Abstract
Delayed memory deficits are common for patients with mild traumatic brain injury (mTBI), according to a recent systematic review of meta-analyses (Karr et al., 2014). However, there has been little work to identify different cognitive processes that may be underpinning these delayed memory deficits for mTBI. Frontal cortex is important for delayed memory, and is implicated in the pathophysiology of mTBI; moreover, frontal lobes are typically considered the locus of executive abilities. To further explore these relationships, we sought to partly explain delayed memory deficits after mTBI by examining behavioral indicators of executive function. Results showed that sub-acute as well as chronic mTBI patients performed worse than controls on the delayed memory trial of the Hopkins Verbal Learning Test-Revised (Brandt & Benedict, 2001), recalling approximately 18% and 15% fewer words, respectively. Furthermore, worse delayed memory performance was associated with less use of the cognitive strategy of semantic clustering, and with lower scores for the executive function composite from a standardized neuropsychological battery (NIH EXAMINER; Kramer et al., 2014). In contrast, serial clustering, a memory organizational strategy thought to be less dependent on executive function, did not show strong relationships to clinical status or delayed memory performance. This exploratory work suggests novel hypotheses to be tested in future, confirmatory studies, including that general executive functions and/or semantic clustering will mediate delayed memory deficits following mTBI.
Keywords: concussion, delayed free recall, delayed memory, executive function, mild traumatic brain injury, semantic clustering
1. Introduction
Traumatic brain injury (TBI) is a prevalent social health concern (Karr et al., 2014; Ponsford et al., 2012). Delayed memory deficits are among the most robust findings in studies of mild TBI (mTBI), according to a recent systematic review of meta-analyses (Karr et al., 2014). However, there has been little work so far to identify different processes that may be underpinning these delayed memory deficits for mTBI. It has long been recognized that encoding, storage, and retrieval processes of long-term memory depend on the intact hippocampus and surrounding medial temporal lobe (MTL) structures (Simons & Spiers, 2003). More recently as well, the important roles of frontal cortex, and frontal-MTL interactions, have been increasingly acknowledged (Simons & Spiers, 2003; Wagner, 2002). Indeed, the frontal cortex is currently recognized as critical to cognitive-control functions across an array of memory systems dealing with explicit, declarative knowledge (i.e., working memory, episodic/autobiographical memory, semantic memory; see e.g., Burianova, McIntosh, & Grady, 2010; Martin & Chao, 2001; Nyberg et al., 2003). Moreover, frontal and temporal brain areas, as well as white matter connections between them, are consistently implicated in the pathophysiology of mTBI (Bondi et al., 2014; McCrea et al., 2009; Stuss, 2011). Frontal lobes are typically considered the locus of executive abilities (e.g., Miyake et al., 2000; Stuss & Alexander, 2000), although it would oversimplify matters to strictly identify executive function with frontal lobe activity (Alvarez & Emory, 2006). Based on these considerations we sought to partly explain delayed memory deficits after mTBI by examining behavioral indicators of executive function during delayed memory.
In this study we assessed delayed memory with a widely-used neuropsychological test requiring immediate and delayed free recall of a word list, the Hopkins Verbal Learning Test-Revised (HVLT-R; Brandt & Benedict, 2001). We conjectured that variance in behavioral indicators of executive function might partly account for the delayed memory deficits for mTBI. As our primary assessment of executive function, we examined the use of the organizational strategy of semantic clustering in the memory test, considered to depend on central-executive processes associated with frontal cortex (Moscovitch, 1992; Woods et al., 2005). Indeed, individuals with frontal lobe lesions have been shown to use less semantic clustering in free recall (Gershberg & Shimamura, 1995).
For converging indicators of executive function, measured independently of HVLT-R performance, we also examined performance on a standardized neuropsychological battery that has been shown to be sensitive to differences in frontal lobe function (NIH EXAMINER; Kramer et al., 2014). This battery yields three scores for executive function domains s (cognitive control, working memory, and verbal fluency), as well as an executive function composite score combining across these domains.
Moreover, we examined the contributions to memory performance from these executive function indicators while also controlling for years of education. Notably, educational attainment has been investigated previously as a marker of cognitive reserve, and has been shown to attenuate effects of moderate and severe TBI on measures of cognitive performance, including episodic memory (Sumowski et al., 2013).
It is generally accepted that the majority of individuals with mTBI will recover from most symptoms and cognitive deficits within a few months of injury, while a smaller fraction will continue to have symptoms and cognitive difficulties after many months or even years (Mayer, Quinn, & Master, 2017; Ponsford et al., 2012). However, the nature of cognitive dysfunction for so-called "post-concussive syndrome" remains controversial. In particular, it remains unclear exactly which cognitive domains remain affected over the long-term in chronic-stage mTBI (Ponsford et al., 2012). To address this question, we tested the generality of delayed memory deficits in mTBI by examining the performances of widely-differing mTBI groups: sub-acute (within two weeks of injury) and chronic (three or more months from injury). We sought to evaluate delayed memory as a candidate core element of post-concussive syndrome. An observation of delayed memory deficits for both sub-acute and chronic mTBI would be a novel contribution to identifying the set of cognitive domains that remain affected in chronic, post-concussive syndrome.
2. Method1
2.1. Ethics statement
We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study. The University of New Mexico Health Sciences Center Human Research Protections Office approved the study. Participants gave written informed consent. Participants were compensated with cash cards.
