Abstract
Functional neuroimaging studies have attempted to explore brain activity that occurs with tic occurrence in subjects with Tourette syndrome (TS). However, they are limited by the difficulty of disambiguating brain activity required to perform a tic, or activity caused by the tic, from brain activity that generates a tic. Inhibiting ticcing following the urge to tic is important to patients’ experience of tics and we hypothesize that inhibition of a compelling motor response to a natural urge will differ in TS subjects compared to controls. This study examines the urge to blink, which shares many similarities to premonitory urges to tic. Previous neuroimaging studies with the same hypothesis have used a one-size-fits-all approach to extract brain signal putatively linked to the urge to blink. We aimed to create a subject-specific and blink-timing-specific pathophysiological model, derived from out-of-scanner blink suppression trials, to eventually better interpret blink suppression fMRI data. Eye closure and continuously selfreported discomfort were reported during five blink suppression trials in 30 adult volunteers, 15 with a chronic tic disorder. For each subject, data from four of the trials were used with an empirical mathematical model to predict discomfort from eye closure observed during the remaining trial. The blink timing model of discomfort during blink suppression predicted observed discomfort much better than previously applied models. Combining this approach with observed eye closure during fMRI blink suppression trials should therefore extract brain signal more tightly linked to the urge to blink. The simple mean of time-discomfort curves from each subject’s other trials also outperformed older models. The TS group blinked more than twice as often during the blink suppression block, and reported higher baseline discomfort, smaller excursion from baseline to peak discomfort during the blink suppression block, and slower return of discomfort to baseline during the recovery block.
Keywords: Tourette syndrome, tic disorders, inhibition (psychology), urge, blinking
1. Introduction
Often the effort to control these wild sensations seems to be more than the human spirit can bear. … At the onset of an impulse … it does not seem possible for the state to be relieved by anything other than the action in progress.
— Bliss, Cohen, & Freedman (1980), discussing premonitory urges to tic
Tourette syndrome (TS) is a chronic neuropsychiatric developmental disorder characterized by motor and vocal tics beginning in childhood (American Psychiatric Association, 2013). Tics differ from other abnormal movements because they can be suppressed for some period of time, although suppression is associated with increasing discomfort (Jankovic, 1997). Behavior therapies are first-line treatments for TS and focus on the link between tics and their preceding urges. Inhibition of tics is part of daily life for most people with TS and it is also an important part of the theory and technique of exposure and response prevention (Verdellen, Keijsers, Cath, & Hoogduin, 2004; Woods et al., 2008). Previous functional imaging studies attempting to explore brain activity involved with tic suppression have been complicated by the fact that successful suppression is unavoidably accompanied by a decrease in movement, which reflects inverse changes in brain activity related to tics during suppression.
Over 90% of people with tics report premonitory urges (PU), meaning an uncomfortable sensation or the perceived need to perform the tic (Kwak, Dat, & Jankovic, 2003; Reese et al., 2014; Crossley & Cavanna, 2013; Miguel et al., 2000; Cavanna, Black, Hallett, & Voon, 2017). Such urges build up prior to a tic and then are transiently relieved by the tic before building up again (Leckman, Walker, & Cohen, 1993; Reese et al., 2014). In fact, many patients with TS feel that the premonitory phenomena are primary, rather than the tic per se (Bliss, Cohen, & Freedman, 1980; Bullen & Hemsley, 1983). This view contributes to the interpretation that tics may be maintained and reinforced by the consequent reduction in discomfort (premonitory urge); premonitory urges thus “may represent an important enduring etiological consideration in the development and maintenance of tic disorders” (Specht et al., 2013). Supporting evidence for this view includes the observation that patients who believe tics are unavoidable or barely suppressible once they experience a premonitory urge have higher impairment ratings on the YGTSS and higher premonitory urge severity (Steinberg et al., 2013) A tic—especially one associated with a premonitory urge—may therefore be viewed as a transient failure of motor inhibition. The precise relationship of premonitory urges to tics remains a debated topic among researchers.
