | ↓ | SOE=High |
| GI bleeding | ↓ | SOE=Low |
| All-cause mortality | ↓ | SOE=Low |
| Death from cardiovascular causes | ≈ | SOE=Moderate |
| Myocardial infarction | ≈ | SOE=High |
| Adverse events | ≈ | SOE=Moderate |
| Xa Inhibitor (Rivaroxaban) vs. Warfarin | ||
| Stroke or systemic embolism | ≈ | SOE=Moderate |
| Ischemic stroke | ≈ | SOE=Moderate |
| Hemorrhagic stroke | ↓ | SOE=Low |
| Systemic embolism | ↓ | SOE=Moderate |
| Major bleeding | ≈ | SOE=Low |
| Intracranial bleeding | ↓ | SOE=High |
| GI bleeding | ↑ | SOE=Low |
| All-cause mortality | ≈ | SOE=Moderate |
| Death from cardiovascular causes | ≈ | SOE=Moderate |
| Myocardial infarction | ≈ | SOE=High |
| Medication adherence | ↓ | SOE=Moderate |
| Xa Inhibitor (Edoxaban 30 mg, 60 mg) vs. Warfarin | ||
| Stroke or systemic embolism | ≈ | SOE=Moderate |
| Ischemic stroke | 30 mg: ↑ 60 mg: ≈ |
SOE=Moderate |
| Hemorrhagic stroke | ↓ | SOE=Moderate |
| Systemic embolism | ≈ | SOE=Moderate |
| Major bleeding | ↓ | SOE=Moderate |
| Intracranial bleeding | ↓ | SOE=Moderate |
| All-cause mortality | 30 mg: ↓ 60 mg: ≈ |
SOE=Low SOE=Moderate |
| Death from cardiovascular causes | ↓ | SOE=Moderate |
| Myocardial infarction | ≈ | SOE=Moderate |
| Percutaneous LAA Closure vs. Warfarin | ||
| Ischemic stroke | ≈ | SOE=Low |
| All strokes | ≈ | SOE=Low |
| Major bleeding | ↓ | SOE=Low |
| All-cause mortality | ≈ | SOE=Low |
| Adverse events | ↑ | SOE=Moderate |
Abbreviations: LAA=left atrial appendage; mg=milligram; SOE=strength of evidence
Conclusion
AF is a widely prevalent atrial arrhythmia with significant morbidity and mortality (1, 62). Overall, our review had four key findings. First, there is evidence that, compared with warfarin, dabigatran and apixaban are superior and rivaroxaban and edoxaban are similar to warfarin in the prevention of stroke or systemic embolism. Second, apixaban and edoxaban are superior to warfarin in reducing the risk of major bleeding, while rivaroxaban and dabigatran are similar to warfarin in the rates of major bleeding. Third, consistent treatment effects for the factor Xa inhibitors versus warfarin were observed across many patient subgroups of interest including patients with a history of stroke, concomitant aspirin treatment, heart failure and duration of AF, among others. Finally, LAA closure devices decrease the risk of major bleeding and show a trend toward a lower risk of stroke, but with higher rates of procedural adverse events such as significant pericardial effusion, major bleeding, and device embolization.
There have been significant efforts to explore the comparative effectiveness of the DOAC medications with VKA therapy. Large RCTs have provided a foundation of evidence for each DOAC, and substudies of these trials examining specific patient groups of interest have largely corroborated results from the main trials. Additionally, a large number of substudies of these trials have now been completed to evaluate the efficacy and safety of the DOAC medications in patient subgroups. The results of the main trials remained consistent in these subgroups of interest. Notably, the relative risk of bleeding was reduced further by treatment with apixaban in patients with GFR<50 mL/min. Overall, this large body of evidence provides guidance toward the safety and efficacy of DOACs in the treatment of patients with a wide range comorbidities. The data for use of LAA closure devices is less robust but does give some direction in the treatment of patients at high risk for bleeding with anticoagulation.
