Abstract
Background
The LRRK2 gene is associated with Parkinson's disease (PD) as a number of mutations within the gene have been shown to be susceptibility factors. Studies on various global populations have determined that mutations such as G2019S, G2385R, and R1628P in LRRK2 increase the risk of developing PD while the N551K‐R1398H haplotype is associated with conferring protection against developing PD. Here we report a study looking at the N551K and R1398H variants for the first time in the Malaysian population.
Methods
Cases (523) which conformed to the United Kingdom PD Brain Bank Criteria for PD were recruited through trained neurologists and age‐ and ethnically matched controls (491) were individuals free of any neurological disorder. The N551K and R1398H mutations were genotyped using the Taqman SNP genotyping assay.
Results
A significant protective association for N551K was found in those of Malay ancestry, with a protective trend seen for R1398H. A meta‐analysis of Chinese individuals in this cohort with other published cohorts of Chinese ancestry indicated a significant protective role for N551K and R1398H.
Conclusion
This study reports that the N551K‐R1398H haplotype is also relevant to the Malaysian population, with a significant protective effect found in those of Malay and Chinese ancestries.
Keywords: LRRK2, N551K, Parkinson's disease, R1398H
1. INTRODUCTION
Parkinson's disease (PD) is an age‐related neurodegenerative disease, caused by the loss of dopaminergic neurons in the substantia nigra pars compacta of the brain. The loss of the dopaminergic neurons leads to a range of movement problems including rigidity, bradykinesia, and tremors.
The leucine‐rich repeat kinase 2 (LRRK2) gene has been extensively studied in relation to both familial and sporadic forms of Parkinson's disease. The PD mutation database reports 127 mutations in this gene, with the G2019S mutation accounting for 40% of North African Arab and 20% of Ashkenazi Jewish PD cases (Ozelius et al., 2006). The G2019s mutation has been consistently shown to result in hyperactivation of the LRRK2 kinase, which has been associated with defects in protein synthesis and degradation, apoptosis, inflammatory responses, and oxidative damage (Rui et al., 2018; Smith et al., 2006). However, this mutation is almost completely absent in Asian PD populations studied thus far (Japanese, Chinese, and Koreans) (Bekris, Mata, & Zabetian, 2010; Guedes et al., 2010; Lesage et al., 2006). In contrast, the G2385R and R1628P mutations are relatively common in Asian PD patients, suggesting differing ethnic‐specific patterns of inheritance for LRRK2 mutations (Gopalai et al., 2014; Zhang et al., 2017).
The N551K and R1398H variants were first described in a PD study looking at linkage disequilibrium within LRRK2 (Paisan‐Ruiz et al., 2006). A multicenter case–control study suggested that individuals carrying the N551K (c.1653C > G, rs7308720) and R1398H (c.4193G > A, rs7133914) variants had a 20% reduced risk of developing PD (Ross et al., 2011). This was replicated in two Asian studies in Singapore and Taiwan (Tan et al.., 2010; Wu et al., 2013). These variants have not previously been screened in a Malaysian PD population and constitutes a significant gap in our understanding of the associated genetic factors in this population. With the advent of targeted therapies (Chan & Tan, 2017), an improved understanding of the genetic and mechanistic factors underlying PD is becoming increasingly important. Therefore, this study aimed to investigate the association between these protective alleles with the risk of PD in a multi‐ethnic Malaysian population.
2. MATERIALS AND METHODS
2.1. Sample recruitment and genetic analysis
A total of 523 PD cases and 491 controls were screened. The patients were recruited from neurology clinics throughout Peninsular Malaysia. PD patients were diagnosed based on the United Kingdom PD Brain Bank Criteria by movement disorder specialists or neurologists with an interest in PD. Control subjects were recruited from spouses and from outpatient clinics. The controls were age and gender‐matched and were not suffering from any neurological disorder. Institutional ethical approval was obtained and all participants provided written informed consent.
The N551K and R1398H variants were genotyped using TaqMan® SNP genotyping assays (Applied Biosystems) on a 7,500 Fast Real‐Time PCR machine. Genotypes were confirmed by sequencing in a subset of 20 individuals to confirm the genotypes and determine the error rate.
