Abstract
Introduction:
The TF-FVIIa complex is the primary activator of coagulation. Elevated levels of microvesicle (MV) bearing tissue factor (TF)-dependent procoagulant activity are detectable in patients with an increased risk of thrombosis. Several methods have been described to measure MV TF activity but they are hampered by limited sensitivity and specificity. The aim of this work was to increase the sensitivity of the MV TF activity assay (called Chapel Hill assay).
Material and Methods:
Improvements of the MV TF activity assay included i/ speed and time of centrifugation, ii/ use of a more potent inhibitory anti-TF antibody iii/ use of FVII and a fluorogenic substrate to increase specificity
Results:
The specificity of the MV TF activity assay was demonstrated by the absence of activity on MV derived from a knock-out-TF cell line using an anti-human TF monoclonal antibody called SBTF-1, which shows a higher TF inhibitory effect than the anti-human TF monoclonal antibody called HTF-1. Experiments using blood from healthy individuals, stimulated or not by LPS, or plasma spiked with 3 different levels of MV, demonstrated that the new assay was more sensitive and this allowed detection of MV TF activity in platelet free plasma (PFP) samples from healthy individuals. However, the assay was limited by an inter-assay variability, mainly due to the centrifugation step.
Conclusions:
We have improved the sensitivity of the MV TF activity assay without losing specificity. This new assay could be used to evaluate levels of TF-positive MV as a potential biomarker of thrombotic risk in patients.
Keywords: microvesicles, extracellular vesicles, tissue factor, procoagulant test
Introduction
Microvesicles (MV) are extracellular vesicles released from the cellular membrane which have been described as procoagulant entities since their first report by Peter Wolf, 50 years ago [1]. This procoagulant phenotype relies on the exposure of anionic phospholipids, especially the phosphatidylserine (PS) on the external leaflet of the membrane, allowing the binding of coagulation factors at the MV surface by their carboxylglutamic acid-rich (GLA)-domains [2]. In addition, the presence of the coagulation initiator tissue factor (TF) on subsets of MV also significantly contributes to their procoagulant activity. Different studies that infused MV into mouse models of venous or arterial thrombosis demonstrate the procoagulant activity of MV in vivo [3–5]. Special attention has been given to cancer-associated thrombosis and the underlying mechanisms linking MV and venous thromboembolism (VTE) [6,7]. Data from animal models show that tumour-derived TF-positive MV are key players of thrombus formation by activating both the coagulation system and platelets [8–12].
These mechanistical data in murine model unequivocally demonstrate the contribution of MV TF in thrombus formation. Indeed, in humans, elevated plasma levels of MV TF have been associated with an increased risk of developing VTE in cancer patients. [13–18]. However, the association between levels of MV TF activity and VTE has been shown in patients with pancreatic cancer but no other types of cancer. This may be due to different pathophysiological mechanisms involved in the VTE formation in cancer [12] but also limited sensitivity of the MV TF activity.
Several methods have been described to measure MV TF in clinical samples using either activity or antigen-based assays [6,19]. However, the specificity and sensitivity of these assays is a concern. Among these assays, antigenic detection of TF on circulating MV provides the advantage to detect both cryptic and decrypted TF but the measurement of TF by flow cytometry remains very challenging because of the low levels of TF and concerns about some anti-TF antibodies [20]. Currently, there are two non-commercial methods that have been reported for MV TF activity that use either a kinetic monitoring of the specific substrate (Leiden assay) or a end point (Chapel Hill assay) to measure factor Xa (FXa) generation [6,21,22]. These assays use an antibody which inhibits TF activity. A good correlation was found between these two versions of the FXa generation assay in 54 pancreatic cancer patients [24] and they proved to be more sensitive than commercial assays [24]. A recent paper described the Chapel Hill assay in detail [25].
The aim of this work was to improve the MV TF-dependent FXa generation assay (MV TF activity assay) and evaluate its analytical performances in comparison with a currently used test (Chapel Hill assay).
Materials and Methods
Blood sample processing
Blood samples from healthy donors, who signed an informed consent form, were collected and processed according to the current International Society on Thrombosis and Haemostasis guidelines [19,26]. Briefly, after a light tourniquet was applied, samples were drawn from the antecubital vein using a butterfly device with a 21-gauge needle. Blood was collected into 5 mL Vacutainer tubes containing 0.129 mol/L sodium citrate (BD Diagnostics, Franklin Lakes, NJ, US), and the first few milliliters were discarded. The samples were subjected to two successive centrifugations (2,500 g for 15 min at room temperature (RT)) to prepare platelet-free plasma (PFP). The PFP was homogenized before being aliquoted and stored at −80°C until use.
For specific experiments, whole blood was incubated with bacterial lipopolysaccharide (LPS) (10 μg/mL, Escherichia coli O111: B4; Sigma Aldrich, St. Louis, MO, USA) for 5h at 37°C. Then PFP were prepared with two successive centrifugations (2,500g, 15 min, RT with a Multifuge X3R centrifuge, rotor TX-1000, k-factor : 9470, Thermofisher, Courtaboeuf, France).
MV preparation
Human myeloid leukemia HL60 cells (Sigma Aldrich, Lyon, France) and human pancreatic BxPC3 cells (Sigma Aldrich, Lyon, France), regularly tested for mycoplasmas with Mycoalert (Lonza Biosciences, Basel, Switzerland) and DAPI (Sigma Aldrich, Lyon, France) were cultured in RPMI 1640 medium (GIBCO BRL, Gaithersburg, MD, USA) supplemented with 10% of fetal bovine serum (FBS) 1% of penicillin and 1% of streptomycin (GIBCO BRL, Gaithersburg, MD, USA), in humidified atmosphere at 37 °C, 5% CO2. Cell viability was assessed by trypan blue dye exclusion. Haploid human cell line (HAP1) cells and its derivative KO-TF-HAP1 made by CRISPR/Cas9 (Thermofisher, Courtaboeuf, France) (TF-protein expression was tested by flow cytometry and TF-gene expression was tested by qPCR) were grown at 37°C and 5% CO2 in Iscove’s Modified Dulbecco’s Medium (IMDM) (GIBCO BRL, Gaithersburg, MD, USA) supplemented with 10% of FBS, 1% of penicillin and 1% of streptomycin. All mediums were filtrated at 0.22 μm (Corning, New York, USA).
MV purified from culture supernatants : HL60-MV, BxPC3-MV and HAP1-MV were purified from conditioned medium after cells and debris by two successive centrifugations at 300g, 5 min and an additional centrifugation at 2,500g centrifugation, 10 min.