Other reviews have also examined the comparative effectiveness of the available treatments for the prevention of thromboembolic disease in patients with AF. The most recent SR (63), examined all RCTs of DOACs, warfarin or antiplatelet therapies for AF. This network meta-analysis showed similar results to our study where the DOACs were generally superior to warfarin with regard to the prevention of stroke and in the reduced risk of major bleeding. Another network meta-analysis from 2016 also included evaluation of the Watchman device. Results showed that the DOACs and the Watchman device were most efficacious in preventing stroke or systemic embolism when compared to aspirin, VKA or placebo (64). Our review similarly summarizes the data from these RCTs but also provides data on the use of DOACs in unique patient populations and more recent observational data comparing DOACs to each other.
Several limitations of this review should be acknowledged. First, our review was limited to English-language publications. Cost analyses comparing treatment modalities were not specifically included in this review. However, costs related to health services utilization can be found in the full report. Next, the studies examined, particularly the observational analyses, were heterogeneous in patient populations, methodology, setting, and outcomes of interest; thus some direct comparisons could not be examined and limited our ability to perform a network meta-analysis. Additional information on the individual observational studies in terms of their characteristics and quality are included Appendix Table 3 and in the full SR. Also, observational studies are prone to selection bias and confounding that even the most robust statistical methods cannot fully adjust for. Additionally, in many of the sub-studies of the RCTs, the outcomes and patient populations were not pre-specified as analyses at the start of the RCT and thus these results do not provide the same SOE associated with the results from pre-specified endpoints and analyses. Finally, the majority of RCT data available compare new treatment options to warfarin—further high-quality evidence is needed to fully compare these treatments to each other. Unfortunately, given the unlikelihood that RCTs comparing DOACs will be completed, observational data comparing these medications may be the most thorough analysis available.
While the available evidence provides ongoing data for the treatment of patients with AF, there remain significant gaps that should be addressed by future research. There is currently only observational data comparing DOACs with each other; RCTs would provide a stronger base of evidence to guide patient care in the selection of which DOAC to use for treatment. Additionally, the shorter half-life of the DOACs compared to the anticoagulation effects of warfarin may lead to differential treatment effects when medication adherence is a concern. Similarly, data regarding persistence of therapy and transitions of therapies between anticoagulation strategies remain inadequate.
The development of DOACs and procedural treatment options have led to the availability of a wide range of treatments to prevent thromboembolic complications in patients with AF. The availability of evidence suggests that these DOACs are noninferior to warfarin in preventing thromboembolic complications and are generally superior to warfarin in reducing major bleeding events. Additionally, the current evidence holds promise that the treatment effects observed in the overall populations of the large RCTs holds true in many subsets of patients. LAA closure devices also hold promise for treatment of patients at high risk for bleeding complications. Ongoing analyses are needed to understand the treatment benefits of these newer treatment options against each other and the unique patient populations who may benefit most from treatment with each specific treatment modality.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Jamie Conklin, M.S.L.I.S., for help with the literature search and retrieval; Samantha E. Bowen, Ph.D., and Amanda J. McBroom, Ph.D., for assistance with project leadership; and Liz Wing, M.A., for editorial assistance.
DISCLAIMER
This project was funded under Contract No. HHSA-290-2015-00004-I from the Agency for Healthcare Research and Quality (AHRQ), U.S. Department of Health and Human Services. The authors of this manuscript are responsible for its content. Statements in the manuscript should not be construed as endorsement by the Patient-Centered Outcomes Research Institute (PCORI), AHRQ, and the U.S. Department of Health and Human Services. AHRQ retains a license to display, reproduce, and distribute the data and the report from which this manuscript was derived under the terms of the agency’s contract with the author.
Appendix
Appendix Figure 1. Analytic framework.
