Abstract
Background:
Multiple androgens drive prostate cancer progression and higher pre-treatment levels of androgens, even within the castrate range, have been previously shown to be associated with an improved overall survival (OS) in mCRPC. Docetaxel impairs microtubules, has androgen receptor (AR) inhibitory effects and is used in both the castration resistant and sensitive settings, where androgen dynamics may impact outcome. The present analysis evaluates the association of decline in serum androgen levels (Testosterone (T), Androstenedione (A) and DHEA in docetaxel-treated mCRPC patients with OS.
Methods:
Data from 1,050 men treated on CALGB 90401 with docetaxel, prednisone and either bevacizumab or placebo were evaluated. Eligibility required progressive mCRPC and no prior chemotherapy. Pre-treatment, 6 week and progression serum assays for T, A and DHEA were performed via tandem Liquid Chromatography-Mass Spectrometry (LC-MS/MS). Changes in T, A and DHEA levels from baseline to 6 weeks were calculated as the ratio of 6-week over baseline. The proportional hazards model was used to assess the prognostic significance of changes in T, A, and DHEA from baseline to 6 weeks in predicting OS adjusting for known prognostic factors.
Results:
Median baseline values for T, A, and, DHEA were 1.0, 13.5 and 8.1, ng/dL respectively while 6 week levels were 0.64, 7.0 and 6.8, ng/dL respectively. Median OS for low testosterone decline is 20.9 months vs 26.3 months for high testosterone decline. In multivariable analysis including known prognostic variables, change in testosterone levels was independently associated with greater OS; the hazard ratio for death with each unit increase in the 6-week/baseline ratio is 1.02 (95% CI=1.01–1.03, p=0.001). Decline in A and DHEA were not significant predictors of OS. In multivariable analysis change in the serum changes did not predict PFS however the ratio of T at 6-weeks over baseline was prognostic of ≥50% decline in PSA with an odds ratio of 0.93 (95% CI=0.85–0.98, p-value=0.039).
Conclusions:
Declines in testosterone during docetaxel treatment is associated with a longer survival, consistent with a favorable prognostic significance of higher serum androgens in the CRPC.
Keywords: Androgens, Metastatic Castration-Resistant Prostate Cancer, Testosterone, Prognostic biomarker Docetaxel
Introduction:
A key characteristic of castration resistant prostate cancer is its continued reliance on androgen: androgen receptor (AR) interactions for tumor survival, proliferation and metastatic spread. Notably, this biological reliance occurs even in the setting of very low androgen levels likely through the combined effects of extragonadal and intracrine production of molecules with androgenic activity such as testosterone, DHEA and androstenedione [1] [11]. Yet the availability of androgens, coupled with an amplified or altered androgen receptor may lead to heightened sensitivity to pharmacologic androgen manipulation.
Prior work from our group has demonstrated that patients who have higher levels of serum androgens, even within the narrow “castrate” range of 0–50ng/dL, tend to survive longer, independent of the therapy they receive[2, 3]. The mechanism of this effect is not known and these associations have been made in the context of the evaluation of androgen lowering therapies such as ketoconazole plus hydrocortisone or abiraterone plus prednisone, in which it is reasonable to hypothesize that higher baseline androgens may exert their prognostic influence only insofar as they predict response to androgen lowering therapy.
In a recent analysis, we demonstrated that the prognostic significance of baseline serum androgens persists even in the context of docetaxel chemotherapy [10], a treatment not generally considered to have an influence on androgen levels. That analysis focused on the prognostic importance of androgen levels on docetaxel treatment, not the effect of docetaxel treatment on androgen levels. In the current analysis, we hypothesize that androgen dynamics and microtubule targeted chemotherapy may interact, and we evaluate the relationship of docetaxel chemotherapy on subsequent androgen levels and their relationship to overall survival, progression-frees survival and PSA decline from a cohort of patients treated on a randomized phase III clinical trial.