MV purified from clinical samples : Platelet-derived MV (PMV) were generated from PRPs as already described [27]. Erythrocyte-derived MV (Ery-MV) were purified generated from purified red blood cells either by aging (48h) or sonication by VIBRA Cell 75186; Pulse S9 (60%) 3 times for 60s. All MV subsets were pelleted at 70,000g, 90 min, 4°C (JA-30.50 Ti fixed-angle rotor, k-factor: 280, Beckman Coulter, Villepinte, France) and washed twice in PBS buffer (2× 70,000g, 90 min). Isolated MV were enumerated by flow cytometry (Gallios, Beckman Coulter, Villepinte, France) standardized by Megamix strategy [28] by reference to counting beads (MP-Count beads, BioCytex, Marseille, France) [29]. Finally, MV were spiked in MV-free plasma (removing MV by high-speed centrifugation 3×70,000g, 90 min) before performing TF-dependent procoagulant testings.
Optimized MV TF dependent FXa generation assay design
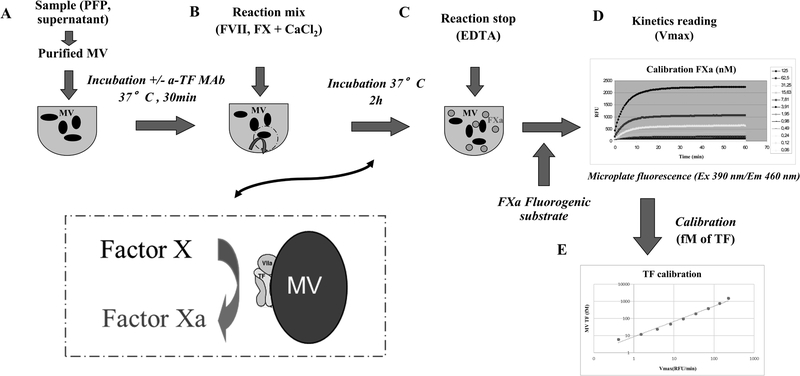
An optimized TF-dependent FXa generation assay (MV TF activity assay) was adapted from the Chapel Hill TF-dependent FXa generation assay [22,30] as described [31]. Briefly, MV were pelleted by centrifugation at 24,000 g for 60 min at RT from 500 μl of plasma 1:2 diluted in HEPES buffer (150 mM NaCl, 20 mM HEPES and 0.1% NaN3, pH 7.4, 0.22 μm filtrated), washed in HEPES buffer and resuspended in 140 μl of HEPES buffer. Aliquots (70 μl) were pre-incubated for 30 min at 37°C with either an inhibitory anti-TF monoclonal antibody (10 μg/ml final, clone SBTF-1, BioCytex, Marseille, France) or a control antibody (10 μg/ml, clone a-DNP 2H11–2H12, BioCytex, Marseille, France) (Figure 1A). Then, 7 μl HEPES-Ca2+ buffer (150 mM NaCl, 20 mM HEPES and 0.1% NaN3, 50mM CaCl2, pH 7.4, 0.22 μm filtrated) containing purified human FVII and FX (Stago BNL, JV Leiden, Netherland) was added to each 70μl sample, to produce final concentrations of 10 nM, 190 nM and 5 mM CaCl2 respectively and incubated for another 2 h at 37°C (Figure 1B). FXa generation was halted by the addition of 8 μl of EDTA buffer (150 mM NaCl, 20 mM HEPES and 0.1% NaN3, 200 mM EDTA, pH 7.4, 0.22 μm filtrated) and a FXa fluorogenic substrate (1 mM final, BioCytex, Marseille, France) was added (Figure 1C). Finally, the fluorescence at 390 nm (excitation) and 460 nm (emission) was monitored for 15 min at 37°C on a microplate fluorescence reader (Fluoroskan, CAT instrument, Stago, Asnières-sur-Seine, France) (Figure 1D). Maximum reaction velocity (Vmax) was calculated with the associated software (Ascent Software, Luqa, Malta). Vmax were corrected by subtracting those generated in the presence of SBTF-1 from those generated in the presence of the control antibody. Data from plasma-purified MV were expressed as fmol/L by comparison to a calibration curve generated using recombinant TF (Figure 1E).
Figure 1. Schematic sketch of the MV TF activity assay.
A. Microvesicles (MV) were extracted from platelet-free plasma (PFP) using ultracentrifugation. Blocking anti-TF (or sham) antibody was reacted with MV for specificity evaluation. B. Reaction mix containing factor VII (FVII), factor X (FX) and calcium (CaCl2) was added, FX cleavage into activate factor X (FXa) was induced by tissue factor (TF)/ activated FVII (FVIIa) complex during incubation at 37°C. C. The reaction was stopped by ethylenediaminetetraacetic acid (EDTA) that captures the calcium. D. The generated FXa was quantified by fluorometry using a specific fluorogenic substrate, and the fluorescence (excitation 390nm/emission 460 nm) was monitored in RFU/min. E. Calibration range of liposome associated recombinant TF (PRP-reagent) allow us to convert the values in femtomolar of TF.
For comparison experiments, different centrifuge rotors were tested (FA45-24-11, k-factor: 321; FA45-24-11, k-factor: 321; F15-6×100y, k-factor: 1536, Thermofisher, Courtaboeuf, France; JA-30.50, k-factor: 280; Beckman Coulter, Villepinte, France). SBTF-1 anti-TF inhibitory antibody was compared to HTF-1 (BD Biosciences, San Jose, CA) at various concentration (0.3–20 μg/ml) and incubation time (5–10 min). Purified human FVII was compared to purified human FVIIa (Stago BNL, JV Leiden, Netherland).
Chapel Hill assay
This assay has already been described in details in Khorana et al. 2008 [21,25,30]. Briefly, the measurement of MV TF activity in plasma is based on an end point FXa generation chromogenic assay, the use of a monoclonal antibody to inhibit TF activity (clone HTF-1, BD Biosciences, San Jose, CA) and the use of FVIIa as TF cofactor.
Statistical analysis
All statistical analyses were performed with GraphPad Prism software version 5.0 (GraphPad Software, San Diego, CA, US). Significant differences were determined using a non-parametric Mann-Whitney test or a paired t-test. Analysis of variances (ANOVA) was used to compare rotors. A p-value less than 0.05 was considered statistically significant. Spearman’s rank correlation was used as a measure of the correlations between assays.