Statistical analysis was performed using open‐source software (OpenEpi) while Review Manager 5 (RevMan 5) (Collaboration, 2014) was used to conduct the meta‐analysis among the Chinese cohort. Heterogeneity among the studies was assessed with the I2 statistics (Higgins & Thompson, 2002).
3. RESULTS AND DISCUSSION
The mean age at PD diagnosis was 57.4 ± 11.8 years and the mean age of controls was 59.3 ± 9.4 years (p = 0.0048). Sixty per cent of PD patients and 51% of controls were male. The cohort consisted of 168 Malay PD cases, 133 Malay controls, 279 Chinese PD cases, 269 Chinese controls, 76 Indian PD cases, and 89 Indian controls.
Linkage analysis (Haploview 4.2) indicated that N551K and R1398H are in linkage disequilibrium (D’ = 0.959, r 2 = 0.906), similar to what has been reported by Tan et al., 2010 (Tan et al., 2010). Genotypes for both variants were in Hardy–Weinberg equilibrium. The error rate of the assay was 0%.
The mutant alleles for both variants were more frequent in the controls by almost twofold. An odds ratio (OR) of 0.623 (95% CI 0.44–0.88, p = 0.007) was obtained for N551K, and an OR of 0.699 (95% CI 0.50–0.98, p = 0.036) was obtained for R1398H, suggesting a reduced risk of developing PD in carriers (Table 1).
Table 1.
Details of genotypes and allele frequencies of N551K and R1398H by ethnicity
| PD cases | Controls | |||||
|---|---|---|---|---|---|---|
| Malay | Chinese | Indian | Malay | Chinese | Indian | |
| N551K (c.1653C>G), rs7308720 | ||||||
| Wild type (C/C) | 155 | 239 | 72 | 113 | 214 | 81 |
| Heterozygous mutant (C/G) | 13 | 38 | 4 | 18 | 54 | 8 |
| Homozygous mutant (G/G) | – | 2 | – | 2 | 1 | – |
| Allelic frequency (%) | ||||||
| Wild type (C) | 96.1 | 92.5 | 97.4 | 91.7 | 89.6 | 95.5 |
| Mutant (G) | 3.9 | 7.5 | 2.6 | 8.3 | 10.4 | 4.5 |
| R1398H (c. 4193G>A), rs7133914 | ||||||
| Wild type (G/G) | 155 | 233 | 71 | 115 | 215 | 80 |
| Heterozygous mutant (G/A) | 13 | 45 | 5 | 16 | 53 | 8 |
| Homozygous mutant (A/A) | – | 1 | – | 2 | 1 | 1 |
| Allelic frequency (%) | ||||||
| Wild type(G) | 95.6 | 91.5 | 96.7 | 92.5 | 89.8 | 93.6 |
| Mutant (A) | 4.4 | 8.6 | 3.3 | 7.5 | 10.2 | 5.4 |
When analyzed according to ethnicities, a protective association for N551K was detected in Malays (OR 0.446, 95% CI 0.22–0.90, p = 0.025). No significant difference was found between the Chinese case and controls (OR 0.700, 95% CI 0.46–1.07, p = 0.096). The Indian subgroup showed a similar, albeit nonsignificant trend (OR 0.574, 95% CI 0.17–1.95, p = 0.373), likely due to the small sample size. When we performed a meta‐analysis for N551K on the combined Chinese datasets from this study, and those by Tan et al. and Wu et al., the analysis showed a significant protective effect with an OR of 0.79 (95% CI 0.67‐0.92, p = 0.003 (Table 2, Figures 1a, 2a). No heterogeneity was detected amongst the studies included in the meta‐analysis for N551K (pheterogeneity = 0.57, I2 = 0%). Meta‐analysis on Malay and Indian samples was not performed as there are no other published studies on these populations for N551K.
Table 2.