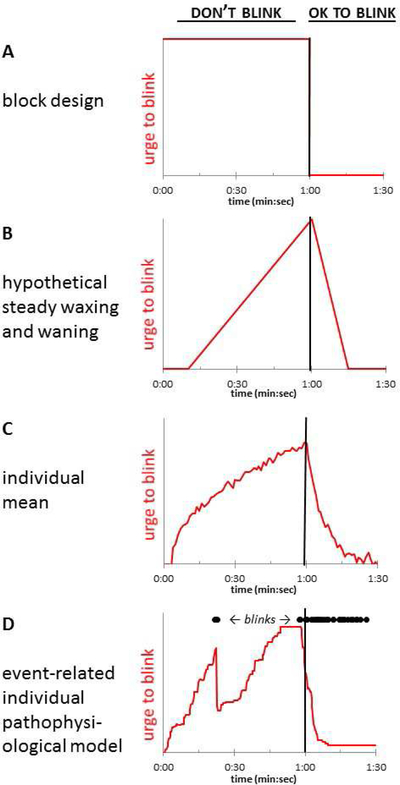
The urge to tic shares many characteristics with natural urges, such as the urge to blink after keeping one’s eyes open for an extended period of time (Jackson, Parkinson, Kim, Schüermann, & Eickhoff, 2011; Belluscio, Tinaz, & Hallett, 2011). The present report was motivated by a study intended to examine the brain activity correlated with the urge to blink, or with imminent failure of blink inhibition, in TS and control subjects. Importantly, the urge to blink can be examined in subjects with or without tics. A necessary early step in such an effort is to identify the timing of changes in the severity of the urge to blink. Previous functional MRI (fMRI) studies examining the urge to blink associated with voluntary eye blink suppression used two approaches to do so. The simplest approach compared mean BOLD signal during blink suppression blocks to mean signal during blocks in which subjects blinked normally, represented by the square-shaped (red) function in Figure 1A (Lerner et al., 2009; Mazzone et al., 2010). Berman et al. hypothesized that relevant brain activity might be identified better by a linear model of urge build-up during blink suppression, as in Figure 1B (Berman, Horovitz, Morel, & Hallett, 2012). In fact, their study reported urge severity on a group level that somewhat resembled this sawtooth model.
Figure 1.
(A) Block design comparing “OK to blink” and “don’t blink” blocks (Mazzone et al., 2010). (B) The sawtooth approximation of urge to blink used by Berman and colleagues (Berman, Horovitz, Morel, & Hallett, 2012). (C) An example average of self-rated time–urge curves from all of a given subject’s trials. (D) An event-related, individualized model proposed for this study, in which the time–urge curve depends on the specific timing of the blinks observed in a single trial, with urge to blink decreasing after each eye closure. In this model, a given time–urge curve would thus apply only to a single experimental block in a single subject. The curves in (C) and (D) were drawn for illustration, without reference to data.
These models enabled analysis of fMRI blink suppression data, but we saw potential for improvement in trying to achieve the same goal. First, these block and sawtooth patterns of discomfort or urge to blink during blink suppression were hypothesized rather than derived from subject-reported data. We found early on that self-reported discomfort during blink suppression followed neither the box nor the sawtooth model (Claudio Torres, Black, Richards, & Black, 2014). Second, urge ratings over time may vary by subject. Figure 1C represents a simple average of several self-report trials from one subject, an approach that would address these two concerns. Third, neither model takes into account the likely effects on urge from any eye closures that occur during a blink suppression block. Such effects would depend on the timing of blinks observed in a specific block; at the extreme, if a subject continued blinking at his/her baseline rate when instructed not to blink, one would expect no buildup in that subject’s urge to blink. Thus we developed a model to predict urge to blink based on observed blink timing, expecting that the urge to blink would decrease somewhat after each period of eye closure, as diagrammed in Fig. 1D. We hypothesized that such a model, taking into account observed eye closures, would better fit subjects’ reported discomfort when instructed not to blink.