Results
Specificity of the MV TF activity assay
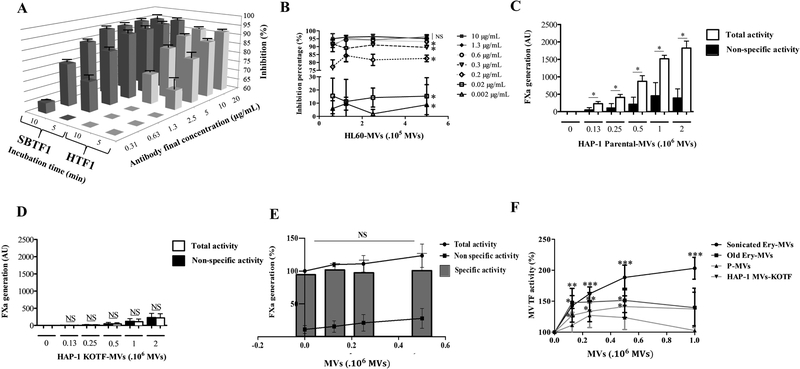
A major modification of the new MV TF activity assay relies on using a novel inhibitory anti-TF antibody (SBTF-1) which ensures the assay specificity. The SBTF-1 clone was compared to the HTF-1 clone used in the Chapel Hill assay at different concentrations and incubation times with all other parameters being the same. As demonstrated on figure 2A, compared to HTF-1, SBTF-1 showed a significantly more potent inhibition of the TF-dependent FXa generation (95±1% vs 92±0.4%, at 20 μg/mL, 5 min, p=0.04). The superiority of SBTF-1 over HTF-1 was confirmed at lower concentration. Twenty-two percent of inhibition were lost at 2.5μg/mL compared with 20 μg/mL with HTF-1 while the inhibitory effect of SBTF-1 was unchanged. At 10μg/ml with both antibodies, we show a significant increase in the inhibition % with SBTF-1 compared to HTF-1 (95±0.7% vs 90±0.5%, p<0.001). This difference is amplified at 5 μg/mL (94±1% vs 86±1%, p<0.001) (Figure 2A). Moreover, the inhibitory effect of SBTF-1 was maintained over a broad range of spiking doses of HL60 MV (0.6 – 5×105) with a saturating concentration above 2.5 μg/ml that decrease significantly an in a concentration dependent manner at 0.63 μg/mL (p=0.03), 0.32 μg/mL (p=0.03) and 0.16 μg/mL (p=0.03) (Figure 2B) and dramatically decrease at 0.02 μg/mL (p=0.03) and at 0.002 μg/mL (p=0.03) (Figure 2B). Thus, these data demonstrated that the SBTF-1 clone has a more potent inhibitory activity than HTF-1 clone, whatever the concentration used. For further experiments, a concentration of 10 μg/ml of SBTF-1 was chosen.
Figure 2. Specificity of the MV TF activity assay.
A. Comparison of blocking effect between SBTF-1 and HTF-1 blocking antibodies on HL60-derived MV as function of antibody concentration with two times of incubation. The results are expressed in inhibition percentage (n=3). B. Comparison of different antibody concentrations (0,002–10 μg/mL) on a range of HL60-MV (0,6–5.105 MV). The results are expressed in inhibition percentage (n=3). C. FXa generation measured on a range of parental HAP1-MV. White histograms represent total activity and black histograms represent non-specific activity. Results are expressed in arbitrary unit (AU) (n=3). D. FXa generation measured on a range of KO-TF-HAP1 derivative MV (KO-TF-MV). White histograms represent total activity and black histograms represent non-specific activity. Results are expressed in AU (n=3). E. FXa generation measured on 105 parental HAP1-MV added to a range of KO-TF-MV. Curve with circles represent the total activity, curve with squared represent non-specific activity and column bar represents the specific activity. Results are expressed in percentage (n=4). F. MV TF activity measured on 105 HL60-MV added to a range of erythrocyte-derived MV (Ery-MV) obtain either by sonication or blood aging and platelet-derived MV (PMV). Results are expressed in percentage compared with HL60-MV alone (n=4).
The TF-dependent FXa generation of the MV TF activity assay was then calculated by the difference between the total FXa activity and the residual FXa activity which is not inhibited by the SBTF-1 antibody (non-specific activity). As illustrated on a range of spiked TF-positive HAP1-MV (Figure 2C), a significant difference of TF activity was noted between, total and non-specific activity. Interestingly, when the assay was performed in presence of the same amount of parental MV which have been knocked-out for TF (KO-TFMV) no TF specific activity was measured in contrast to parental MV (Figure 2D). This result demonstrates the TF specificity of the SBTF-1 antibody and therefore the specificity of the MV TF activity assay for TF.
The impact of MV surface phospholipids (PLs) was also tested. As illustrated in figure 2E, the addition of a range of KO-TF-MV to MV of the same PLs nature (parental HAP1-MV) results in a slight increase in the FXa activity (+17±17% with 0.25×106 KO-TF-MV). This increase of FXa activity was only due to an increase in the non-specific activity (+11±7% with 0.25×106 KO-TF-MV) while the TF specific activity remained unchanged (Figure 2E). In contrast, a significant increase of the specific activity generated by HL60-MV was observed after spiking of sonicated Ery-MV, old Ery-MV, KO-TF-MV and PMV. As shown on Figure 2F, the extent of the increase varies according to the PL origins ranging from 100±20% with 1×106 sonicated Ery-MV (p<0.001), 50±20% with 0.5×106 old Ery-MV (p=0.01), 40±20% with 0.5×106 KO-TF-MV (p=0.02) to no significant impact with PMV. These results demonstrate that MV TF specific activity can be impacted according to the origin of MV surface phospholipids.