Methods:
Patient Population
Data from 1,050 men treated on CALGB 90401 with docetaxel, prednisone and either bevacizumab or placebo were used. Eligibility required progressive mCRPC and no prior chemotherapy. Pre-treatment, 6 week and progression serum assays for T, A and DHEA were performed via tandem Liquid Chromatography-Mass Spectrometry (LC-MS/MS) at a commercial laboratory (NMS labs, Willow Grove PA). Among these patients, serum samples were collected from 730 patients at baseline. Moreover, out of these 730 patients, serum were collected from 583 patients at 6 weeks (Figure 1).
Figure 1.
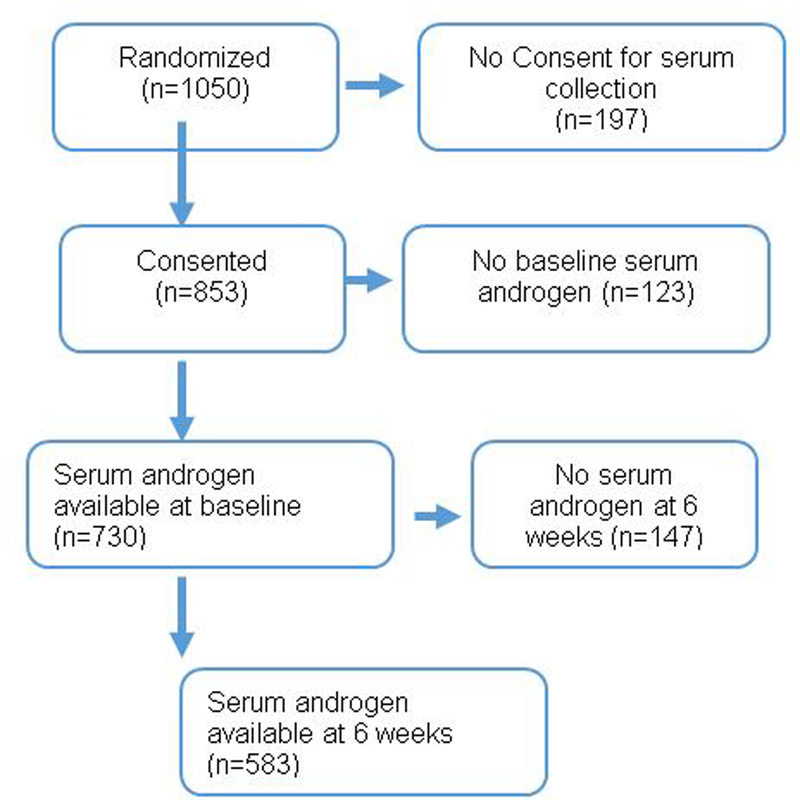
CONSORT Diagram
Data Analysis
The primary endpoint of this biomarker analysis is overall survival (OS) defined according the overall clinical trial protocol for CALGB 90401as survival from the date of randomization to death among dead patients. Secondary endpoints were progression-free survival (PFS) and ≥50% decline in PSA from baseline. PFS was defined from the date of randomization to progression or death, whichever occurred first. Progression was assessed using the PCWG2 soft tissue and bone scan criteria. PSA decline required confirmation of PSA value at least two weeks.
We compare the association of levels of testosterone, androstenedione and DHEA at baseline with their serum values at 6 weeks. The partially missing values of the serum androgens were treated as left-censored values and were imputed based on the other risk factors using the R software package zCompositions [9]. Changes in T, A and DHEA levels were calculated as the ratio of serum androgen levels measured at 6-week over serum androgen levels at baseline. The ratio decreases if the serum level at 6-weeks is lower than serum levels at baseline. Thus, the lower the ratio the greater is the decline in serum levels at 6-weeks from baseline. In addition, the ratio in each of T, A, and DHEA levels were dichotomized based on the median ratio into no/low-decline (ratio ≥ median ratio) or high-decline (ratio < median ratio).