Our objectives with this study were:
To create a subject-specific and blink-timing-specific model from out-of-scanner blink suppression trials in the same subject (as in Fig. 1D), to facilitate eventual application to identify brain regions with discomfort-related activity during a “don’t blink” fMRI session.
To test whether the new model more accurately predicted discomfort based on blink timing than did the square or sawtooth models shown in Fig. 1 A,B, or the individual mean model in Fig. 1C.
To estimate the accuracy of the new model compared to direct subject report, using a leave-one-out cross-validation (LOOCV) approach.
To compare blinking, as well as discomfort with and efficacy of blink suppression, between subjects with and without Tourette syndrome.
2. Materials & Methods
2.1. Ethics approvals
This research was approved in advance by the Washington University Human Research Protection Office (protocols #201303016 and #201506018). All subjects participated only after written informed consent.
2.2. Subjects
We examined data from two studies. We report how we determined our sample size, all data exclusions, all inclusion/exclusion criteria, whether inclusion/exclusion criteria were established prior to data analysis, all manipulations, and all measures in the study.
Study 1. This study included 32 adult and 2 youth volunteers who continuously reported urge to blink during blink suppression; these data were examined only to improve our methods for measuring urge to blink, and to select models to analyze future data.
Study 2. This study provided the data reported below. It included 15 adults with TS (age 28 ± 3.8 years, range 18–30; 6 Male) and 15 control subjects (age 26 ± 2.2 SD; 6 Male). Eligible participants for the TS group included those who met the criteria for DSM5 Tourette’s Disorder or Persistent Motor or Vocal Tic Disorder (American Psychiatric Association, 2013) with tics in the past week. Another experiment being conducted using the same subject group also required that they also must have had a tic involving the right eyebrow, eyelid or cheek at some point in their life. Nine subjects had OCD and four had ADHD. Exclusion criteria for all subjects included contraindication to MRI, intellectual disability, substantial autistic traits (SRS T score ≥ 75; (Constantino, 2013)), dementia, currently active major depression or substance abuse, a neurological or general medical condition that would interfere with study participation, or lifetime history of psychosis, mania or somatization disorder. Potential controls were also excluded for a past or present tic disorder in the subject or a first-degree relative. TS symptom severity was assessed using the Yale Global Tic Severity Scale (YGTSS; (Leckman et al., 1989)) and TS symptom history using the Diagnostic Confidence Index (DCI; (Robertson et al., 1999)). The overall severity of premonitory urges was assessed using the Premonitory Urge for Tics Scale (PUTS; (Woods, Piacentini, Himle, & Chang, 2005)), and each subject also completed the Beliefs About Tics Scale (BATS; (Steinberg et al., 2013)). Symptoms of OCD and ADHD were rated using the Yale-Brown Obsessive Compulsive Disorder Scale (Y-BOCS; (Goodman, 1989)) and ADHD Rating Scale (Barkley, 1990) respectively.
2.3. Data collection
Demographic data were collected and managed using REDCap electronic data capture tools hosted at Washington University in St. Louis (Harris et al., 2009). Each subject was seen twice, first for clinical information and to perform the “out of scanner” blink and tic suppression tasks, and then they returned for a session in the MRI suite where they performed the blink suppression and other tasks in the scanner. This report will focus exclusively on the ratings reported during the out-of-scanner tasks. In these, they were observed for a two-minute baseline period to measure the baseline blink rate, and then performed two blink suppression tasks. In each of these tasks, the subject performed 5 trials seated in front of a computer monitor, each characterized by 60 seconds of blink suppression (the monitor read “DON’T BLINK”), followed by 30 seconds during which they were allowed to blink freely (“OK TO BLINK”). During one task (“discomfort trials”), each subject continuously rated discomfort using the vertical position of a mouse-controlled pointer, with continuous feedback that interpreted the position on a 0–9 scale (Subjective Units of Distress) (Benjamin et al., 2010; Specht et al., 2013). The other task (“effort trials”) was identical except that, rather than rating discomfort, subjects were instructed to rate the effort being used to keep the eyes open. Order of tasks (discomfort, effort) was balanced across subjects. The baseline blinking condition was included to ensure that subjects exhibited relative suppression during the “don’t blink” task. Eye closures were recorded using a video camera with 30 or 60 Hz frame rates (we switched halfway through the study to an infrared camera).