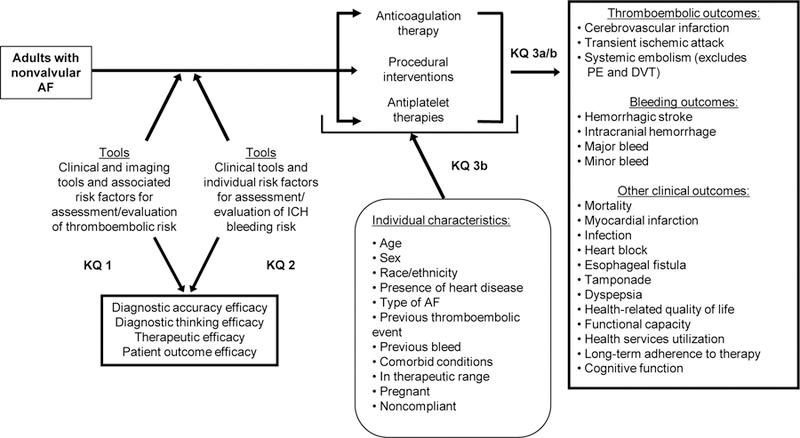
AF=atrial fibrillation; DVT=deep vein thrombosis; KQ=Key Question; ICH=intracranial hemorrhage; PE=pulmonary embolism
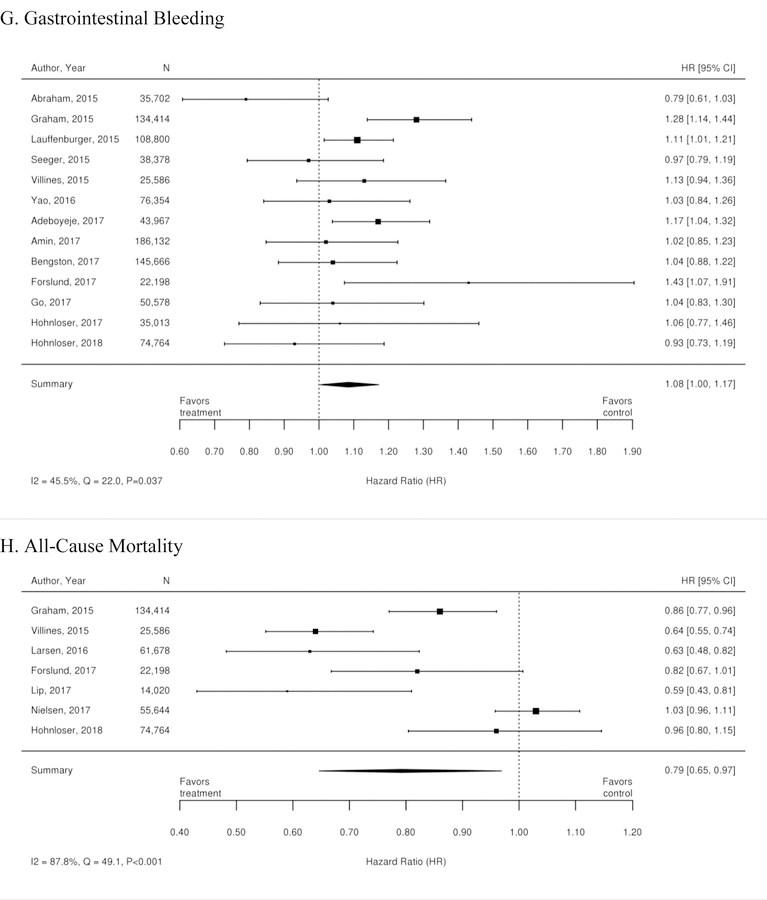
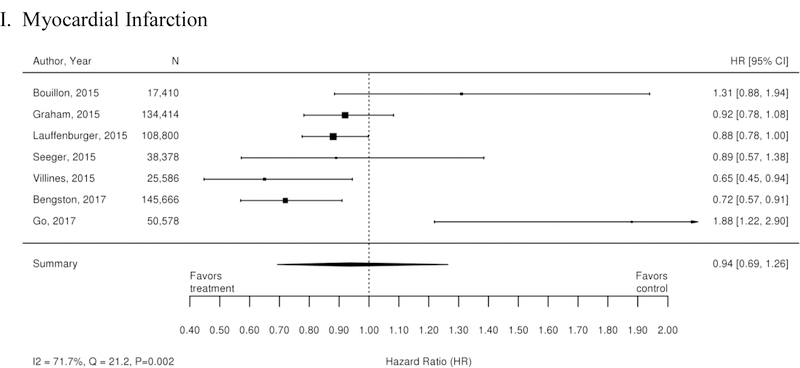
Appendix Figure 2. Forest plots for comparison of warfarin versus dabigatran (observational studies).