2.2. Participants
Participants reported normal or corrected-to-normal vision, and English as primary language. A total of 84 eligible individuals participated (23 control, 38 sub-acute, 23 chronic). Sample sizes were determined prior to conducting the study to be similar to those in previously published mTBI studies (e.g., Geary, Kraus, Rubin, Pliskin, & Little, 2011). Sub-acute and chronic mTBI patients were recruited to participate in two separate, longitudinal studies conducted concurrently by overlapping research teams (henceforth referred to in this paper as Cavanagh et al. and Quinn et al., respectively. Please see Acknowledgements for fully detailed identifying information for these studies). In one of these studies (Cavanagh et al.), control and sub-acute mTBI were recruited as participants, returning to the lab for three sessions to track recovery (HVLT-R was only administered in the first session). In the other study (Quinn et al.), chronic mTBI were recruited as participants, receiving either an intervention (brain stimulation) or sham treatment over multiple (> 3) sessions. Here we report on the first session from these studies (session 1 for control and sub-acute, Cavanagh et al., and pre-treatment baseline session for chronic, Quinn et al.).
2.2.1. Eligibility criteria.
Eligibility criteria were determined prior to analyses of data. Sub-acute mTBI were initially screened for possible inclusion by a nurse in the University of New Mexico Hospital Emergency Department. Candidate sub-acute participants: 1) were restricted in age to be between 18 - 55 years (inclusive), 2) had a Glasgow Coma Scale score of 13 – 15 (if available), and 3) were required to have experienced loss of consciousness (LOC) at the time of injury (≤30 minutes in duration). In a subsequent phone interview, candidate sub-acute mTBI participants were excluded if they reported any of the following: 1) currently taking medications other than SSRI, 2) current or past substance abuse, 3) primary language other than English, 4) history of seizures, or 5) prior head injury. Sub-acute participants were recruited if they were able to come to the lab within 14 days of head injury.
Healthy, non-injured controls were recruited by advertisement in the community. Phone interview screened controls using the same criteria as for sub-acute. Controls were excluded if they reported prior head injury with LOC. Additionally, we wished to limit between-groups variance related to age and gender. However, in this we did not aim for 1:1 matching. For complete details of age- and gender-matching, see Table 1. To limit variance related to age, we recruited some control participants to match individual sub-acute participants for age (± 2 years). A number of controls were matched for age in this manner to more than one sub-acute individual. To limit variance in the study related to gender, we recruited some control participants to match individual sub-acute participants for gender (in most cases also matched individually for age).
Table 1.
Group characteristics.
| Control | Sub-acute | Chronic | ||
|---|---|---|---|---|
| 1. | N | 21 | 37 | 21 |
| 2. | # female | 13 | 13 | 8 |
| 3. | # w/ mild TBI | NA | 37 | 15 |
| 4. | # w/ moderate TBI | NA | 0 | 6 |
| 5. | # w/ previous TBI | 0 | 0 | 15 |
| 6. | Duration LOC (minutes) | NA | 5.77 ± 7.29 (4.00, 5.00, .10, 30.00) | 153.57 ± 429.57 (5.00, 1.00, 1.00, 1440.00) |
| 7. | # w/ LOC > 30 minutes | NA | 0 | 5 |
| 8. | # control w/ one age- and gender-matching sub-acute | 13 (8 female, 5 male) | NA | NA |
| 9. | # control w/ more than one age- and gender-matching sub-acute | 6 (3 female, 3 male) | NA | NA |
| 10. | # control w/ zero age- and gender-matching sub-acute | 2 (2 female, 0 male) | NA | NA |
| 11. | # control matched to each sub-acute (when sub-acute matched to more than one control) | 1 ± 1 (1, 1, 0, 4) | NA | NA |
| 12. | # sub-acute w/ one age- and gender-matching control | NA | 29 (9 female, 20 male) | NA |
| 13. | # sub-acute w/ more than one age- and gender-matching control | NA | 0 (0 female, 0 male) | NA |
| 14. | # sub-acute w/ zero age- and gender-matching control | NA | 8 (4 female,4male) | NA |
| 15. | # sub-acute matched to each control (when control matched to more than one sub-acute) | NA | 1 ± .417 (1, 1, 0,1) | NA |
Note. Key characteristics of groups in analyses in main text (total N = 79). Abbreviations: LOC = loss of consciousness. NA = Not applicable. LOC is reported in minutes: Mean ± SD (Median, Mode, Min, Max). (The upper bound was used whenever self-reported LOC was given as an approximation or range (e.g., "< 1 min" was coded as 1, "< 5 min was coded as 5, "5 - 10 min" was coded as 10, etc.). Rows 8 through 15 report details about matching of control and sub-acute individuals for age and gender. (For purposes of this tabulation, age matched ± 2 years). Number of # sub-acute matched to each control (when control matched to more than one sub-acute) reported as M ± SD (Median, Mode, Min, Max). Number of # control matched to each sub-acute (when sub-acute matched to more than one control) reported as M ± SD (Median, Mode, Min, Max).
Chronic mTBI participants were recruited from the University of New Mexico Hospital Emergency Department and from other local Emergency Departments and clinics, from patient recruitment services, and through advertisements. Eligibility criteria were the same as for sub-acute participants, except that LOC could have lasted up to 24 hours, they must have been injured between 3 months and 15 years prior to the time of testing, and they must have reported having current problems with at least one of the cognitive symptoms on the Neurobehavioral Symptom Inventory (Cicerone & Kalmar, 1995). Unlike sub-acute patients, chronic patients were not excluded for prior head injuries, and moreover the chronic sample included some individuals with moderate TBI as well as some with mTBI (see Table 1).