We had hypothesized that effort or ability to suppress blinks might vary independently of discomfort: One person might have relatively high discomfort while holding the eyes open, but due to greater effort or greater inhibitory ability might blink no more than another person who experienced relatively little discomfort. Thus we chose to separate urge to blink into two hypothesized components, discomfort and effort, and tracked each separately. However, in our initial pilot data, mean self-rated discomfort and effort curves were fairly similar (Claudio Torres, Black, Richards, & Black, 2014), so we focused our effort on the discomfort ratings (see also section 3.5).
Tic subjects were also observed by video recording of the upper body on the screening day for 5 minutes of baseline (“OK to tic”) and 5 minutes of tic suppression.
2.3.1. Blink detection
Blinks from the first two seconds of each trial were ignored to allow for the participant to adjust to the start of each condition. Because many subjects used only a subset of the full range from 0–9, the discomfort scores were z-standardized for each subject to reduce variance across subjects, as suggested by (Brandt et al., 2016): . The SD was computed over all ratings recorded by the subject in all blocks. A custom program written in C++ was modified from code kindly provided by Dr. Aleksandra Królak to identify whether on each frame of the video the subject’s eyes were closed. The new program compares the intensity histogram of a region-of-interest (ROI) centered on each iris in each video frame to a baseline frame in which the eyes were open. The pupils and iris widths had been marked manually on the baseline frame. The ROIs of the subsequent frames were centered automatically using a motion detection algorithm to follow head movement. The root mean squared error (RMSE) of the ROI intensity histogram comparison was computed for each frame, and frames in which the RMSE value exceeded a specified threshold were interpreted as eye closures. The results were then visually checked to see if the program happened to miss a blink due to head movement and timing was adjusted as needed. A Python script was used to eliminate suprathreshold deviations lasting less than 100 ms and to create a binary time series indicating eye closure at each video frame. The eye closure time series and discomfort ratings, which were recorded at 4 Hz, were synchronized and resampled to identical 0.25 s blocks. For each quarter-second time bin, the eye closure fraction fi was defined as the number of eyes-closed frames within a given bin, divided by the total number of frames in that bin. Blink rate appeared similar over repeated trials, so means across trials were used in subsequent analyses. Several subjects were outliers, as defined by a blink rate more than 1.5 interquartile ranges below the first quartile or above the third quartile, so blink rates were compared between groups using the nonparametric Mann-Whitney test.
2.3.2. Tic detection
To analyze tic suppression, the videos were clipped and renamed so that the rater was blind to the condition. Each subject was recorded while sitting alone in a quiet room and tic detection included the upper body. These tic suppression trials were done separately from the blink suppression trials and the subjects did not continuously self-report their discomfort. Author KU, a movement disorders neurologist, reviewed these recordings and indicated the presence of a tic with a button press using a slightly modified version of our TicTimer software (Black, Koller, & Black, 2017). Tic frequency and the number of 10-second tic free intervals per minute were calculated from the TicTimer output and tic suppression was calculated using the difference between the two conditions as a fraction of the baseline.
2.4. Model development
We examined data from Study 1 and Study 2 to choose the form of an empiric mathematical model of self-reported discomfort. In order to build a subject-specific and blink-timing-specific model we first looked at subjects’ raw discomfort scores and settled on a model in which discomfort during the “don’t blink” block increases as the square root of time, with brief dips in discomfort after eye closure, and discomfort during the “OK to blink” block decreases exponentially back to its baseline level when the eyes are closed. Details on how the model was created are provided in the Appendix. We also compared these results to a simpler “individual mean model” comprising the average across trials of a subject’s z-scored discomfort ratings.