A. Ischemic or Hemorrhagic Stroke; B. Stroke or Systemic Embolism; C. Ischemic or Uncertain Stroke; D. Hemorrhagic Stroke; E. Major Bleeding; F. Intracranial Bleeding; G. Gastrointestinal Bleeding; H. All-Cause Mortality; I. Myocardial Infarction
CI=confidence interval
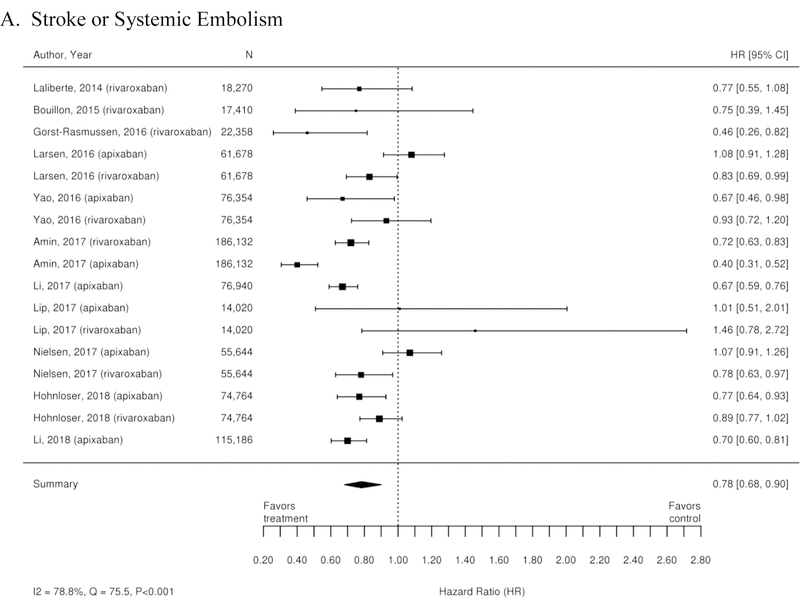
Appendix Figure 3. Forest plots for comparisons of factor Xa inhibitors versus warfarin (observational studies).
A. Stroke or Systemic Embolism; B. Ischemic or Uncertain Stroke; C. Hemorrhagic Stroke; D. Major Bleeding; E. Intracranial Bleeding; F. Gastrointestinal Bleeding; G. All-Cause Mortality
CI=confidence interval
REFERENCES
- 1.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114(7):e257–354. [DOI] [PubMed] [Google Scholar]
- 2.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. [DOI] [PubMed] [Google Scholar]
- 3.Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med. 2006;119(5):448 e1–19. [DOI] [PubMed] [Google Scholar]
- 4.Stewart S, Hart CL, Hole DJ, McMurray JJ. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med. 2002;113(5):359–64. [DOI] [PubMed] [Google Scholar]
- 5.Lee WC, Lamas GA, Balu S, Spalding J, Wang Q, Pashos CL. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ. 2008;11(2):281–98. [DOI] [PubMed] [Google Scholar]
- 6.Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology. 2003;22(2):118–23. [DOI] [PubMed] [Google Scholar]
- 7.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–4. [DOI] [PubMed] [Google Scholar]
- 8.Caro JJ. An economic model of stroke in atrial fibrillation: the cost of suboptimal oral anticoagulation. Am J Manag Care. 2004;10(14 Suppl):S451–58; discussion S8–61. [PubMed] [Google Scholar]
- 9.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. [DOI] [PubMed] [Google Scholar]
- 12.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. [DOI] [PubMed] [Google Scholar]
- 13.Lopes RD, Crowley MJ, Shah BR, Melloni C, Wood KA, Chatterjee R, et al. Stroke Prevention in Atrial Fibrillation. AHRQ Comparative Effectiveness Reviews. 2013. [PubMed]
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 15.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22(17):2693–710. [DOI] [PubMed] [Google Scholar]
- 16.Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions-Agency for Healthcare Research and Quality and the Effective Health Care Program. Journal of Clinical Epidemiology. 2010;63(5):513–23. [DOI] [PubMed] [Google Scholar]
- 17.Agency for Healthcare Research and Quality. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Available at: https://www.effectivehealthcare.ahrq.gov/topics/cer-methods-guide/overview. Accessed November 27, 2017. [PubMed] [Google Scholar]
- 18.Connolly SJ, Ezekowitz MD, Yusuf S, Reilly PA, Wallentin L. Newly identified events in the RE-LY trial. N Engl J Med. 2010;363(19):1875–6. [DOI] [PubMed] [Google Scholar]
- 19.Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129(9):961–70. [DOI] [PubMed] [Google Scholar]
- 20.Verdecchia P, Reboldi G, Angeli F, Mazzotta G, Lip GYH, Brueckmann M, et al. Dabigatran vs. warfarin in relation to the presence of left ventricular hypertrophy in patients with atrial fibrillation- the Randomized Evaluation of Long-term anticoagulation therapY (RE-LY) study. Europace. 2017. [DOI] [PMC free article] [PubMed]
- 21.Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, et al. The Long-Term Multicenter Observational Study of Dabigatran Treatment in Patients With Atrial Fibrillation (RELY-ABLE) Study. Circulation. 2013;128(3):237–43. [DOI] [PubMed] [Google Scholar]
- 22.Food and Drug Administration. FDA labeling for Pradaxa. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022512s028lbl.pdf. Accessed Septermber 21, 2018.