As noted, these data were collected as part of larger scale studies (Cavanagh et al., Quinn et al.). However, only the data specific to the cognitive strategies underlying HVLT-R performance are reported here.
To address sources of heterogeneity in the samples described above we performed supplemental analyses in which control and sub-acute groups were matched 1:1 for age and gender; and the chronic group included only mTBI (total N = 49; control n = 17, 10 female; sub-acute n = 17, 10 female; chronic N = 15, 6 female). (See Supplemental Results).
2.3. Procedure
2.3.1. Education.
A self-reported measure of years of education was obtained by a demographic questionnaire.
2.3.2. Executive function.
The executive function composite, obtained from a standardized neuropsychological battery assessing diverse facets of executive function (NIH EXAMINER; Kramer et al., 2014), was our main indicator of executive function measured independently of HVLT-R performance. The three executive function domains contributing to the executive function composite score were cognitive control, working memory, and verbal fluency.
2.3.3. Hopkins Verbal Learning Test-Revised (HVLT-R).
There were four trials in HVLT-R (Brandt & Benedict, 2001). The first three trials were verbal learning trials (Trials 1 - 3), in which the same list of words was read aloud in the same order for successive tests of immediate free recall. The fourth trial was the delayed memory trial (Trial 4): After performing unrelated tasks for 20 minutes, the participant was asked to repeat the earlier-learned list without hearing it again, for test of delayed free recall. We examined total-correct, the total number of words correctly recalled in any order. Delayed memory was operationally defined as performance on Trial 4. Additionally, we report repetitions and intrusions across Trials 1 - 4. Following the delayed memory trial, a test of recognition memory was administered, in which 24 words were read aloud, 12 of which were from the original list, and 12 of which were new words (intermixed). The participant was tasked with indicating which words were from the original list. From this assessment we report hits and false alarm errors.
2.3.3.1. Semantic clustering.
For our indicator of executive function during HVLT-R performance, we computed the semantic clustering index for each trial. Briefly, a semantic cluster was counted each time that two correct words were recalled consecutively from the same semantic category (e.g., lion, horse). Intrusions and repetitions were ignored. Then, the total number of observed semantic clusters was corrected by the number of semantic clusters that would be expected due to chance, according to the list-based method described in detail in Stricker et al. (2002)2.
2.3.3.2. Serial clustering.
We also examined serial clustering as an alternative method of organization in free recall, considered to be less dependent on executive function, and more dependent on cue-driven associative processes (Gershberg & Shimamura, 1995; Woods et al., 2005). Briefly, a serial cluster was counted each time two correct words were recalled in the same order in which they had been presented in the list (again, intrusions and repetitions were ignored). Then, the total number of observed serial clusters was corrected by the number expected due to chance, again according to the list-based method described in detail in Stricker et al. (2002).
3. Results
From the number of eligible participants (N = 84; 23 control, 38 sub-acute, 23 chronic), data were missing for three individuals (2 control, 1 sub-acute). Two chronic patients were determined to be making insufficient effort during testing, due to failing the Test of Memory Malingering (TOMM; Tombaugh, 1996), and their data were excluded. From the available data-set (N = 79), we formed three groups for analyses (control n = 21, 13 female; sub-acute n = 37, 13 female; chronic n = 21, 8 female). Data are available, absent any personally identifiable information, in Supplemental Materials and at Open Science Framework, https://osf.io/b3epc/. Please note that given the exploratory nature of this report, in the following sections we take a descriptive approach, focused on estimating the strength of relationships, and we do not report results of null hypothesis significance testing.
Descriptive statistics for the variables of main interest are reported in Table 2. Notably, sub-acute and chronic patients showed delayed memory deficits, recalling on Trial 4 approximately 18% and 15% fewer words than controls, respectively.
Table 2.
Descriptive statistics.
| Control | Sub-acute | Chronic | |
|---|---|---|---|
| M (SD) | M (SD) | M (SD) | |
| Age | 31.10 (12.01) | 28.24 (9.49) | 36.95 (11.31) |
| Education | 15.76 (2.19) | 13.38 (1.96) | 15.05 (2.91) |
| Executive | 1.03 (.53) | .63 (.50) | .73 (.66) |
| Cog-ctl | 1.05 (.48) | .88 (.60) | .56 (.75) |
| WM | .62 (.77) | .30 (.58) | .60 (.47) |
| Fluency | .96 (.61) | .45 (.65) | .68 (.78) |
| Sem clust T1 | .49 (1.83) | .14 (1.13) | .50 (1.52) |
| Sem clust T2 | 1.62 (2.51) | .57 (1.67) | 1.40 (2.07) |
| Sem clust T3 | 1.57 (2.40) | 1.30 (1.87) | 2.01 (2.15) |
| Sem clust T4 | 2.98 (2.94) | 1.29 (1.89) | 1.91 (1.65) |
| Ser clust T1 | .63 (1.36) | .41 (.92) | .33 (1.11) |
| Ser clust T2 | .51 (.94) | .53 (1.21) | .37 (1.18) |
| Ser clust T3 | .63 (1.51) | .57 (1.33) | .40 (1.63) |
| Ser clust T4 | .46 (1.83) | .05 (.69) | .19 (.75) |
| Tot-cor T1 | 6.52 (1.94) | 5.43 (1.44) | 6.14 (2.18) |
| Tot-cor T2 | 9.19 (2.56) | 7.95 (1.99) | 8.62 (2.25) |
| Tot-cor T3 | 10.62 (1.43) | 9.41 (1.74) | 9.86 (1.69) |
| Tot-cor T4 | 10.33 (1.74) | 8.16 (2.28) | 8.48 (2.36) |
Note. Descriptive statistics. (N = 79; control n = 21, sub-acute n = 37, chronic n = 21). Abbreviations: Cog-ctl = Cognitive-control. WM = Working memory. Sem clust. = Semantic clustering. Ser clust = Serial clustering. Tot-cor = total-correct. T1 - T4 refers to Trials 1 – 4.