The proportional hazards model was used to assess the prognostic significance of changes in T, A, and DHEA from baseline to 6 weeks in predicting OS and PFS adjusting for known prognostic factors: ECOG performance status, LDH greater than upper limit of normal (ULN), opioid analgesic use, disease site (defined as lymph node only, bone metastases with no visceral involvement, or any visceral metastases), hemoglobin, PSA, albumin, and alkaline phosphate. The Kaplan-Meier method was used to estimate OS and PFS distributions for the no/low-decline and the high-decline groups of patients. Lastly, the logistic regression was utilized to assess the prognostic significance of changes in T, A, and DHEA baseline from 6 weeks in predicting ≥50% decline in PSA from baseline
Results
Table 1 compares the baseline characteristics of patients having serum androgen values at both baseline and 6-weeks with patients having only baseline serum values. At baseline, serum levels were below the limit of detection (LOD) in 39% of T samples, 19% of A samples, and 57% of DHEA samples. While at 6-weeks post-randomization, 69% of T samples, 30% of A samples, and 79% of DHEA samples were below LOD. Overall, there were no differences between the baseline characteristics of these two groups of patients. The median baseline values for T, A, and, DHEA were 1.0, 13.5 and 8.1, ng/dL respectively – all within the range of eligibility and below the ‘castrate’ threshold. The median androgen levels at 6 weeks post randomization were 0.64, 7.0 and 6.8, ng/dL respectively. The median ratio for T, A, and DHEA were 0.93, 0.56 and 0.86 respectively. We present the box plots of T, A, and DHEA at baseline and 6 weeks for both the placebo and bevacizumab arms (Figure 2). There is an overall trend of decline in each of T, A, and DHEA, and this is clearly evident in both arms.
Table 1:
Baseline characteristics of patients with missing and available serum androgens at 6 weeks
| Characteristic | Patients with SA at baseline and 6-weeks (n=583) |
Patients with SA at baseline only (n=147) |
|---|---|---|
| Median Age in years (range) | 69 (42–93) | 68 (42– 93) |
|
Race White Others |
515 (88%) 68 (12%) |
126 (86%) 21 (14%) |
|
Opioid Analgesic use Yes No Missing |
187 (32%) 325 (56%) 71 (12%) |
39 (26%) 48 (33%) 60 (41%) |
| LDH > 1 ULN | 208 (36%) | 61 (41%) |
|
Performance Status 0 1 2 |
333 (57%) 233 (40%) 17 (3%) |
81 (55%) 58 (40%) 8 ( 5%) |
|
Disease Site Lymph Node Bone/ Bone + Lymph Node Any Visceral |
62 (11%) 425 (73%) 96 (16%) |
19 (13%) 102 (69%) 26 (18%) |
|
Hemoglobin
Median 25th, 75th percentile |
12.7 11.7,13.8 |
12.9 11.4, 13.8 |
|
Alkaline Phosphatase U/L
Median 25th, 75th percentile |
112 82.0, 195.0 |
149 84.0, 299.5 |
|
Albumin g/dL Median 25th, 75th percentile |
4.0 3.7, 4.2 |
4.0 3.6, 4.3 |
|
PSA ng/ml
Median 25th, 75th percentile |
72.6 26.8, 212.0 |
84.8 33.4, 226.7 |
|
Treatment Arm D/P+B D/P+ Placebo |
283 (49%) 300 (51%) |
79 (54%) 68 (46%) |
Figure 2.