- 23.Geller BJ, Giugliano RP, Braunwald E, Murphy SA, Hanyok JJ, Jin J, et al. Systemic, noncerebral, arterial embolism in 21,105 patients with atrial fibrillation randomized to edoxaban or warfarin: results from the Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction Study 48 trial. Am Heart J. 2015;170(4):669–74. [DOI] [PubMed] [Google Scholar]
- 24.Easton JD, Lopes RD, Bahit MC, Wojdyla DM, Granger CB, Wallentin L, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11(6):503–11. [DOI] [PubMed] [Google Scholar]
- 25.Hankey GJ, Patel MR, Stevens SR, Becker RC, Breithardt G, Carolei A, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012;11(4):315–22. [DOI] [PubMed] [Google Scholar]
- 26.Rost NS, Giugliano RP, Ruff CT, Murphy SA, Crompton AE, Norden AD, et al. Outcomes With Edoxaban Versus Warfarin in Patients With Previous Cerebrovascular Events: Findings From ENGAGE AF-TIMI 48 (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Stroke. 2016;47(8):2075–82. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JH, Lopes RD, Thomas L, Alings M, Atar D, Aylward P, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2014;35(4):224–32. [DOI] [PubMed] [Google Scholar]
- 28.Shah R, Hellkamp A, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G, et al. Use of concomitant aspirin in patients with atrial fibrillation: Findings from the ROCKET AF trial. Am Heart J. 2016;179:77–86. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Ruff CT, Giugliano RP, Murphy SA, Nordio F, Patel I, et al. Concomitant Use of Single Antiplatelet Therapy With Edoxaban or Warfarin in Patients With Atrial Fibrillation: Analysis From the ENGAGE AF-TIMI48 Trial. J Am Heart Assoc. 2016;5(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hylek EM, Held C, Alexander JH, Lopes RD, De Caterina R, Wojdyla DM, et al. Major bleeding in patients with atrial fibrillation receiving apixaban or warfarin: The ARISTOTLE Trial (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation): Predictors, Characteristics, and Clinical Outcomes. J Am Coll Cardiol. 2014;63(20):2141–7. [DOI] [PubMed] [Google Scholar]
- 31.Bahit MC, Lopes RD, Wojdyla DM, Held C, Hanna M, Vinereanu D, et al. Non-major bleeding with apixaban versus warfarin in patients with atrial fibrillation. Heart. 2017;103(8):623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopes RD, Guimaraes PO, Kolls BJ, Wojdyla DM, Bushnell CD, Hanna M, et al. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation therapy. Blood. 2017;129(22):2980–7. [DOI] [PubMed] [Google Scholar]
- 33.Cowper PA, Sheng S, Lopes RD, Anstrom KJ, Stafford JA, Davidson-Ray L, et al. Economic Analysis of Apixaban Therapy for Patients With Atrial Fibrillation From a US Perspective: Results From the ARISTOTLE Randomized Clinical Trial. JAMA Cardiol. 2017;2(5):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. European Heart Journal. 2012. [DOI] [PubMed]
- 35.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34(31):2464–71. [DOI] [PubMed] [Google Scholar]
- 36.Guimaraes PO, Wojdyla DM, Alexander JH, Thomas L, Alings M, Flaker GC, et al. Anticoagulation therapy and clinical outcomes in patients with recently diagnosed atrial fibrillation: Insights from the ARISTOTLE trial. Int J Cardiol. 2017;227:443–9. [DOI] [PubMed] [Google Scholar]