Effect sizes and 95% confidence intervals (CIs) are reported in Table 3, for contrasts between sub-acute versus control, chronic versus control, and sub-acute versus chronic. Standardized effect size (Hedges' g) was calculated as the difference between means divided by the pooled estimate of standard deviation, corrected for bias using the method in Hedges & Olkin (1985). Effect sizes for total-correct on Trial 4 were relatively large for the sub-acute minus control contrast, g = −1.02, 95% CI = (−1.58, −.45). Likewise for the chronic minus control contrast, g = −.88, 95% CI = (−1.51, −.24). Results suggest again that both patient groups had delayed memory deficits relative to the control group.
Table 3.
Effect sizes
| Sub-acute minus control |
Chronic minus control | Sub-acute minus chronic |
|
|---|---|---|---|
| ES (CI) | ES (CI) | ES (CI) | |
| Age | −.27 (−.81, .27) | .49 (−.12, 1.11) | −.84 (−1.40, −.29) |
| Education | −1.14 (−1.72, −.57) | −.27 (−.88, .34) | −.70 (−1.25, −.15) |
| Executive | −.77 (−1.33, −.22) | −.49 (−1.11, .12) | −.18 (−.71, .36) |
| Cog-ctl | −.30 (−.84, .24) | −.76 (−1.39, −.14) | .48 (−.06, 1.02) |
| WM | −.48 (−1.03, .06) | −.03 (−.64, .57) | −.54 (−1.09, 0) |
| Fluency | −.79 (−1.35, −.24) | −.39 (−1.00, .22) | −.32 (−.86, .21) |
| Sem clust T1 | −.24 (−.78, .29) | .01 (−.60, .61) | −.28 (−.81, .26) |
| Sem clust T2 | −.52 (−1.06, .03) | −.09 (−.70, .51) | −.45 (−.99, .09) |
| Sem clust T3 | −.13 (−.66, .41) | .23 (−.38, .84) | −.40 (−.94, .14) |
| Sem clust T4 | −.72 (−1.27, −.17) | −.44 (−1.05, .17) | −.34 (−.88, .20) |
| Ser clust T1 | −.20 (−.73, .34) | −.24 (−.84, .37) | .08 (−.46, .62) |
| Ser clust T2 | .02 (−.52, .55) | −.13 (−.73, .48) | .13 (−.40, .67) |
| Ser clust T3 | −.04 (−.58, .49) | −.14 (−.75, .46) | .12 (−.42, .65) |
| Ser clust T4 | −.33 (−.87, .21) | −.19 (−.80, .42) | −.19 (−.73, .34) |
| Tot-cor T1 | −.66 (−1.21, −.11) | −.18 (−.79, .43) | −.40 (−.94, .14) |
| Tot-cor T2 | −.55 (−1.10, −.01) | −.23 (−.84, .37) | −.32 (−.86, .22) |
| Tot-cor T3 | −.73 (−1.28, −.18) | −.48 (−1.09, .14) | −.26 (−.80, .28) |
| Tot-cor T4 | −1.02 (−1.58, −.45) | −.88 (−1.51, −.24) | −.14 (−.67, .40) |
Note. Effect sizes. (N = 79; control n = 21, sub-acute n = 37, chronic n = 21). The standardized effect size (ES) was calculated as the difference between means divided by the pooled estimate of standard deviation, and was corrected for bias using the method in Hedges & Olkin (1985; Hedges' g). Abbreviations: Cog-ctl = Cognitive-control. WM = Working memory. Sem clust. = Semantic clustering. Ser clust = Serial clustering. Tot-cor = total-correct. T1 - T4 refers to Trials 1 – 4.
Correlations among key variables of interest are reported in Table 4. For this analysis we restricted our attention to Trial 4 (delayed memory trial) for semantic clustering, serial clustering, and total-correct recall. Notably, total-correct on Trial 4 was most strongly positively correlated with semantic clustering on Trial 4 and the executive function composite (r= .52 and r = .56, respectively). It was less strongly positively correlated with the three executive function domains of cognitive control, working memory, and fluency (r = .39, r = .34, and r = .42, respectively), and education (r = .28). It was only weakly, negatively correlated with age (r = −.11) and very nearly unrelated to serial clustering (r = .03). Also of interest, education was positively correlated with semantic clustering on Trial 4 and the executive function composite (r = .40 and r = .31, respectively), which were likewise positively correlated with each other (r = .27).
Table 4.
Correlations.