Optimizing sensitivity of the MV TF activity assay
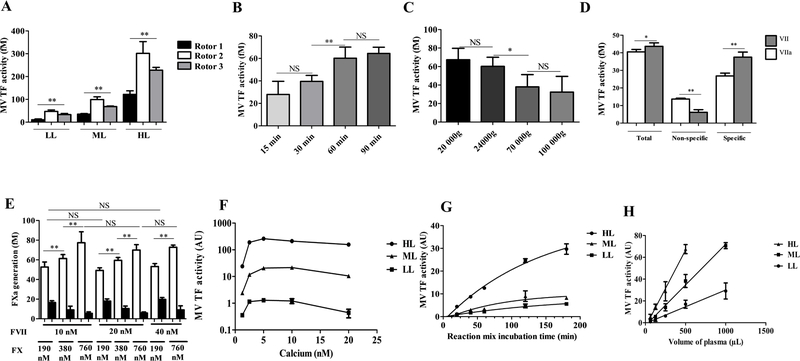
The MV TF activity assay was optimized in order to improve its sensitivity. First, the impact of the centrifugation protocol was evaluated by comparing MV TF activity in MV-free plasma spiked by three different levels of BxPC3-MV. As illustrated in figure 3A, the experiment was performed at the same centrifuge force (24,000g) but with different rotors. We observed a significant different activity in all concentrations (LL : 11±3; 47±6 and 33±5 fM for rotor 1, 2 and 3 respectively, p = 0.003; ML : 35±3; 100±10; 70±2 fM, p = 0.002; HL: 120±16; 300±50; 230±13 fM, p = 0.002). Because of this variation, all further comparison was performed with a same rotor. (rotor 2). Regarding the centrifugation time, a significantly increased activity was observed with 60 min compared to 15 or 30 min as performed on the Chapel Hill assay (28±10 fM vs 60±10 fM, p = 0.009, at 15 and 60 min, respectively) while no further increase was observed with 90 min (64±6 fM) (Figure 3B). Regarding the centrifugation speed, no significant difference was observed between the speed used in the Chapel Hill assay protocol 20,000g and 24,000g. Surprisingly, further increase in the centrifugation speed results in a significant decrease in the activity (60±10; 38±13; 32±17 fM at 24.000g, 70,000g and 100,000g, respectively, p = 0.02) (Figure 3C). Therefore, centrifugation of PFP at 24,000 g for 60 min at RT, using the same rotor were delineated as optimized preanalytical conditions to measure TF activity in a controlled range.
Figure 3. Increasing sensitivity of the MV TF activity assay.
A. Comparison of MV TF activity in plasma spiked with BxPC3-MV between three different rotors (n=6 with rotor 1 and 2, n=3 with rotor 3). Results are expressed in fM of TF. B. Comparison of MV TF activity on PFP between four centrifugation times at 24.000g (15 min, 30 min, 60 min, 90 min) (n=6). Results are expressed in fM of TF. C. Comparison of MV TF activity on PFP between four centrifugation speeds during 60 min (20.000g, 24.000g, 70.000g, 100.000g) (n=6). Results are expressed in fM of TF. D. Comparison between FVII and FVIIa in the reaction mix on HL60-MV (n=3). Results are expressed in AU. E. Comparison between different reaction mixes with different concentrations of FVII and FX on HL60-MV. White histograms represent total activity and black histograms represent non-specific activity. Results are expressed in AU. F. MV TF activity measured on HL60-MV with a reaction mix that contain a range of concentrations of calcium. Results are expressed in AU. G. MV TF activity measured on HL60-MV with different reaction mix incubation times. H. Evaluation of linearity by measured MV TF on a range of volumes of the same plasma. MV-free plasma spiked by three various levels of HL60-MV are used: high level (HL), medium level (ML) and low level (LL) (HL = 7500 MV/μL, ML = 2500/μL, LL = 750 MV/μL). Results are expressed in AU (n=3).
The Chapel Hill assay includes as reaction mix FX, CaCl2 and FVIIa to generate FXa. First, we compare FVII with FVIIa. As a result, FVII generated significantly increased MV TF activity compared to FVIIa (38±3 fM vs 27±2 fM, p=0.02) with significantly less non-specific activity (6±2 fM vs 14±1 fM, p=0.004) (Figure 3D). Secondly, we determined the optimal concentrations of the FX or FVII to be used in the reaction mix without increasing the non-specific activity. We compared different concentration of FX and FVII. As shown in figure 3E, a concentration of FVII above 10 nM does not improve the sensitivity. In contrast, increasing the FX concentration results in a significant gain in the MV TF activity without increasing the non-specific activity (36±6, 52±4, and 72±10 fM, at 190, 380 and 760 nM (10 nM FVII), respectively, p=0.002). Finally, a concentration of FX of 190 nM was chosen for cost reasons. Regarding calcium concentration, an activity plateau was reached at 5 mM regardless of the levels of MV TF activity (Figure 3F) showing that this concentration is sufficient for the FXa generation assay. Thirdly, we determine the optimal incubation time with the reaction mix. As observed in figure 3G, MV TF activity increased with incubation time. A 2h of incubation was chosen for further experiments as a good sensitivity/time ratio (Figure 3G). Therefore, the incubation time was the same as the Chapel Hill assay.
Taken together, these results established the optimal experimental conditions to improve sensitivity of the MV TF activity assay without losing specificity: centrifugation at 24,000 g for 60 min; 10 nM FVII, 190 nM FX and 5 mM CaCl2 incubated for 2 h instead of centrifugation at 20,000g for 15 min; 10 nM FVIIa, 300 nM FX and 10 mM CaCl2 incubated for 2h in the Chapel Hill assay.
Linearity and reproducibility of the MV TF activity assay
The impact of the MV TF activity assay optimizations was evaluated, on linearity and reproducibility. First, to demonstrate the linearity of the assay, a dose-effect relationship of the MV TF activity was measured on different volumes of plasma spiked with HL60-MV. A significant linear relationship between plasma volume centrifugate and MV TF activity measured (HL, r2=0.97, p<0.0001; ML, r2=0.98, p<0.0001; LL, r2=0.91, p<0.0001) (Figure 3H).
The variability of the MV TF activity assay was evaluated either after MV purification or on PFP with the aim to include the impact of the centrifugation procedure. As shown in Table 1, the repeatability and reproducibility over a 12 month-period of three levels of purified MV were low (4% and 4–13% respectively) while the repeatability and reproducibility over time of the assay in PFP was above 20% (20%, 22–26%, respectively) due to the centrifugation step. Because the quality of the recovery of the MV pellet may vary between operators, the inter-operator reproducibility (n=4) was evaluated using samples containing three different levels of MV TF activity. As a result, the variability gradually decreases with the FXa activity, (LL, CV=38%, ML, CV=17%, HL, CV=3%, Table 1). While the direct comparison was not made, no difference in variability is expected between the MV TF activity assay and the Chapel Hill assay because both assays share a centrifugation step which is the main cause of variability within the assays.
Table 1.