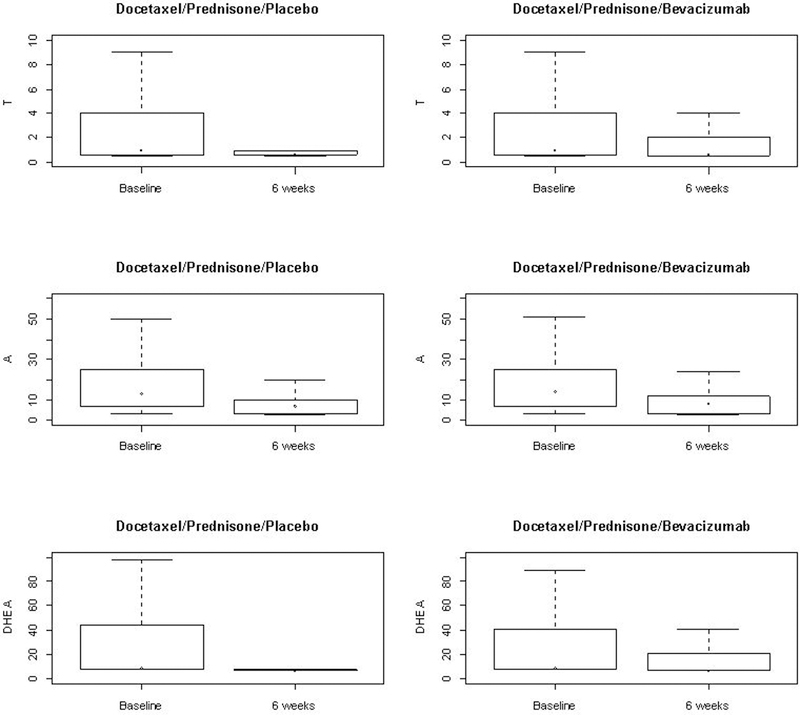
Serum androgens at baseline and 6 weeks by Treatment Arm
Table 2 provides a 3-way classification of patients based on their T, A and DHEA levels. In patients who had T values below LOD at baseline, 76% had T values below LOD at 6-weeks. On the other hand, 73% of patients with T levels ≤3 ng/dL at baseline but their T levels were below LOD at 6-weeks. Among patients who had their baseline T values above 3 ng/dL, 24% of them had their T values decreased to below 3 ng/dL while 52% declined below the LOD.
Table 2:
Association of serum levels at baseline with serum levels at 6 weeks
| Testosterone at 6-weeks | ||||
| Baseline T | Below LOD | ≤3 | >3 | |
| Below LOD | 179 (76%) | 39 (17%) | 18 (7%) | |
| ≤3 | 152 (73%) | 33 (16%) | 24 (11%) | |
| >3 | 71 (52%) | 33 (24%) | 34 (24%) | |
| Androstenedione at 6-weeks | ||||
| Baseline A | Below LOD | ≤17 | >17 | |
| Below LOD | 38 (37%) | 60 (58%) | 6 (5%) | |
| ≤17 | 81 (31%) | 151 (58%) | 30 (11%) | |
| >17 | 53 (24%) | 134 (62%) | 30 (14%) | |
| DHEA at 6-weeks | ||||
| Baseline DHEA | Below LOD | ≤51 | >51 | |
| Below LOD | 289 (85%) | 37 (11%) | 13 (4%) | |
| ≤51 | 95 (74%) | 28 (21%) | 6 (5%) | |
| >51 | 73 (64%) | 25 (22%) | 17 (15%) | |
In patients who had A values below LOD at baseline, 37% had values below LOD and 58% had values ≤17 ng/dL at 6-weeks. In patients with A levels ≤17 ng/dL at baseline, 31% had their A levels below LOD at 6-weeks. Among patients who had their baseline A values above 17 ng/dL, 62% of them had A values that decreased to below 17 ng/dL while 24% declined below LOD.
In patients who had DHEA values below LOD at baseline, 85% had values below LOD and 11% had values ≤51 ng/dL at 6-weeks. In patients with DHEA levels ≤51 ng/dL at baseline, 74% had their DHEA levels below LOD at 6-weeks. Among patients who had their baseline DHEA values above 51 ng/dL, 22% of them had their DHEA values decreased to ≤51 ng/dL while 64% declined below LOD.
Clinical Outcomes
Overall Survival
The median OS for the entire study cohort was 22 months. Figures 3A–3C present the overall survival Kaplan-Meier by high and low/no decline for the three serum androgens. Patients with high-decline in T (greater decline than the median) had higher median survival duration of 26.3 months (95%CI=23.4–28.1) compared with patients with a median of 20.9 months in no/low-decline in T (95%CI=19.3–23.4, p=0.003, Figure 3A). On the other hand, the median OS for high-decline in A was 23.3 months (95% CI= 21.0–26.3) compared with median OS =23.1 in no/low-decline in A (95% CI= 21.1–26.7, p=0.163, Figure 3B). Patients with high-decline in DHEA had higher median OS of 26.3 months (95% CI=23.4–28.5) compared to median OS =20.4 in patients with no/low-decline in DHEA (95% CI= 19.4–23.4, p=0.07 Figure 3C). In multivariable analysis adjusting for known prognostic variables, decline in T levels was independently associated with a decreased hazard of death. The hazard ratio for death with a unit increase in the 6-week/baseline ratio is 1.02 (95% CI=1.01–1.03, p=0.001 Table 3).