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Age | - | ||||||||
| 2. | Education | .23 | - | |||||||
| 3. | Executive | −.10 | .31 | - | ||||||
| 4. | Cog-ctl | −.31 | .02 | .66 | - | |||||
| 5. | WM | −.22 | .17 | .60 | .28 | - | ||||
| 6. | Fluency | .05 | .32 | .82 | .26 | .33 | - | |||
| 7. | Sem clust T4 | .08 | .40 | .27 | .05 | .25 | .28 | - | ||
| 8. | Ser clust T4 | −.21 | −.10 | .16 | .14 | .13 | .12 | −.42 | - | |
| 9. | Tot-cor T4 | −.11 | .28 | .56 | .39 | .34 | .42 | .52 | .03 | - |
Note. Correlations among selected variables. Coefficient is Pearson's r. (N = 79; control n = 21, sub-acute n = 37, chronic n = 21). Abbreviations: Cog-ctl = Cognitive-control. WM = Working memory. Sem clust. = Semantic clustering. Ser clust = Serial clustering. Tot-cor = total-correct. T4 refers to Trial 4, the delayed memory trial in HVLT-R.
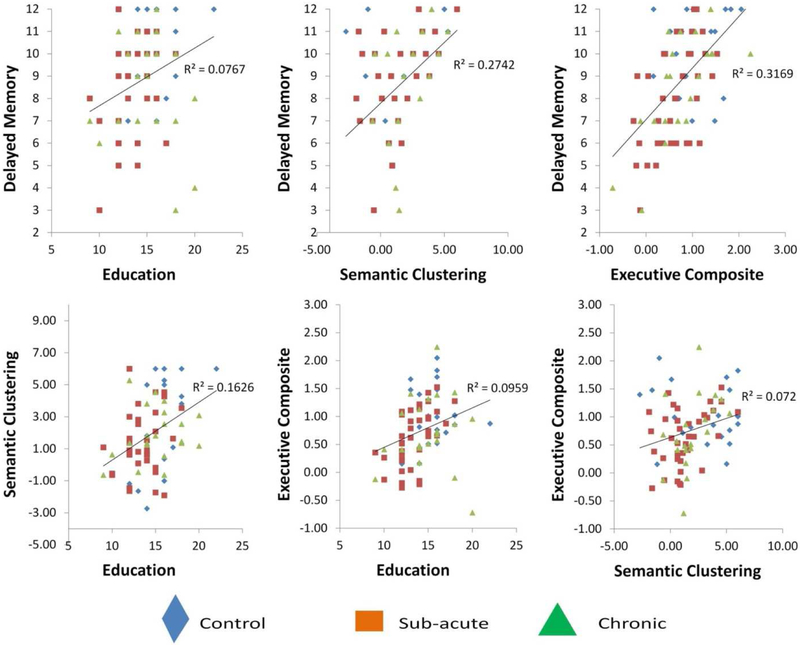
These results show that delayed memory performance was associated with better executive function abilities as well as with greater semantic clustering and higher levels of education. Additionally, executive function abilities, semantic clustering, and education were all positively inter-correlated with each other. For these reasons, we wished to further examine the degrees to which these variables were individually predictive of delayed memory performance, while statistically controlling for variance shared amongst them, using multiple regression. These results also show that the executive function composite was more strongly correlated with delayed memory performance than were the separate executive function domains. For this reason, in the following multiple regression model we restricted our focus to the executive function composite, among variables obtained from the NIH Examiner battery. Scatterplots are in Figure 1, depicting relationships among delayed memory performance, education, semantic clustering, and the executive composite, separately for the three groups.
Figure 1.
Scatterplots for key relationships among variables in the study (Total correct on Trial 4, the delayed memory trial, predicted by education, executive function, and semantic clustering (upper row), and inter-relationships among these predictors (lower row). Individuals in control, sub-acute TBI, and chronic TBI groups are represented separately (see legend).
Next, we used a multiple regression model to assess the degree to which variance in delayed memory performance (total-correct on Trial 4) was related to variance between groups, or to inter-individual variance in education, semantic clustering on Trial 4, serial clustering on Trial 4, and the executive function composite. These predictors were entered simultaneously, thus controlling for variance shared amongst them. The group factor was represented by two dummy-coded variables entered together, DC1 and DC2, respectively representing the contrasts between sub-acute (coded as '1') versus control (coded as '0'), and between chronic (coded as '1') versus control (coded as '0'). Thus the control group was the base level and the group effect was fully represented when both dummy-coded variables were entered simultaneously. Unstandardized B-weights and 95% CIs are reported in Table 5. About half the variance in delayed memory performance was accounted for by the model, Total R2 = .51, R2adj = .47.
Table 5.
Regression weights
| B (unstandardized) |
95% CI for B (Lower) |
95% CI for B (Upper) |
|
|---|---|---|---|
| Education | −.06 | −.24 | .13 |
| Sem clust T4 | .47 | .25 | .69 |
| Ser clust T4 | .26 | −.15 | .68 |
| Executive | 1.63 | .87 | 2.39 |
| DC1 (sub-acute vs control) | −.75 | −1.84 | .35 |
| DC2 (chronic vs control) | −.83 | −1.93 | .27 |
Note. Multiple regression model predicting the dependent variable Total-Correct on Trial 4, the delayed memory trial in HVLT-R (N = 79; control n = 21, sub-acute n = 37, chronic n = 21). Predictors were entered simultaneously: Education, Semantic clustering (Trial 4), Serial clustering (Trial 4), Executive function composite, as well as dummy coded contrasts representing sub-acute versus control (DC1) and chronic versus control (DC2). Abbreviations: Sem clust = Semantic clustering. Ser clust = Serial clustering. T4 = Trial 4. Total R2 = .51, R2adj = .47.