Reproducibility of the MV TF activity assay. Different reproducibility expressed with the coefficient of variation. Type of sample, number of experiment and number of operators are indicated. MV-free plasma spiked by three distinct levels of BxPC3-MV : HL = high level, ML = medium level, LL = low level.
| Sample nature | Number of samples | Number of operators | Coefficient of variation | ||
|---|---|---|---|---|---|
| Repeatability | Purified MVs | 4 | 4 | 4% | |
| Reproducibility over time | Purified MVs | 52 | 1 | LL | 13% |
| ML | 9% | ||||
| HL | 4% | ||||
| Repeatability | PFP | 4 | 1 | 20% | |
| Reproducibility over time | PFP | 25 | 1 | LL | 26% |
| ML | 22% | ||||
| HL | 25% | ||||
| Inter-operator reproducibility | PFP | 4 | 3 | LL | 38% |
| ML | 17% | ||||
| HL | 3% | ||||
Evaluation of the MV TF activity assay sensitivity
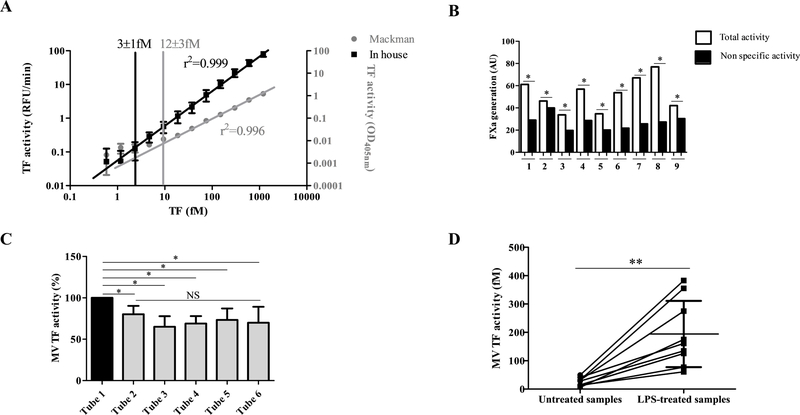
The sensitivity of the MV TF activity assay was first compared to the Chapel Hill assay evaluating the detection limit of the method which was defined as the linearity breakpoint in a serial dilution of recombinant source of TF. As shown in the figure 4A, a lower detection limit was found for the new assay compared to the Chapel Hill assay (3±1 vs 12±3 fM, p=0.03). This low detection limit permitted the detection of MV TF activity in plasma samples from healthy individuals (26±15 fM) which was specifically inhibited by the anti-TF antibody SBTF-1 (Figure 4B). After adjusting for the enrichment factor of 3.6 between the sample of 500μL and the pellet recovered in 140 μL of buffer, this value represents 7±4 fmol/mL of PFP. This result was obtained after discarding the first milliliters of blood because of a potential release of subendothelial TF during the venipuncture [32]. Indeed, as shown in figure 4C, when measured on 6 successive 5 ml tubes from the same donor, the activity was significantly higher in the first tube compared to the following (− 20±10%, p= 0.05). This result suggests that the first tube was contaminated by subendothelial TF and therefore should be discarded from the analysis. Next, the ability of MV TF activity assay to discriminate MV TF activity from unstimulated blood compared to the same blood stimulated with LPS was measured. As illustrated in figure 4D, the activity was significantly increased in LPS-stimulated compared to unstimulated conditions (190±120 fM vs 26±15 fM, p = 0.002).
Figure 4. Validation of MV TF assay sensitivity.
A. Limit of linearity determined by a range of successive two-fold dilutions of PRP reagent. Results are expressed in relative fluorescence unit per minute (RFU/min) with the MV TF assay and in optical density at 405 nm (OD405nm) with the Chapel Hill assay. B. MV TF activity measured on healthy individuals. White histograms represent total activity and black histograms represent non-specific activity. Results are expressed in AU (n=9). C. Measurement of MV TF activity on 6 successive blood collection tubes. Results are expressed in percentage compared to the first tube (N=5). D. MV TF activity compared between PFP extracted from untreated blood and LPS-treated blood. Results are expressed in fM TF (n=9).
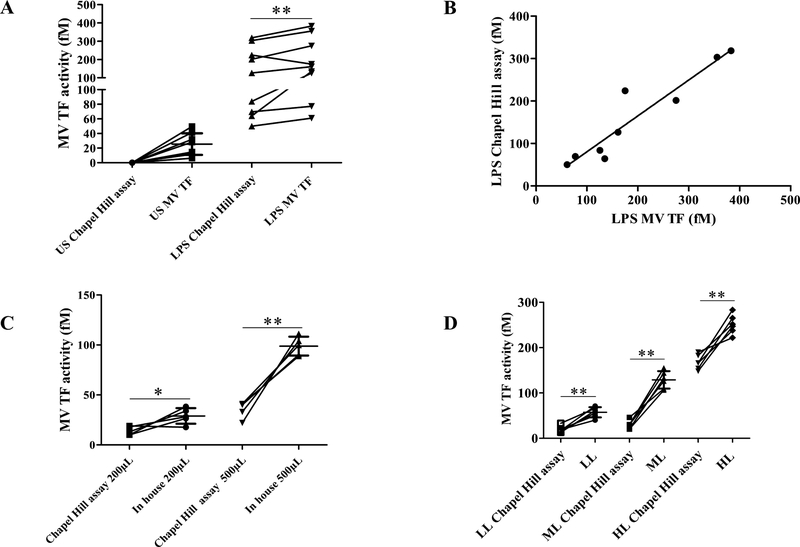
Finally, the sensitivity of the MV TF activity assay was directly compared to the Chapel Hill assay. In healthy plasma the FXa generation specifically inhibited by the anti-TF antibodies was significantly detected with the MV TF activity assay (26±15 fM) while it remains undetectable with the Chapel Hill assay (Figure 5A). In LPS-stimulated plasma, a significant increase of 30±40% with the MV TF activity assay was measured (p = 0.04) (Figure 5A) with a good correlation between assays (r2 = 0.952; p<0.0001, Figure 5B). Both assays were performed with a different plasma volume (500μL vs 200μL). After normalization of this volume (200μL or 500μL) the MV TF activity remains significantly higher with the MV TF activity assay than the Chapel Hill assay (29±8 vs 14±4 fM; p=0.03; 99±9 vs 35±8 fM; p=0.008 for 200 and 500μL, respectively, figure 5C). These results were confirmed in plasma spiked with three different levels of TF+MV (BxPC3). As shown in figure 5D, a significantly higher activity was found in the MV TF activity assay compared to the Chapel Hill assay (57±11 vs 20±7 fM; p=0.003, 129±19 vs 30±9 fM; p=0.002 and 251±21 vs 168±16 fM; p=0.002; for LL, ML and HL, respectively). Altogether these results demonstrate that the MV TF activity assay is more sensitive than the Chapel Hill assay and that the difference of initial plasma volume was not the key factor explaining this difference.
Figure 5. Comparison of the in-house MV TF activity method and pre-existing assays.
A. MV TF activity measured with the MV TF activity assay (In-house) and the Chapel Hill assay described by Khorana et al. in 2008 were compared on the PFP of 9 healthy individuals from untreated and LPS-treated blood. B. Correlation between in-house method and Chapel Hill assay for LPS-treated samples, r2=0952, p<0.0001, significant. C. MV TF activity measured with the MV TF activity assay (In-house) and the Chapel Hill assay describes by Khorana et al. in 2008 were compared on spiked MV TF in plasma featuring three levels of activity (HL = 7500 MV/μL, ML = 2500/μL, LL = 750 MV/μL). D. MV TF activity measured with the MV TF activity assay and the Chapel Hill assay with the same PFP volume 200μL and 500μL. Results are expressed in fM.