Summary of published reports on genetic studies of N551K and R1398H in Parkinson's disease and meta‐analysis results
| Study | Country/Population | Sample size | Results |
|---|---|---|---|
| N551K (c.1653C>G), rs7308720 | |||
| Tan et al., 2010 (Han Chinese) | Singapore | 250 PD, 250 controls | OR 0.60 (p = 0.019) |
| Singapore | 192 PD, 192 controls | OR 0.92 (p = 0.757) | |
| Taiwan | 293 PD, 299 controls | OR 0.62 (p = 0.021) | |
| China | 628 PD, 510 controls | OR 0.91 (p = 0.570) | |
| Combined | 1363 PD, 1251 controls | OR 0.74 (p = 0.004) | |
| Ross et al., 2011 | Caucasian | 6995 PD, 5595 controls | OR 0.88 (p = 0.025) |
| Asian | 1376 PD, 962 controls | OR 0.73 (p = 0.0017) | |
| Arab‐Berber | 240 PD, 372 controls | OR 0.83 (p = 0.47) | |
| Wu et al., 2013 | Taiwan | 573 PD, 503 controls | OR 0.91 (p = 0.577) |
| Current study | Malaysia | 523 PD, 491 controls | OR 0.623 (p = 0.007) |
| Meta‐analysis on Chinese samples | This study, | 2215 PD, 2023 controls | OR 0.79 (p = 0.003) |
| Tan et al., and | |||
| Wu et al | |||
| R1398H (c. 4193G>A), rs7133914 | |||
| Tan et al., 2010 Han Chinese | Singapore | 250 PD, 250 controls | OR 0.64 (p = 0.038) |
| Singapore | 192 PD, 192 controls | OR 0.85 (p = 0.559) | |
| Taiwan | 293 PD, 299 controls | OR 0.64 (p = 0.033) | |
| China | 628 PD, 510 controls | OR 0.90 (p = 0.566) | |
| Combined | 1363 PD, 1251 controls | OR 0.75 (p = 0.005) | |
| Ross et al., 2011 | Caucasian | 6995 PD, 5595 controls | OR 0.89 (p = 0.034) |
| Asian | 1376 PD, 962 controls | OR 0.73 (p = 0.002) | |
| Arab‐Berber | 240 PD, 372 controls | OR 1.00 (p = 1.00) | |
| Wu et al., 2013 | Taiwan | 573 PD, 503 controls | OR 0.84 (p = 0.2418) |
| Wu‐Chou et al., 2013 | Taiwan | 519 PD, 434 controls | OR 0.89 (p = 0.45) |
| Current study | Malaysia | 523 PD, 491 controls | OR 0.699 (p = 0.036) |
| Meta‐analysis on Chinese samples | This study, | 2734 PD, 2457 controls | OR 0.81 (p = 0.002) |
| Tan et al., | |||
| Wu et al., and | |||
| Wu‐Chou et al. | |||
Figure 1.
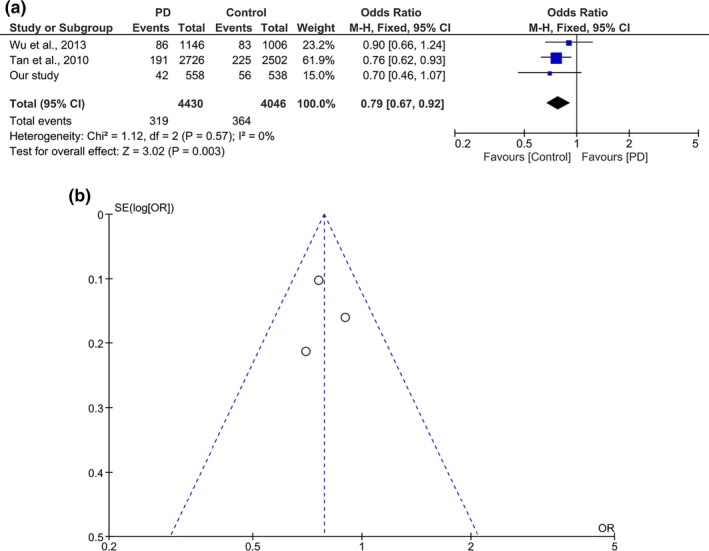
(a) Forest plot of pooled analysis for N551K among Chinese PD cohort. (b) Forest plot of pooled analysis for R1398H among Chinese PD cohort
Figure 2.