Semantic clustering and the executive function composite were relatively strong predictors of delayed memory performance. In practical terms of the original units, and holding other variables constant, for each additional unit of semantic clustering, individuals recalled about an additional half-word, B = .47, an increment that plausibly ranged from about one-quarter to two-thirds, 95% CI = (.25, .69). For general executive function, they recalled about one-and-a-half words more, B = 1.63, an increment plausibly ranging from almost a word, to almost two-and-a-half words extra, 95% CI = (.87, 2.39) (again while holding other variables constant). In contrast, serial clustering and education were noticeably weaker and more variable as predictors of delayed memory performance, B = .26, 95% CI = (−.15, .68), and B = −.06, 95% CI = (−.24, .13), respectively; with each variable sometimes associated with fewer words recalled, sometimes with more.
Clinical status remained strongly related to delayed memory performance while holding other variables constant, with sub-acute and chronic patients recalling about three-fourths of a word less than controls (B = −.75 and B = −.83, respectively). However, these parameter estimates were quite variable for both the sub-acute-minus-control contrast (DC1) and the chronic-minus-control contrast (DC2), 95% CI = (−1.84, .35), and (−1.93, .27), respectively; with each contrast sometimes associated with nearly two fewer words recalled, sometimes with a fraction of a word more.
Finally, we report in Supplemental Table 1 descriptive statistics and effect sizes for repetitions and intrusions (summed across trials), as well as hits and false alarm errors in the recognition test. These results show that sub-acute patients committed more repetitions than chronic patients, and chronic patients had fewer hits in the recognition portion of the test than controls. Repetitions and intrusions were only weakly negatively correlated with total-correct on Trial 4 (r = −.10, and r = −.15, respectively). In contrast, hits were strongly positively correlated with total-correct on Trial 4 (r = .57), while false alarm errors were moderately negatively correlated with total-correct on Trial 4 (r = −.33).
3.1. Supplemental analyses.
As noted in Method, the matching of control to sub-acute for age and gender was not 1:1, and the chronic group included 6 individuals with moderate TBI as well as those with mTBI (see again Table 1). To address these sources of heterogeneity, we reexamined in Supplemental Results all of the preceding analyses with fully matched groups, in which control and sub-acute groups were individually matched 1:1 for age and gender and the chronic group excluded the 6 individuals with moderate TBI. These Supplemental Results were overall similar to those reported here in the main text.
3.2. Results summary
Results showed that sub-acute as well as chronic patients showed delayed memory deficits compared to controls as measured by Trial 4 of HVLT-R, recalling approximately 18% and 15% fewer words, respectively. Both the executive process of semantic clustering and the general executive function composite from a standard neuropsychological test battery were strong predictors of delayed memory in both zero-order correlations and in multiple regression.
4. Discussion
According to a recent systematic review of meta-analyses (Karr et al., 2014), delayed memory deficits are among the most robust findings in studies of mTBI. However, there has been little work so far to identify different processes that may be underpinning these delayed memory deficits for mTBI. Given the apparent robustness of widely-observed delayed memory deficits for mTBI, it is remarkable that few previous studies have explored delayed memory performance in order to try to identify underlying mechanisms. Some studies have shown that mTBI patients engage in less semantic clustering during verbal learning (Bruce & Echemendia, 2003; Geary et al., 2011). But these studies did not show a direct relationship between semantic clustering and delayed memory performance. Here we went further than previous studies by showing that less use of semantic clustering was directly related to worse delayed memory performance. In contrast, serial clustering, an alternative method of organization in free recall considered to be less dependent on executive function, did not show strong relationships to delayed memory performance or clinical status. mTBI has long been associated with problems with executive function (Stuss, 2011). Here we showed that poorer general executive abilities were directly related to worse delayed memory performance. Regression results showed that semantic clustering and general executive function each accounted for unique variance in delayed memory performance. These are novel findings implicating executive functions as underpinning delayed memory deficits following mTBI.
Additionally, we advanced the study of delayed memory deficits for mTBI by examining the generality of these deficits across widely-differing mTBI groups, i.e., sub-acute (within two weeks of injury) and chronic (more than three months from injury). As noted, the nature of cognitive dysfunction for chronic post-concussive syndrome remains controversial (Mayer et al., 2017; Ponsford et al., 2012). Specifically, it remains unclear exactly which cognitive domains remain affected over the long-term in chronic, post-concussive syndrome (Ponsford et al., 2012). Our results showed that delayed memory deficits were observed in both sub-acute and chronic mTBI. This novel finding suggests that delayed memory may indeed belong in the set of cognitive domains that remain affected in chronic, post-concussive syndrome. However, this testable hypothesis must be investigated further in confirmatory studies with larger samples.
4.1. Effect sizes.
The Hedges' g effect size estimate for the delayed memory deficit for sub-acute patients was estimated at 1.02 (positive sign indicating worse performance by patients versus controls), with 95% CI indicating a range from .45 to 1.58. For chronic patients, Hedges' g was estimated at .88, ranging from .24 to 1.51. To make direct comparisons with published meta-analytic effect sizes for delayed memory deficits for mTBI (Karr et al., 2014), we converted our Hedges' g effect sizes to Cohen's d. For sub-acute versus controls our Cohen's d effect size was estimated to be 1.07, while for chronic it was .89. For comparison Karr et al. (2014) reported Cohen's d estimates for delayed memory deficits for mTBI patients ranging from .13 to .71 across six published meta-analyses. Thus the effect sizes for delayed memory deficits in our study were at least as high as published meta-analytic values. This robustness of mTBI-related delayed memory deficits across a large number of studies including our own strongly suggests that delayed memory is a core cognitive domain that is predictably impaired by mTBI. It is recommended therefore that delayed memory should be a major priority for mTBI clinicians and researchers.