Discussion
Although many studies have suggested that MV TF activity may be a useful biomarker to identify patients with an increased risk of thrombosis, the most convincing results were published in patients with cancer [14,15,22,24,33,34]. In contrast, significant increases in MV TF activities were not observed in cardiovascular disorders [6]. It is thought that the major part of TF-positive MV are derived from tumor cells in cancer in particular for pancreatic cancer displaying the highest MV TF activity [12,18], whereas they are derived from hematopoietic cells in non-tumoral disease. But one can also hypothesized that the current tests are hampered by a lack of sensitivity. In the present study, we showed that the sensitivity of MV TF activity can be significantly improved by 1/ increasing the plasma volume, the speed and time of centrifugation, the FX concentration, 2/using an anti-TF antibody (Clone SBTF-1) with a more potent inhibitory and, 3/ using kinetic monitoring of a fluorogenic substrate that measures Xa generation and the use FVII instead of FVIIa to reduce TF-independent FXa generation.
We demonstrated that this new assay was able to measure MV TF activity with a high specificity and an improved sensitivity, especially in the low concentration range of MV TF activity. Indeed, we were able to detect MV TF activity in normal PFP samples. However, the assay was still limited by an inter-assay variability, mainly due to the centrifugation step.
The MV TF activity assay developed in the present study combines an enzymatic assay of generated FXa, with MV purification by centrifugation. A first step was to improve some parameters that influence the preanalytical step, one of the most important issue, as extensively discussed in previous reviews [35,36]. Centrifugation is frequently used to pellet MV because it can be performed easily. However, as illustrated in our study, isolation of the MV introduces some variability, as shown by the CV of MV TF activity. We found significantly less variability with MV isolated using purified MV. Consistent with previous studies showing that the recovery of the pellet depend on the rotor type, the centrifugation speed (g-force) and the centrifugation time [37], we demonstrated that MV TF activity is significantly affected by 1) type of rotor 2) speed of centrifugation, 3) centrifugation time. According to our result, centrifugation of PFP at 24,000 g for 60 min at RT, using the same rotor were delineated as optimized preanalytical conditions to measure TF activity in a controlled range.
Another disadvantage of centrifugation is to cause the aggregation of MV and/or their contaminations by unwanted elements, such as protein/lipid aggregates [38]. Accordingly, in the future, an option to overcome the disadvantages of centrifugation would be to use anti-TF antibody coated magnetic beads to specifically capture MV from larger volume of blood, thus reducing the time to isolate MV and avoiding washing steps We recently used such a strategy in a new assay for the measurement of plasmin activity of MV [29], with an improvement of time, sensitivity, specificity and reproducibility.
We also focused on improving the analytical settings of the FXa generation assay and showed that the sensitivity of the MV TF activity was optimized by 1) adding factor VII instead of VIIa, 2) an optimized calcium concentration, and 3) an optimized incubation time that allows a better recovery of activated FX and a better cleavage of the fluorescent substrate.
An important modification was provided by introducing a new inhibitory anti-TF antibody with a high inhibitory potential (SBTF-1). Using MV generated from the cell line HAP1 that has been KO for TF and MV generated from their derivative HAP1 parental cell, no TF specific activity was generated in MV from KO-TF-MV, indicating that SBTF-1 confers a high specificity to the novel assay. In comparison with the commercially available anti-TF antibody HTF-1 (the most widely used antibody in the previous studies [22,25,30,39]), the SBTF-1 exhibited an increased inhibitory effect, as attested by the difference of inhibition of the FXa generation at different time and concentration. Moreover, the SBTF-1 inhibitory effect operated over a broad range of MV concentration.
Non TF elements that can modify TF activity in blood are widely described in the previous studies, such notably negatively charged PLs [40–42]. Indeed, we showed that TF activity can be influenced by MV generated by erythrocyte-derived MV (Ery-MV) probably by increasing the rate of FX availability for the TF-FVIIa complex. We have shown that hemolysis increase the non-specific FXa generation in plasma samples from dogs with immune-mediated hemolytic anemia[43]. This emphasizes the importance of the pre-analytical treatment of samples and the interpretation of data from hemolysis samples.
Having optimized the analytical settings of MV TF activity assay, we challenged its sensitivity and specificity. We illustrate here the sensitivity by showing 1/ a lower detection limit, 2/ the existence of a basal level of MV TF activity in blood plasma from healthy donors and, 3/ a significant increase of activity for MV from LPS stimulated blood.
Finally, we demonstrated that MV TF activity assay presented a higher sensitivity than the end point Xa generation assay compared with the Chapel Hill assay where methods differences are summarized in table 2. Indeed, significantly more TF activity was always measured by the optimized Xa generation assay 1/ using plasma from healthy controls, untreated or LPS treated 2/ using plasma enriched with low, medium and high known concentrations of MV from the pancreatic cancer cell line BxPC3, with a better correlation between assays observed for higher TF activity plasma in contrast to low TF activity samples.
Table 2.
Differences between the MV TF activity assay and the Chapel Hill assay
| MV TF activity assay | Mackman Assay | |
|---|---|---|
| Sample | 500 μL plasma | 200 μL plasma |
| Isolation of MVs | 1h, 24.000g centrifugation with one wash | 15 min, 20.000g centrifugation with two washes |
| Blocking antibody | SBTF1 (10 μg/mL) | HTF1 (4 μg/mL) |
| Reaction MIX | 10 nM VII, 190 nM X and 5 mM CaCL2 | 10 nM FVIIa, 300 nM FX and 10 mM CaCL2 |
| Substrate monitoring | Kinetic | End-point |
| Substrate | Fluorogenic | Chromogenic |
In conclusion, the MV TF activity assay presented here shows a higher sensitivity without reducing specificity. Therefore, this modified assay could provide a significant improvement to measure TF-positive MV as a potential biomarker of thrombotic risk in patients.
Highlights:
Current methods to measure MV TF activity have limited sensitivity
An improved TF-dependent FXa generation assay was developed
The updated MV TF activity assay includes a new anti-TF inhibitory antibody (SBTF-1)
The MV TF activity assay improve sensitivity as compared with previous test
SOURCES OF FUNDING
This work was partly funded by INSERM and Aix-Marseille University (France). Financial support was also received from the Stago Company. This work was supported by funds from the John C. Parker Endowed Professorship and the National Institute of Health (T32HL007149).