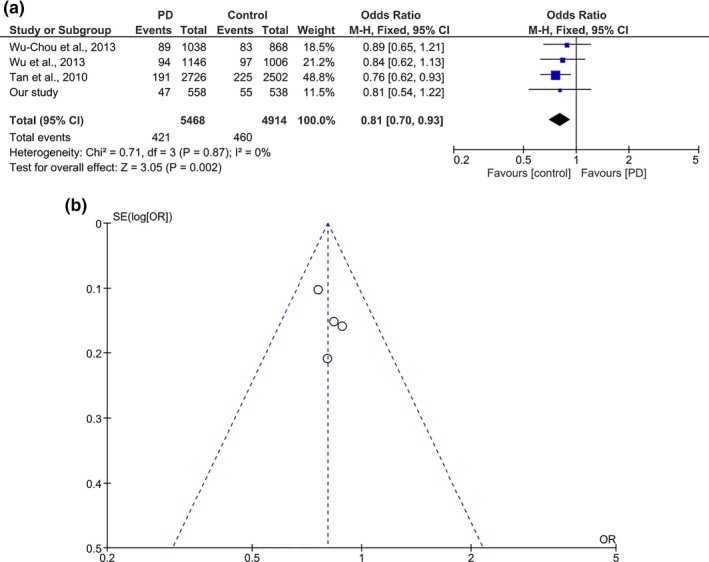
(a) Funnel plot of pooled analysis using fixed effect model for N551K among Chinese PD cohort. (b) Funnel plot of pooled analysis using fixed effect model for R1398H among Chinese PD cohort
When analyzing R1398H according to ethnicities, the protective association was detectable with borderline significance in Malays (OR 0.495, 95% CI 0.24–1.01, p = 0.055), but the results were not significant in the Chinese (OR 0.808, 95% CI 0.54–1.22 p = 0.306) and Indians (OR 0.571, 95% CI 0.19–1.71 p = 0.317). We performed a meta‐analysis of R1398H (combined Chinese datasets from this study, and those by Tan et al., Wu et al., and Wu‐Chou et al.) and this showed an OR of 0.81 (95% CI 0.70‐0.93, p = 0.002), (Table 2, Figures 1b, 2b). Similar to N551K, meta‐analysis on Malays and Indians was not possible as this is the first report on these populations for these variants. No heterogeneity was detected amongst the studies included in the meta‐analysis for R1398H (pheterogeneity = 0.87, I2 = 0%).
4. CONCLUSION
We have previously reported an association between PD in our Malaysian cohort with the risk alleles G2385R and R1628P within the LRRK2 gene (Gopalai et al., 2014). However, the association with the R1398H and N551K protective alleles have not previously been determined in this population. Here we report that N551K variant is associated in a protective manner in the Malay population, with the R1398H variant also showing a similar protective trend. N551K and R1398H showed a significant protective association when we pooled our Chinese cohort with other reported studies. The N551K‐R1398H association was unable to be detected in the Malaysian Indian cohort, likely due to the relatively small sample size.
The exact mechanism(s) underlying the protective effects of the N551K‐R1398H haplotype are not clear at present. The N551K variant is not within any domain of the LRRK2 protein, but is in linkage disequilibrium with the R1398H variant, which lies within the Ras‐of‐complex (ROC) GTPase domain, and enables the binding of guanine nucleotides via a phosphate‐binding motif.
The kinase activity of LRRK2 is modulated by GTPase activity, GTP hydrolysis, and GTP binding (Cookson, 2010). Pathogenic mutations in LRRK2 such as G2019S elevate the level of kinase activity (West et al., 2005), which in turn has been shown to cause neuronal toxicity (Smith et al., 2006). Lower levels of GTPase activity lead to a lower level of LRRK2 kinase activity (Biosa et al., 2012). Studies on R1398H have indicated that it plays a role in decreasing GTP‐bound LRRK2, in addition to positive effects on axon outgrowth and activation of associated Wnt signaling pathways (Nixon‐Abell et al., 2016). This may be one mechanism through which it may be conferring a protective effect on the cell.