As an indicator of cognitive reserve, educational attainment has been previously shown to attenuate effects of moderate and severe TBI on cognitive performance (Sumowski et al., 2013). Somewhat differently, our results showed that years of education was not a strong predictor of delayed memory performance, while controlling for variance shared with semantic clustering and general executive function. While many TBI studies typically report education as part of the standard demographic information, few have examined education as a predictor of cognitive deficits after mTBI. In this respect the present work makes another novel contribution to better understanding the cognitive effects of mTBI.
4.2. Limitations.
First we must note that our samples were modest in size (N = 79; control n = 21, sub-acute n = 37, chronic n = 21). Future work should re-examine these results in a confirmatory design (please see also section 4.3) with larger samples. Additionally, it should be noted that our examination of the generality of delayed memory deficits across mTBI subpopulations (i.e., sub-acute versus chronic) was made in a cross-sectional design. Future work should implement a longitudinal design, to better assess the extent to which delayed memory deficits for some individuals with mTBI-related problems persist over the longer-term (again, please see also section 4.3). It should be noted again that the matching of control to sub-acute for age and gender was not 1:1, and there were several additional differences between chronic and sub-acute mTBI besides time since injury (see again Table 1). However, we addressed these sources of heterogeneity in supplemental analyses, in which control and sub-acute groups were individually matched 1:1 for age and gender and chronic group included only mTBI, and results were much the same as in the main text (Supplemental Results).
4.3. Hypothesis-generation.
Findings of this exploratory study immediately suggest the following basic hypotheses, which could be examined in future confirmatory work with larger samples.
H1a. Accounting for general executive functions will mediate delayed memory deficits observed for individuals with mTBI at the sub-acute stage.
H1b. Accounting for semantic clustering, a cognitive strategy employed during the memory task, will mediate delayed memory deficits observed with mTBI at the sub-acute stage.
H2a. In a longitudinal design, delayed memory deficits will persist over time for a subset of individuals with mTBI-- specifically those who develop chronic-stage, post-concussive syndrome (determined independently of memory test).
H2b. In a longitudinal design, general executive functions at the sub-acute stage will predict which individuals will continue to show delayed memory deficits, among those who develop chronic-stage, post-concussive syndrome.
5. Conclusion.
Overall, results provide novel evidence implicating executive dysfunction as contributing to the widely-observed delayed memory deficits for mTBI. Delayed memory is an important cognitive ability, critical for successful functioning across a wide variety of daily-life situations. Understanding the mechanisms underlying delayed memory deficits in sub-acute and chronic mTBI patients may facilitate the development of much needed treatments.
Supplementary Material
Acknowledgements
This study was a component of two larger studies supported by a grant from the National Institute of General Medical Sciences (NIGMS) 1P20GM109089-01A1. Details of these two projects can be found at the NIH Research Portfolio Online Reporting Tools (RePORTER; https://projectreporter.nih.gov/reporter.cfm). For the study referred to in the paper as Cavanagh et al., please use the following link. https://projectreporter.nih.gov/project_info_description.cfm?aid=9525979&icde=45112451&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC&pball=
For the study referred to in the paper as Quinn et al., please use the following link. https://projectreporter.nih.gov/project_info_description.cfm?aid=9525980&icde=45112506&ddparam=&ddvalue=&ddsub=&cr=5&csb=default&cs=ASC&pball=
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No part of the study procedures or analyses was pre-registered prior to the research being conducted.
The equation for expected semantic clustering includes a numerator term for the number of correct responses. However, the semantic clustering index is in-principle independent of total-correct. Correct responses can be achieved by other organization methods (e.g., serial clustering) or even by no strategy at all. Semantic clustering index and total-correct are only asymmetrically related. As semantic clustering increases, total-correct necessarily increases. However, it is not true that as total-correct increases, semantic clustering necessarily increases. Observed semantic clusters are required to be correct responses by definition. But correct responses are not required by definition to belong to semantic clusters. Correct responses may be the result of semantic clustering or other strategies such as serial clustering, or of no strategy at all. Likewise, the equation for expected serial clustering includes a numerator term for the number of correct responses. However, the serial clustering index is in principle independent of total-correct for the same reasons that apply to semantic clustering.
Contributor Information
James M. Broadway, University of New Mexico Health Sciences Center, Department of Neurosciences
Rebecca E. Rieger, University of New Mexico, Department of Psychology
Richard A. Campbell, University of New Mexico Health Sciences Center, Department of Psychiatry and Behavioral Sciences
Davin K. Quinn, University of New Mexico Health Sciences Center, Department of Psychiatry and Behavioral Sciences
Andrew R. Mayer, Mind Research Network
Ronald A. Yeo, University of New Mexico, Department of Psychology
J. Kevin Wilson, University of New Mexico, Department of Psychology.
Darbi Gill, University of New Mexico Health Sciences Center, Department of Neurosciences.
Violet Fratzke, University of New Mexico, Department of Psychology.