Figure 3.
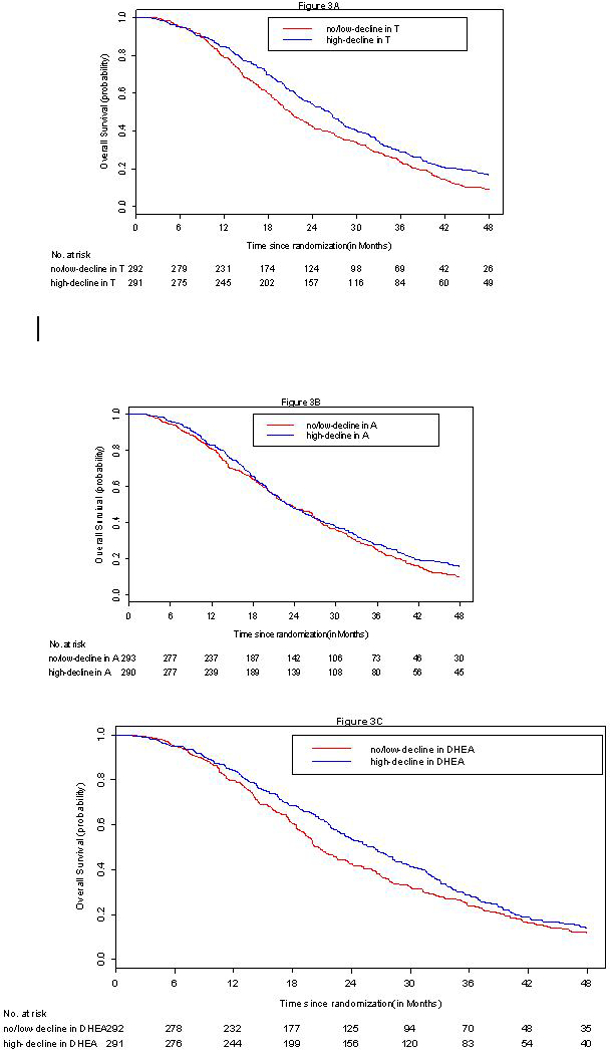
A-3C Kaplan Meier overall survival curves by change in serum androgen at 6-weeks from baseline.
Table 3:
Proportional hazards model with change in serum androgen predicting overall survival
| Variable | Hazard Ratio (95% CI) |
p-value |
|---|---|---|
| LDH> 1ULN (Yes vs. no) |
1.48 (1.20–1.83) | <0.001 |
| Opioid use (Yes vs. no) |
1.22 (0.99–1.50) | 0.063 |
| Performance status (1,2 vs. 0) |
1.30(1.09–1.54) | 0.003 |
| Bone/Bone+LN vs LN | 1.41 (1.02–1.95) | 0.041 |
| Any visceral site vs LN | 1.59 (1.09–2.23) | 0.015 |
| PSA | 1.00 (1.00–1.00) | <0.001 |
| Hemoglobin | 0.89(0.84–0.95) | <0.001 |
| Alkaline Phosphate | 1.00 (1.00–1.00) | 0.847 |
| Albumin | 1.00 (0.92–1.08) | 0.909 |
| T change (6-weeks/Baseline ratio) |
1.02 (1.01–1.03) | 0.001 |
| A change (6-weeks/Baseline ratio) |
0.97 (0.90–1.05) | 0.475 |
| DHEA change (6-weeks/Baseline ratio) |
0.97 (0.93–1.02) | 0.224 |
In an exploratory analysis, the greatest survival difference observed (26.6 mo vs 21.7 mo) is between patients who converted from a ‘high’ Testosterone (above the median) to a low (below the median) testosterone level. Notably, patients experiencing the change of high to low had inferior survival to those converting from low to high ( 26.0 months) or those remaining above the median (24.9 months). Similar patterns were observed with other androgens. Statistical analysis of these results is not appropriate due to their exploratory nature and the fact that many values subsequent values were below the limits of detection. (Supplemental Table 1)
Progression-free Survival
The median PFS for high-decline in T was 9.2 months (95% CI = 8.2–10.1) and 8.5 months in no/low-decline in T (95% CI = 7.9–9.5, Figure 4A). For high-decline in A, the median PFS is 9.5 months (95% CI =8.2–10.5) while median the PFS for no/low-decline in A was 8.5 months (95% CI = 8–9.2, Figure 4B). The median PFS for high-decline in DHEA was 9.1 months (95% CI=8.1–10.3), while the median PFS is 8.7 months (95% CI = 7.8–9.7) for no/low-decline in DHEA. In multivariable analysis, change in the serum changes did not predict PFS (Table 4).