Apart from having a protective effect in PD, another study investigated a possible link with rapid eye movement‐sleep behavior disorder (RBD), a condition now regarded as a prodromal symptom of synucleinopathies, most commonly PD. In a case–control study involving 350 RBD patients and 869 controls, the N551K‐R1398H haplotype was significantly associated with a reduced risk of developing RBD (Bencheikh et al., 2018). No association was found in studies of Alzheimer's disease patients (Ng, Ng, Tan, Kandiah et al., 2018) or essential tremor (Ng, Ng, Tan, Prakash et al., 2018).
In conclusion, we show that consistent with other published reports on the protective effect of N551K and R1398H, these variants are also protective in the Malaysian Malay and Chinese ethnicities. Further studies will need to be done to determine the cellular mechanism of how this protective effect is mediated.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
We thank the patients and controls for participating in the study. This study was funded by the Fundamental Research Grant Scheme (Malaysia) awarded to AAA (FP017); the University of Malaya High‐Impact Research (HIR) grant awarded to LSY (UM.0000017/HIR.C3) and grants from the National Medical Research Council and Singapore Millennium Foundation awarded to TEK.
Gopalai AA, Lim JL, Li H‐H, et al. LRRK2 N551K and R1398H variants are protective in Malays and Chinese in Malaysia: A case–control association study for Parkinson's disease. Mol Genet Genomic Med. 2019;7:e604 10.1002/mgg3.604
REFERENCES
- Bekris, L. M. , Mata, I. F. , & Zabetian, C. P. (2010). The genetics of Parkinson disease. Journal of Geriatric Psychiatry and Neurology, 23(4), 228–242. 10.1177/0891988710383572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bencheikh, B. O. A. , Ruskey, J. A. , Arnulf, I. , Dauvilliers, Y. , Monaca, C. C. , De Cock, V. C. , … Högl, B. (2018). LRRK2 protective haplotype and full sequencing study in REM sleep behavior disorder. Parkinsonism & Related Disorders, 52, 98‐101. [DOI] [PubMed] [Google Scholar]
- Biosa, A. , Trancikova, A. , Civiero, L. , Glauser, L. , Bubacco, L. , Greggio, E. , & Moore, D. J. (2012). GTPase activity regulates kinase activity and cellular phenotypes of Parkinson's disease‐associated LRRK2. Human Molecular Genetics, 22(6), 1140–1156. 10.1093/hmg/dds522 [DOI] [PubMed] [Google Scholar]
- Chan, S. L. , & Tan, E.‐K. (2017). Targeting LRRK2 in Parkinson's disease: An update on recent developments. Expert Opinion on Therapeutic Targets, 21(6), 601–610. 10.1080/14728222.2017.1323881 [DOI] [PubMed] [Google Scholar]
- Collaboration, C . (2014). Review Manager (RevMan)[Computer Program] Version 5.2. 3. Copenhagen: The Nordic Cochrane Centre; 2012. HEALTH PSYCHOLOGY REVIEW, 17. [Google Scholar]
- Cookson, M. R. (2010). The role of leucine‐rich repeat kinase 2 (LRRK2) in Parkinson's disease. Nature Reviews Neuroscience, 11(12), 791 10.1038/nrn2935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalai, A. A. , Lim, S.‐Y. , Chua, J. Y. , Tey, S. , Lim, T. T. , Mohamed Ibrahim, N. , Puvanarajah, S. D. (2014). LRRK2 G2385R and R1628P mutations are associated with an increased risk of Parkinson's disease in the Malaysian population. BioMed Research International, 2014, 1–4. 10.1155/2014/867321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes, L. C. , Ferreira, J. , Rosa, M. , Coelho, M. , Bonifati, V. , & Sampaio, C. (2010). Worldwide frequency of G2019S LRRK2 mutation in Parkinson's disease: A systematic review. Parkinsonism & Related Disorders, 16(4), 237–242. 10.1016/j.parkreldis.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Higgins, J. P. , & Thompson, S. G. (2002). Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine, 21(11), 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- Lesage, S. , Dürr, A. , Tazir, M. , Lohmann, E. , Leutenegger, A.‐L. , Janin, S. , … Brice, A. (2006). LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. New England Journal of Medicine, 354(4), 422–423. [DOI] [PubMed] [Google Scholar]