- 37.Halvorsen S, Atar D, Yang H, De Caterina R, Erol C, Garcia D, et al. Efficacy and safety of apixaban compared with warfarin according to age for stroke prevention in atrial fibrillation: observations from the ARISTOTLE trial. Eur Heart J. 2014;35(28):1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu PT, Lopes RD, Stevens SR, Wallentin L, Thomas L, Alexander JH, et al. Efficacy and Safety of Apixaban Compared With Warfarin in Patients With Atrial Fibrillation and Peripheral Artery Disease: Insights From the ARISTOTLE Trial. J Am Heart Assoc. 2017;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Westenbrink BD, Alings M, Granger CB, Alexander JH, Lopes RD, Hylek EM, et al. Anemia is associated with bleeding and mortality, but not stroke, in patients with atrial fibrillation: Insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Am Heart J. 2017;185:140–9. [DOI] [PubMed] [Google Scholar]
- 40.Durheim MT, Cyr DD, Lopes RD, Thomas LE, Tsuang WM, Gersh BJ, et al. Chronic obstructive pulmonary disease in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Int J Cardiol. 2016;202:589–94. [DOI] [PubMed] [Google Scholar]
- 41.Vinereanu D, Stevens SR, Alexander JH, Al-Khatib SM, Avezum A, Bahit MC, et al. Clinical outcomes in patients with atrial fibrillation according to sex during anticoagulation with apixaban or warfarin: a secondary analysis of a randomized controlled trial. Eur Heart J. 2015;36(46):3268–75. [DOI] [PubMed] [Google Scholar]
- 42.Ezekowitz JA, Lewis BS, Lopes RD, Wojdyla DM, McMurray JJ, Hanna M, et al. Clinical outcomes of patients with diabetes and atrial fibrillation treated with apixaban: results from the ARISTOTLE trial. Eur Heart J Cardiovasc Pharmacother. 2015;1(2):86–94. [DOI] [PubMed] [Google Scholar]
- 43.Rao MP, Vinereanu D, Wojdyla DM, Alexander JH, Atar D, Hylek EM, et al. Clinical Outcomes and History of Fall in Patients with Atrial Fibrillation Treated with Oral Anticoagulation: Insights From the ARISTOTLE Trial. Am J Med. 2018;131(3):269–75.e2. [DOI] [PubMed] [Google Scholar]
- 44.Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, et al. Gastrointestinal Bleeding in Patients With Atrial Fibrillation Treated With Rivaroxaban or Warfarin: ROCKET AF Trial. J Am Coll Cardiol. 2015;66(21):2271–81. [DOI] [PubMed] [Google Scholar]
- 45.Mahaffey KW, Stevens SR, White HD, Nessel CC, Goodman SG, Piccini JP, et al. Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur Heart J. 2014;35(4):233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeVore AD, Hellkamp AS, Becker RC, Berkowitz SD, Breithardt G, Hacke W, et al. Hospitalizations in patients with atrial fibrillation: an analysis from ROCKET AF. Europace. 2016;18(8):1135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, et al. On-Treatment Outcomes in Patients With Worsening Renal Function With Rivaroxaban Compared With Warfarin: Insights From ROCKET AF. Circulation. 2016;134(1):37–47. [DOI] [PubMed] [Google Scholar]
- 48.Halperin JL, Hankey GJ, Wojdyla DM, Piccini JP, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban compared with warfarin among elderly patients with nonvalvular atrial fibrillation in the Rivaroxaban Once Daily, Oral, Direct Factor Xa Inhibition Compared With Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF). Circulation. 2014;130(2):138–46. [DOI] [PubMed] [Google Scholar]
- 49.Bansilal S, Bloomgarden Z, Halperin JL, Hellkamp AS, Lokhnygina Y, Patel MR, et al. Efficacy and safety of rivaroxaban in patients with diabetes and nonvalvular atrial fibrillation: the Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF Trial). Am Heart J. 2015;170(4):675–82.e8. [DOI] [PubMed] [Google Scholar]