James F. Cavanagh, University of New Mexico, Department of Psychology
References
- Alvarez JA & Emory E (2006). Executive function and the frontal lobes: A meta-analytic review. Neuropsychology Review, 16, 17 – 42. [DOI] [PubMed] [Google Scholar]
- Bondi CO, Semple BD, Noble-Haeusslein L, Osier ND, Carlson SW, Dixon CE, … Kline AE (2014). Found in translation: Understanding the biology and behavior of experimental traumatic brain injury. Neuroscience and Biobehavioral Reviews, 58, 123–146. 10.1016/j.neurobirev.2014.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, & Benedict RHB (2001). Hopkins Verbal Learning Test – Revised (HVLT-R). Lutz, FL: Psychological Assessment Resources. [Google Scholar]
- Bruce JM & Echemendia RJ (2003). Delayed-onset deficits in verbal encoding strategies among patients with mild traumatic brain injury. Neuropsychology, 17, 622–629. [DOI] [PubMed] [Google Scholar]
- Burianova H, McIntosh AR, & Grady CL (2010). A common functional brain network for autobiographical, episodic, and semantic memory retrieval. NeuroImage, 49, 865–874. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, & Kalmar K (1995). Persistent Concussion Syndrome: The structure of subjective complaints after mild traumatic brain injury. Journal of Head Trauma Rehabilitation, 10(3), 1–17. [Google Scholar]
- Geary EK, Kraus MF, Pliskin NH, & Little DM (2010). Verbal learning differences in chronic mild traumatic brain injury. Journal of the International Neuropsychological Society, 16, 506–516. [DOI] [PubMed] [Google Scholar]
- Geary EK, Kraus MF, Rubin LH, Pliskin NH, & Little DM (2011). Verbal learning strategy following mild traumatic brain injury. Journal of the International Neuropsychological Society, 17, 709–719. [DOI] [PubMed] [Google Scholar]
- Gershberg FB, & Shimamura AP (1996). Impaired use of organizational strategies in free recall following frontal lobe damage. Neuropsychologia, 13, 1305–1333. [DOI] [PubMed] [Google Scholar]
- Hedges LV & Olkin I (1985). Statistical methods for meta-analysis. Orlando, FL: Academic Press. [Google Scholar]
- Karr JE, Areshenkoff CN, & Garcia-Barrera MA (2014). The neuropsychological outcomes of concussion: A systematic review of meta-analyses on the cognitive sequelae of mild traumatic brain injury. Neuropsychology, 28, 321–336. [DOI] [PubMed] [Google Scholar]
- Kramer JH, Mungas D, Possin KL, Rankin KP, Boxer AL, Rosen HJ, Widmeyer M (2014). NIH EXAMINER: Conceptualization and development of an executive function battery. Journal of the International Neuropsychological Society, 20, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, & Chao LL (2001). Semantic memory and the brain: Structure and processes. Current Opinion in Neurobiology, 11, 194–201. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Quinn DK, & Master CL (2017). The spectrum of mild traumatic brain injury: A review. Neurology, 89, 623–632. 10.1212/WNL.0000000000004214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea M, Iverson GL, McAllister TW, Hammeke TA, Powell MR, Barr WB, & Kelly JP (2009). An integrated review of recovery after mild traumatic brain injury (MTBI): Implications for clinical management. The Clinical Neuropsychologist, 23, 1368–1390. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, & Howerter A (2000). The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology, 41, 49–100. [DOI] [PubMed] [Google Scholar]
- Moscovitch M (1992). Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience, 4, 257–267. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, & Ingvar M (2003). Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropsychologia, 41, 371–377. [DOI] [PubMed] [Google Scholar]
- Ponsford J, Cameron P, Fitzgerald M, Grant M, Mikocka-Walus A, & Schonberger M (2012). Predictors of postconcussive symptoms 3 months after mild traumatic brain injury. Neuropsychology, 26, 304–313. [DOI] [PubMed] [Google Scholar]
- Schneeweis N, Skirbekk V, & Winter-Ebmer R (2014). Does education improve cognitive performance four decades after school completion? Demography, 51, 619–643. [DOI] [PubMed] [Google Scholar]
- Simons JS & Spiers HJ (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews Neuroscience, 4, 637–648. [DOI] [PubMed] [Google Scholar]
- Stricker JL, Brown GG, Wixted J, Baldo JV, & Delis DC (2002). New semantic and serial clustering indices for the California Verbal Learning Test—Second Edition: Background, rationale, and formulae. Journal of the International Neuropsychological Society, 8, 425–435. [DOI] [PubMed] [Google Scholar]
- Stuss DT (2011). Traumatic brain injury: Relation to executive dysfunction and the frontal lobes. Current Opinion in Neurology, 24, 584–589. [DOI] [PubMed] [Google Scholar]
- Stuss DT & Alexander MP (2000). Executive functions and the frontal lobes: A conceptual view. Psychological Research, 63, 289 – 298. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Chiaravallotti N, Krch D, Paxton J, & DeLuca J (2013). Education attenuates the negative impact of traumatic brain injury on cognitive status. Archives of Physical Medicine and Rehabilitation, 94, 2562 – 2564. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN (1996). Test of Memory Malingering (TOMM). New York, NY: :: Multi-Health Systems, Inc. [Google Scholar]
- Wagner AD (2002). Cognitive control and episodic memory: Contributions from prefrontal cortex In Squire LR and Schacter DL (Eds.) Neuropsychology of Memory (pp. 174–192). New York, NY: Guilford Press. [Google Scholar]
- Woods SP, Rippeth JD, Conover E, Gongvatana A, Gonzalez R, Carey CL, … The HIV Neurobehavioral Research Center Group (2005). Deficient strategic control of verbal encoding and retrieval in individuals with methamphetamine dependence. Neuropsychology, 19, 35–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.