Footnotes
DISCLOSURES
We disclose as a conflict of interest that a patent on this topic has been licensed by the Stago Company, and P.Poncelet, T. Bouriche, J. Bez and C. Judicone are full-time employees of Biocytex.
Bibliography
- [1].Wolf P, The nature and significance of platelet products in human plasma, Br. J. Haematol 13 (1967) 269–288. [DOI] [PubMed] [Google Scholar]
- [2].Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat M-L, Boilard E, Buzas EI, Caporali A, Dignat-George F, Evans PC, Lacroix R, Lutgens E, Ketelhuth DFJ, Nieuwland R, Toti F, Tunon J, Weber C, Hoefer IE, Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology, Thromb. Haemost 117 (2017) 1296–1316. doi: 10.1160/TH16-12-0943. [DOI] [PubMed] [Google Scholar]
- [3].Biró E, Sturk-Maquelin KN, Vogel GMT, Meuleman DG, Smit MJ, Hack CE, Sturk A, Nieuwland R, Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner, J. Thromb. Haemost 1 (2003) 2561–2568. [DOI] [PubMed] [Google Scholar]
- [4].Chou J, Mackman N, Merrill-Skoloff G, Pedersen B, Furie BC, Furie B, Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation, Blood. 104 (2004) 3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- [5].Ramacciotti E, Hawley AE, Farris DM, Ballard NE, Wrobleski SK, Myers DD, Henke PK, Wakefield TW, Leukocyte- and platelet-derived microparticles correlate with thrombus weight and tissue factor activity in an experimental mouse model of venous thrombosis, Thromb. Haemost 101 (2009) 748–754. [PMC free article] [PubMed] [Google Scholar]
- [6].Hisada Y, Alexander W, Kasthuri R, Voorhees P, Mobarrez F, Taylor A, McNamara C, Wallen H, Witkowski M, Key NS, Rauch U, Mackman N, Measurement of microparticle tissue factor activity in clinical samples: A summary of two tissue factor-dependent FXa generation assays, Thromb. Res 139 (2016) 90–97. doi: 10.1016/j.thromres.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mege D, Mezouar S, Dignat-George F, Panicot-Dubois L, Dubois C, Microparticles and cancer thrombosis in animal models, Thromb. Res 140 (2016) 21–26. doi: 10.1016/S0049-3848(16)30094-9. [DOI] [PubMed] [Google Scholar]
- [8].Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C, Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo, J. Exp. Med 206 (2009) 1913–1927. doi: 10.1084/jem.20082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang J-G, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N, Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer, Blood. 119 (2012) 5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thomas GM, Brill A, Mezouar S, Crescence L, Gallant M, Dubois C, Wagner DD, Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice, J. Thromb. Haemost 13 (2015) 1310–1319. doi: 10.1111/jth.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Geddings JE, Hisada Y, Boulaftali Y, Getz TM, Whelihan M, Fuentes R, Dee R, Cooley BC, Key NS, Wolberg AS, Bergmeier W, Mackman N, Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice, J. Thromb. Haemost 14 (2016) 153–166. doi: 10.1111/jth.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hisada Y, Mackman N, Cancer-associated pathways and biomarkers of venous thrombosis, Blood. 130 (2017) 1499–1506. doi: 10.1182/blood-2017-03-743211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tesselaar MET, Romijn FPHTM, Van Der Linden IK, Prins FA, Bertina RM, Osanto S, Microparticle-associated tissue factor activity: a link between cancer and thrombosis?, J. Thromb. Haemost. JTH 5 (2007) 520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- [14].Zwicker JI, Liebman HA, Neuberg D, Lacroix R, Bauer KA, Furie BC, Furie B, Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy, Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res 15 (2009) 6830–6840. doi: 10.1158/1078-0432.CCR-09-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bharthuar A, Khorana AA, Hutson A, Wang J-G, Key NS, Mackman N, Iyer RV, Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers, Thromb. Res 132 (2013) 180–184. doi: 10.1016/j.thromres.2013.06.026. [DOI] [PubMed] [Google Scholar]
- [16].Woei-A-Jin FJSH, Tesselaar MET, Garcia Rodriguez P, Romijn FPHTM, Bertina RM, Osanto S, Tissue factor-bearing microparticles and CA19.9: two players in pancreatic cancer-associated thrombosis?, Br. J. Cancer 115 (2016) 332–338. doi: 10.1038/bjc.2016.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Faille D, Bourrienne M-C, de Raucourt E, de Chaisemartin L, Granger V, Lacroix R, Panicot-Dubois L, Hammel P, Lévy P, Ruszniewski P, Ajzenberg N, Rebours V, Biomarkers for the risk of thrombosis in pancreatic adenocarcinoma are related to cancer process, Oncotarget. 9 (2018) 26453–26465. doi: 10.18632/oncotarget.25458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cui C-J, Wang G-J, Yang S, Huang S-K, Qiao R, Cui W, Tissue Factor-bearing MPs and the risk of venous thrombosis in cancer patients: A meta-analysis, Sci. Rep 8 (2018) 1675. doi: 10.1038/s41598-018-19889-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coumans FAW, Brisson AR, Buzas EI, Dignat-George F, Drees EEE, El-Andaloussi S, Emanueli C, Gasecka A, Hendrix A, Hill AF, Lacroix R, Lee Y, van Leeuwen TG, Mackman N, Mäger I, Nolan JP, van der Pol E, Pegtel DM, Sahoo S, Siljander PRM, Sturk G, de Wever O, Nieuwland R, Methodological Guidelines to Study Extracellular Vesicles, Circ. Res 120 (2017) 1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- [20].Poncelet P, Robert S, Bailly N, Garnache-Ottou F, Bouriche T, Devalet B, Segatchian JH, Saas P, Mullier F, Tips and tricks for flow cytometry-based analysis and counting of microparticles, Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapheresis 53 (2015) 110–126. doi: 10.1016/j.transci.2015.10.008. [DOI] [PubMed] [Google Scholar]