- Ng, A. S. , Ng, E. Y. , Tan, Y. J. , Kandiah, N. , Zhou, J. , Hameed, S. , Tan, E.‐K. (2018). Case‐control analysis of leucine‐rich repeat kinase 2 protective variants in Alzheimer's disease. Neurobiology of Aging, 64, 157.e7–157.e9. 10.1016/j.neurobiolaging.2017.11.012. [DOI] [PubMed] [Google Scholar]
- Ng, A. S. , Ng, E. Y. , Tan, Y. J. , Prakash, K. M. , Au, W. L. , Tan, L. C. , & Tan, E.‐K. (2018). Case‐control analysis of LRRK2 protective variants in Essential Tremor. Scientific Reports, 8(1), 5346 10.1038/s41598-018-23711-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon‐Abell, J. , Berwick, D. C. , Grannó, S. , Spain, V. A. , Blackstone, C. , & Harvey, K. (2016). Protective LRRK2 R1398H variant enhances GTPase and Wnt signaling activity. Frontiers in Molecular Neuroscience, 9, 18 10.3389/fnmol.2016.00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozelius, L. J. , Senthil, G. , Saunders‐Pullman, R. , Ohmann, E. , Deligtisch, A. , Tagliati, M. , … Hailpern, S. M. (2006). LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. New England Journal of Medicine, 354(4), 424–425. [DOI] [PubMed] [Google Scholar]
- Paisan‐Ruiz, C. , Evans, E. , Jain, S. , Xiromerisiou, G. , Gibbs, J. , Eerola, J. , … Papadimitriou, A. (2006). Testing association between LRRK2 and Parkinson's disease and investigating linkage disequilibrium. Journal of Medical Genetics, 43(2), e09–e09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, O. A. , Soto‐Ortolaza, A. I. , Heckman, M. G. , Aasly, J. O. , Abahuni, N. , Annesi, G. , … Brice, A. (2011). Association of LRRK2 exonic variants with susceptibility to Parkinson's disease: A case–control study. The Lancet Neurology, 10(10), 898–908. 10.1016/S1474-4422(11)70175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui, Q. , Ni, H. , Gao, F. , Dang, B. , Li, D. , Gao, R. , & Chen, G. (2018). LRRK2 contributes to secondary brain injury through a p38/Drosha signaling pathway after traumatic brain injury in rats. Frontiers in Cellular Neuroscience, 12, 51 10.3389/fncel.2018.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, W. W. , Pei, Z. , Jiang, H. , Dawson, V. L. , Dawson, T. M. , & Ross, C. A. (2006). Kinase activity of mutant LRRK2 mediates neuronal toxicity. NatureNeuroscience, 9(10), 1231 10.1038/nn1776 [DOI] [PubMed] [Google Scholar]
- Tan, E. K. , Peng, R. , Teo, Y. Y. , Tan, C. L. , Angeles, D., Ho, P., … Wu, R. M.(2010). Multiple LRRK2 variants modulate risk of Parkinson disease: A Chinese multicenter study. Human Mutation, 31(5), 561–568. [DOI] [PubMed] [Google Scholar]
- West, A. B. , Moore, D. J. , Biskup, S. , Bugayenko, A. , Smith, W. W. , Ross, C. A. , … Dawson, T. M. (2005). Parkinson's disease‐associated mutations in leucine‐rich repeat kinase 2 augment kinase activity. Proceedings of the National Academy of Sciences, 102(46), 16842–16847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Y.‐R. , Chang, K.‐H. , Chang, W.‐T. , Hsiao, Y.‐C. , Hsu, H.‐C. , Jiang, P.‐R. , … Lee, B.‐H. (2013). Genetic variants of LRRK2 in Taiwanese Parkinson's disease. PLoS ONE, 8(12), e82001 10.1371/journal.pone.0082001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Sun, Q. , Yi, M. , Zhou, X. , Guo, J. , Xu, Q. , … Yan, X. (2017). Genetic analysis of LRRK2 R1628P in Parkinson's disease in Asian populations. Parkinson's Disease, 2017, 1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]