Figure 4.
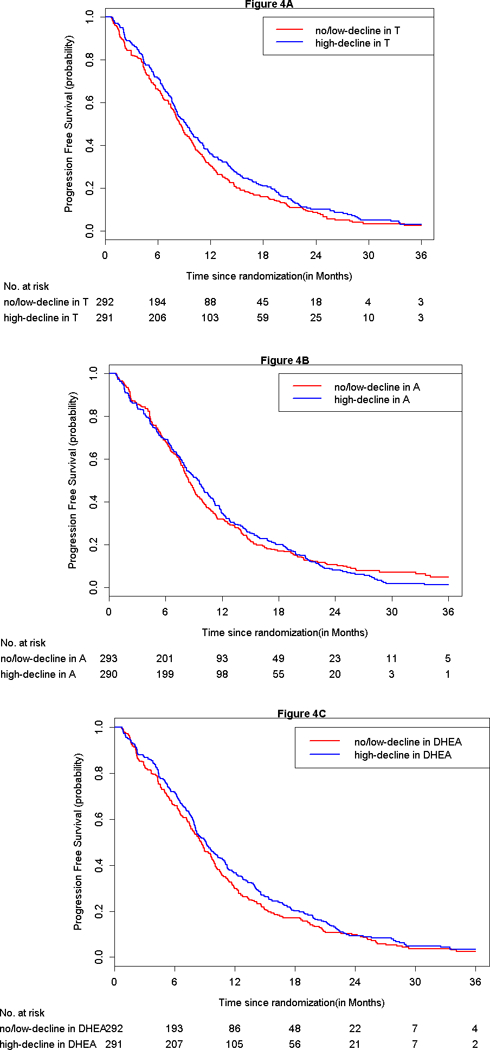
A-AC: Kaplan Meier progression-free survival curves by change in serum androgen at 6-weeks from baseline.
Table 4:
Proportional hazards model with change in serum androgen predicting progression-free survival
| Variable | Hazard Ratio (95% CI) | p-value |
|---|---|---|
| LDH> 1ULN (yes vs. no) |
1.38 (1.13–1.70) | 0.002 |
| Opioid use (yes vs. no) |
1.09 (0.88–1.33) | 0.435 |
| Performance status (1,2 vs. 0) |
1.09 (0.92–1.29) | 0.340 |
| Bone/Bone+LN vs LN | 1.19 (0.85–1.65) | 0.306 |
| Any visceral site vs LN | 1.37 (0.94–2.00) | 0.101 |
| PSA | 1.00 (1.00–1.00) | 0.004 |
| Hemoglobin | 0.96 (0.90–1.02) | 0.255 |
| Alkaline Phosphate | 1.00 (1.00–1.027) | 0.607 |
| Albumin | 1.00 (0.94–1.07) | 0.954 |
| T change (6-weeks/Baseline ratio) |
1.01 (1.00–1.02) | 0.128 |
| A change (6-weeks/Baseline ratio) |
0.93 (0.85–1.01) | 0.089 |
| DHEA change (6-weeks/Baseline ratio) | 0.98 (0.93–1.04) | 0.585 |
PSA decline
Higher decline in T and DHEA levels are significantly associated with higher proportion of PSA response in univariate analysis (Table 5). In multivariable analysis (Table 6), only the ratio of T at 6-weeks over baseline was prognostic of ≥50% decline in PSA with an odds ratio of 0.93 (95% CI=0.85–0.98, p-value=0.039, Table 6). Thus, higher decline in T (lower T ratio of 6-weeks over baseline) is associated with higher likelihood of PSA response.