- 50.Vemulapalli S, Hellkamp AS, Jones WS, Piccini JP, Mahaffey KW, Becker RC, et al. Blood pressure control and stroke or bleeding risk in anticoagulated patients with atrial fibrillation: Results from the ROCKET AF Trial. Am Heart J. 2016;178:74–84. [DOI] [PubMed] [Google Scholar]
- 51.van Diepen S, Hellkamp AS, Patel MR, Becker RC, Breithardt G, Hacke W, et al. Efficacy and safety of rivaroxaban in patients with heart failure and nonvalvular atrial fibrillation: insights from ROCKET AF. Circ Heart Fail. 2013;6(4):740–7. [DOI] [PubMed] [Google Scholar]
- 52.Magnani G, Giugliano RP, Ruff CT, Murphy SA, Nordio F, Metra M, et al. Efficacy and safety of edoxaban compared with warfarin in patients with atrial fibrillation and heart failure: insights from ENGAGE AF-TIMI 48. Eur J Heart Fail. 2016;18(9):1153–61. [DOI] [PubMed] [Google Scholar]
- 53.Zelniker TA, Ruff CT, Wiviott SD, Blanc JJ, Cappato R, Nordio F, et al. Edoxaban in atrial fibrillation patients with established coronary artery disease: Insights from ENGAGE AF-TIMI 48. Eur Heart J Acute Cardiovasc Care. 2018:2048872618790561. [DOI] [PubMed]
- 54.Noseworthy PA, Yao X, Abraham NS, Sangaralingham LR, McBane RD, Shah ND. Direct Comparison of Dabigatran, Rivaroxaban, and Apixaban for Effectiveness and Safety in Nonvalvular Atrial Fibrillation. Chest. 2016;150(6):1302–12. [DOI] [PubMed] [Google Scholar]
- 55.Graham DJ, Reichman ME, Wernecke M, Hsueh YH, Izem R, Southworth MR, et al. Stroke, Bleeding, and Mortality Risks in Elderly Medicare Beneficiaries Treated With Dabigatran or Rivaroxaban for Nonvalvular Atrial Fibrillation. JAMA Intern Med. 2016;176(11):1662–71. [DOI] [PubMed] [Google Scholar]
- 56.Lip GYH, Keshishian A, Kamble S, Pan X, Mardekian J, Horblyuk R, et al. Real-world comparison of major bleeding risk among non-valvular atrial fibrillation patients initiated on apixaban, dabigatran, rivaroxaban, or warfarin: A propensity score matched analysis. Thrombosis and Haemostasis. 2016;116(5):975–86. [DOI] [PubMed] [Google Scholar]
- 57.Lin J, Trocio J, Gupta K, Mardekian J, Lingohr-Smith M, Menges B, et al. Major bleeding risk and healthcare economic outcomes of non-valvular atrial fibrillation patients newly-initiated with oral anticoagulant therapy in the real-world setting. J Med Econ. 2017:1–10. [DOI] [PubMed]
- 58.Adeboyeje G, Sylwestrzak G, Barron JJ, White J, Rosenberg A, Abarca J, et al. Major Bleeding Risk During Anticoagulation with Warfarin, Dabigatran, Apixaban, or Rivaroxaban in Patients with Nonvalvular Atrial Fibrillation. J Manag Care Spec Pharm. 2017;23(9):968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Staerk L, Gerds TA, Lip GYH, Ozenne B, Bonde AN, Lamberts M, et al. Standard and reduced doses of dabigatran, rivaroxaban and apixaban for stroke prevention in atrial fibrillation: a nationwide cohort study. J Intern Med. 2018;283(1):45–55. [DOI] [PubMed] [Google Scholar]
- 60.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–42. [DOI] [PubMed] [Google Scholar]
- 61.Holmes DR Jr., Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1–12. [DOI] [PubMed] [Google Scholar]
- 62.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. [DOI] [PubMed] [Google Scholar]
- 63.Lopez-Lopez JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tereshchenko LG, Henrikson CA, Cigarroa J, Steinberg JS. Comparative Effectiveness of Interventions for Stroke Prevention in Atrial Fibrillation: A Network Meta-Analysis. J Am Heart Assoc. 2016;5(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.