- [21].Khorana AA, Francis CW, Menzies KE, Wang J-G, Hyrien O, Hathcock J, Mackman N, Taubman MB, Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer, J. Thromb. Haemost 6 (2008) 1983–1985. doi: 10.1111/j.1538-7836.2008.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tesselaar MET, Romijn FPHTM, Van Der Linden IK, Prins FA, Bertina RM, Osanto S, Microparticle-associated tissue factor activity: a link between cancer and thrombosis?, J. Thromb. Haemost 5 (2007) 520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- [23].Thaler J, Ay C, Mackman N, Bertina RM, Kaider A, Marosi C, Key NS, Barcel DA, Scheithauer W, Kornek G, Zielinski C, Pabinger I, Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients, J. Thromb. Haemost 10 (2012) 1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- [24].Tatsumi K, Antoniak S, Monroe DM, Khorana AA, Mackman N, Subcommittee on Hemostasis and Malignancy of the Scientific and Standardization Committee of the International Society on Thrombosis and Hemostasis, Evaluation of a new commercial assay to measure microparticle tissue factor activity in plasma: communication from the SSC of the ISTH, J. Thromb. Haemost 12 (2014) 1932–1934. doi: 10.1111/jth.12718. [DOI] [PubMed] [Google Scholar]
- [25].Hisada Y, Mackman N, Measurement of tissue factor activity in extracellular vesicles from human plasma samples, Res Pr. Thromb Haemost 0 (2018). doi: 10.1002/rth2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lacroix R, Dignat-George F, Microparticles: new protagonists in pericellular and intravascular proteolysis, Semin. Thromb. Hemost 39 (2013) 33–39. doi: 10.1055/s-0032-1333310. [DOI] [PubMed] [Google Scholar]
- [27].Lacroix R, Plawinski L, Robert S, Doeuvre L, Sabatier F, Martinez de Lizarrondo S, Mezzapesa A, Anfosso F, Leroyer AS, Poullin P, Jourde N, Njock M-S, Boulanger CM, Anglés-Cano E, Dignat-George F, Leukocyte- and endothelial-derived microparticles: a circulating source for fibrinolysis, Haematologica. 97 (2012) 1864–1872. doi: 10.3324/haematol.2012.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robert S, Lacroix R, Poncelet P, Harhouri K, Bouriche T, Judicone C, Wischhusen J, Arnaud L, Dignat-George F, High-sensitivity flow cytometry provides access to standardized measurement of small-size microparticles--brief report, Arterioscler. Thromb. Vasc. Biol 32 (2012) 1054–1058. doi: 10.1161/ATVBAHA.111.244616. [DOI] [PubMed] [Google Scholar]
- [29].Cointe S, Harti Souab K, Bouriche T, Vallier L, Bonifay A, Judicone C, Robert S, Armand R, Poncelet P, Albanese J, Dignat-George F, Lacroix R, A new assay to evaluate microvesicle plasmin generation capacity: validation in disease with fibrinolysis imbalance, J. Extracell. Vesicles 7 (2018) 1494482. doi: 10.1080/20013078.2018.1494482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, Key NS, Mackman N, Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity, Thromb. Res 129 (2012) 80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Agouti I, Cointe S, Robert S, Judicone C, Loundou A, Driss F, Brisson A, Steschenko D, Rose C, Pondarré C, Bernit E, Badens C, Dignat-George F, Lacroix R, Thuret I, Platelet and not erythrocyte microparticles are procoagulant in transfused thalassaemia major patients, Br. J. Haematol 171 (2015) 615–624. doi: 10.1111/bjh.13609. [DOI] [PubMed] [Google Scholar]
- [32].Tekkeşin N, Esen OB, Kilinç C, Eviyaoğlu O, Discard first tube for coagulation testing, Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb 23 (2012) 299–303. doi: 10.1097/MBC.0b013e328351ebbf. [DOI] [PubMed] [Google Scholar]
- [33].van Doormaal F, Kleinjan A, Berckmans RJ, Mackman N, Manly D, Kamphuisen PW, Richel DJ, Büller HR, Sturk A, Nieuwland R, Coagulation activation and microparticle-associated coagulant activity in cancer patients. An exploratory prospective study, Thromb. Haemost 108 (2012) 160–165. doi: 10.1160/TH12-02-0099. [DOI] [PubMed] [Google Scholar]
- [34].Sartori MT, Della Puppa A, Ballin A, Campello E, Radu CM, Saggiorato G, d’Avella D, Scienza R, Cella G, Simioni P, Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role, Thromb. Haemost 110 (2013) 378–385. doi: 10.1160/TH12-12-0957. [DOI] [PubMed] [Google Scholar]
- [35].Key NS, Mackman N, Tissue factor and its measurement in whole blood, plasma, and microparticles, Semin. Thromb. Hemost 36 (2010) 865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- [36].Lacroix R, Robert S, Poncelet P, Kasthuri RS, Key NS, Dignat-George F, ISTH SSC Workshop, Standardization of platelet-derived microparticle enumeration by flow cytometry with calibrated beads: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop, J. Thromb. Haemost 8 (2010) 2571–2574. doi: 10.1111/j.1538-7836.2010.04047.x. [DOI] [PubMed] [Google Scholar]
- [37].Cvjetkovic A, Lötvall J, Lässer C, The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles, J. Extracell. Vesicles 3 (2014) 23111. doi: 10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Linares R, Tan S, Gounou C, Arraud N, Brisson AR, High-speed centrifugation induces aggregation of extracellular vesicles, J. Extracell. Vesicles 4 (2015) 29509. doi: 10.3402/jev.v4.29509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nielsen T, Kristensen AF, Pedersen S, Christiansen G, Kristensen SR, Investigation of procoagulant activity in extracellular vesicles isolated by differential ultracentrifugation, J. Extracell. Vesicles 7 (2018) 1454777. doi: 10.1080/20013078.2018.1454777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fu Y, Zhou J, Li H, Cao F, Su Y, Fan S, Li Y, Wang S, Li L, Gilbert GE, Shi J, Daunorubicin induces procoagulant activity of cultured endothelial cells through phosphatidylserine exposure and microparticles release, Thromb. Haemost 104 (2010) 1235–1241. doi: 10.1160/TH10-02-0102. [DOI] [PubMed] [Google Scholar]
- [41].Kothari H, Nayak RC, Rao LVM, Pendurthi UR, Cystine 186-cystine 209 disulfide bond is not essential for the procoagulant activity of tissue factor or for its de-encryption, Blood. 115 (2010) 4273–4283. doi: 10.1182/blood-2009-09-241356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhou J, Shi J, Hou J, Cao F, Zhang Y, Rasmussen JT, Heegaard CW, Gilbert GE, Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia, J. Thromb. Haemost 8 (2010) 773–782. doi: 10.1111/j.1538-7836.2010.03763.x. [DOI] [PubMed] [Google Scholar]
- [43].Kidd L, Mackman N, Prothrombotic mechanisms and anticoagulant therapy in dogs with immune-mediated hemolytic anemia, J. Vet. Emerg. Crit. Care San Antonio Tex 2001. 23 (2013) 3–13. doi: 10.1111/j.1476-4431.2012.00824.x. [DOI] [PubMed] [Google Scholar]