Table 5:
Association of Change in Serum Androgen with ≥50% decline in PSA
| <50% Decline in PSA (n=164) |
≥50% Decline in PSA (n=418) |
|
|---|---|---|
| T Levels | ||
| High-decline | 66 (40%) | 226 (54%) |
| No/low- Decline | 98 (60%) | 192 (46%) |
| A levels | ||
| High-decline | 77 (47%) | 215 (51%) |
| No/low- Decline | 87 (53%) | 203 (49%) |
| DHEA levels | ||
| High-decline | 64 (39%) | 227 (54%) |
| No/low- Decline | 100 (61%) | 191 (46%) |
Table 6:
Logistic regression model with change in serum androgen predicting ≥50% Decline in PSA
| Variable | Odds Ratio (95% CI) | p-value |
|---|---|---|
| LDH> 1ULN (yes vs. no) |
0.71 (0.45–1.11) | 0.131 |
| Opioid use (yes vs. no) |
0.93 (0.59–1.47) | 0.742 |
| Performance status (1,2 vs. 0) |
0.96 (0.65–1.42) | 0.827 |
| Bone/Bone+LN vs LN | 0.53 (0.22–1.17) | 0.138 |
| Any visceral site vs LN | 0.50 (0.19–1.24) | 0.147 |
| PSA | 1.00 (1.00–1.00) | 0.172 |
| Hemoglobin | 1.17 (1.02–1.35) | 0.029 |
| Alkaline Phosphate | 1.00 (1.00–1.00) | 0.677 |
| Albumin | 0.96 (0.84–1.08) | 0.420 |
| T change (6-weeks/Baseline ratio) |
0.93 (0.85–0.98) | 0.039 |
| A change (6-weeks/Baseline ratio) |
1.07 (0.90–1.29) | 0.492 |
| DHEA change (6-weeks/Baseline ratio) | 1.01 (0.91–1.15) | 0.807 |
Discussion
In the current study we demonstrate that androgen levels decline in some patients during treatment with docetaxel. Further, we show that a greater decline in androgens during docectaxel therapy is associated with a greater overall survival. This result is notable for the fact that treatment with Docetaxel or Docetaxel plus bevacizumab is not expected to lower androgen levels, as would be the case with androgen synthesis inhibitor therapies like abiraterone.
Although further work will be required to address the underlying mechanisms of this phenomenon, certain facets of these data allow for hypothesis generation. The androgen receptor relies on microtubules for transport from the cytoplasm to the nucleus following ligand stimulation. Via microtubule stabilization, docetaxel impairs androgen transport after ligand activation, thus docetaxel’s activity in CRPC can be attributed in part to its effect on AR transport. These observations suggest dual effects of androgen decline and microtubule targeting, when they occur, may convey additive, if not synergistic, benefits.
The concurrent use of corticosteroids in docetaxel treated patients may contribute to the decline in levels of T, DHEA and androstenedione. Indeed, in several randomized phase III studies in CRPC, prednisone has emerged as a key component of the outcome. In one study, COU-302, abiraterone plus prednisone was compared to prednisone to placebo, in that study, approximately 25% of patients experienced a 50% decline in PSA and the overall survival of this control arm was substantially longer than that observed in the PREAVAIL study that compared Enzalutamide to placebo, wherein the response proportion to placebo was 3%[4][5]. In the control arm of the COU-301 study Abiraterone plus prednisone was compared to prednisone plus placebo in post docetaxel patients. In the prednisone alone arm testosterone, androstenedione and DHEAS declined by 49%, 20% and 48%, respectfully. Abiraterone plus prednisone, incidentally, lowered the androgens by 90%,92% and 86%, respectively. It is not known why, in the current study, Androstenedione decline is less that Testosterone decline although this mirrors the observations with prednisone alone. [14]
Why some patients experience responses to corticosteroids while others do not is not known, nor is the spectrum of decline in androgens that will result from standard doses of corticosteroids. Given our data, it is plausible that patients with a greater degree of corticosteroid-induced decline in Testosterone may be those experiencing the prolonged survival.
Recently published data suggest that polymorphisms in critical androgen synthesis enzymes may mediate response to ADT and ketoconazole, an agent with known androgen synthesis inhibitor effects that was in widespread clinical use in the US prior to the development of abiraterone [6] [12, 13]. Such polymorphisms could also mediate greater than expected responses to even low dose prednisone. Further work by our group will explore these relationships.
In the multivariable analysis, a decline in testosterone was independently associated with an improvement in survival, adding to a predictive model for mCRPC[7]. In prior analysis, we have speculated that the prognostic improvement in the setting of higher androgens arose from the fact that tumors evolving in the context of very low androgens were more virulent, but left open the question of whether or not higher androgens are more protective. Herein we suggest that this relationship is not static and that clinical improvement may in fact be a result of, if not dependent on, androgen suppression during treatment. The plausibility of this arises from the significant clinical activity of therapies like abiraterone, where the clinical benefits are typically, but not always, proportional to degrees of androgen decline [8].
Further mechanistic work is clearly required, as is a more in-depth analysis of androgen metabolism factors. Our study is presented with a full recognition of limitations. We do not have data, for example, on adherence to prednisone and its impact on androgen levels. The mechanism by which docetaxel impact androgen levels remains a subject of investigation.
Conclusion
These data, from a randomized phase III study, demonstrate that a decline in testosterone during docetaxel treatment is associated with more survival that is favourable. Further studies are underway to demonstrate the mechanistic ramifications of these findings and how they can integrated into clinical decision-making.
Supplementary Material
Acknowledgments
Support: R21 CA195424–01, U10CA180821, U10CA180882, W81XWH-15-1-0467; P30 CA 008748
Footnotes
Supplementary information is available at PCAN’s website
The authors declare no potential conflicts of interest.
References:
- 1.Montgomery RB, et al. , Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res, 2008. 68(11): p. 4447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan CJ, et al. , Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: results from a cancer and leukemia group B study. Clin Cancer Res, 2007. 13(7): p. 2030–7. [DOI] [PubMed] [Google Scholar]
- 3.Ryan CJ, et al. , Serum androgens as prognostic biomarkers in castration-resistant prostate cancer: results from an analysis of a randomized phase III trial. J Clin Oncol, 2013. 31(22): p. 2791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer TM, et al. , Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med, 2014. 371(5): p. 424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathkopf DE, et al. , Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol, 2014. 66(5): p. 815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hearn JWD, et al. , HSD3B1 and resistance to androgen-deprivation therapy in prostate cancer: a retrospective, multicohort study. Lancet Oncol, 2016. 17(10): p. 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halabi S, et al. , Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol, 2014. 32(7): p. 671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan CJ, et al. , Androgen dynamics and serum PSA in patients treated with abiraterone acetate. Prostate Cancer Prostatic Dis, 2014. 17(2): p. 192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palarea-Albaladejo J, & Martín-Fernández JA. zCompositions—R package for multivariate imputation of left-censored data under a compositional approach. Chemometrics and Intelligent Laboratory Systems, 2015;143, 85–960) [Google Scholar]
- 10.Ryan et al Androgens and Overall Survival in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Docetaxel ( Submitted)
- 11.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, et al. ,, .Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008. August 1;68(15):6407–15. [DOI] [PubMed] [Google Scholar]
- 12.Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, Gable P, et al. , ,. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004. March 15;22(6):1025–33. [DOI] [PubMed] [Google Scholar]
- 13.Small EJ, Baron AD, Fippin L, Apodaca D. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997. April;157(4):1204–7. [PubMed] [Google Scholar]
- 14., Ryan CJ, Peng W, Kheoh T, Welkowsky E, Haqq CM, Chandler DW, et al. , Androgen dynamics and serum PSA in patients treated with abiraterone acetate. Prostate Cancer Prostatic Dis. 2014. June;17(2):192–8. doi: 10.1038/pcan.2014.8. Epub 2014 Mar 18. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