2.4.1. Bayesian parameter estimation
The parameters a, b, c, h, k and r for the blink timing model were fit to self-reported discomfort over time using observed blink timing and the Bayesian Data-Analysis Toolbox, which implements a Markov chain Monte Carlo method (Bretthorst & Marutyan, 2016; Bretthorst, 1988). Prior probabilities for each parameter were chosen as follows. To minimize the risk that the priors would constrain the estimation, we assumed uniform priors over [0, 104] for a, [−10, 10] (standard deviation units) for b, [0,30] seconds for c, and [0,10] s−1 for r. The data presented in the appendix suggested the following priors for the coordinates (h,k) of the vertex of the parabola representing a transient decrease in discomfort after eye closure: for h, a uniform prior from 0 to 10s, and for k, a Gaussian prior with mean −0.4, S.D. 1.0, range [−1,0]. Mean values of these parameters can be found in Table 2.
Table 2.
Comparison of average parameter values for subjects with TS and control participants.
| Block | Model parameter | Units | TS, N=14 * | Ctrl, N=14 * | p (2-tailed t test) |
|---|---|---|---|---|---|
| both blocks | model fit (Pearson’s r) | — | 0.82 (0.59–0.93) | 0.9 (0.79–0.95) | 3.36×10−5 |
| don’t blink | a (change with time in discomfort) | SUDS·s−½ † | 0.27 ± 0.1 | 0.33 ± 0.05 | 3.49×10−4 |
| don’t blink | b (baseline discomfort) | SUDS | −0. 879 ± 0.29 | −1.06 ± 0.23 | 2.13×10−4 |
| don’t blink | c (time offset of change in discomfort) | s | 6.53 ± 9.58 | 6.41 ± 5.93 | 0.93 |
| don’t blink | h (half duration of reduction with one blink) | s | 4.7 ± 3.22 | 4.89 ± 4.09 | 0.76 |
| don’t blink | k (amplitude of reduction with one blink) | SUDS | −0.31 ± 0.18 | −0.34 ± 0.28 | 0.41 |
| OK to blink | r (recovery rate) | s−1 | 1.72 ± 1.4 | 2.49 ± 2.57 | 0.03 |
values are mean ± SD, except for model fit, for which the t test was applied to Fisher’s z(r) scores; the mean ± SD for each group were converted back to r values and reported here as r(mean) (r(mean−SD)−r(mean+SD)).
SUDS = Z scores for subjective units of distress.
2.4.2. Accuracy
To evaluate the accuracy of a model’s prediction of discomfort, we used data from 4 trials to predict discomfort of the 5th trial, in a leave-one-out cross-validation (LOOCV) approach. Specifically, we excluded one of the 5 suppression trials and estimated model parameters from the data from the remaining 4 trials, using the “Enter ASCII Model” package from the Bayesian Data-Analysis Toolbox. If the model served as a good predictor, then the discomfort predicted by that model with parameters based on the remaining trials should closely match the actual discomfort reported during the excluded trial. For the blink timing model, the parameters based on the remaining trials were applied to the observed blink timing of the excluded trial to estimate discomfort for the excluded trial. Each subject had 5 complete suppression trials, and therefore 5 sets of estimated parameters were computed.
2.5. Comparison to other models
To compare the 4 models, we calculated the correlation values for each LOOCV step, i.e. the Pearson r value comparing the model fit to the remaining 4 trials vs. the data from the trial left out. Correlation was used to measure model accuracy because the individual subject time–urge signal, predicted from blink timing in the fMRI session and the parameters identified from the out-of-scanner blink suppression trials, will be used as a contrast in a general linear model to identify regions in each subject that correspond to subject discomfort during periods of effective blink suppression in the scanner. Pearson r values were compared between models using the t-test after Fisher’s z transform. The models were also compared using the binomial test in terms of how often one model outperformed another, with the null hypothesis that either model is equally likely to be superior on each run or in each subject.
2.6. Availability of data and analysis tools
This study’s procedures and analyses were not pre-registered prior to the research being conducted. Study data, digital study materials, and analysis code can be found in the following publicly accessible repository: https://github.com/BlackHershey/blink-TS.
3. Results
A total of 28 participants completed the blink suppression trials to be analyzed in the results. All subjects were from study 2. Data were excluded from one control subject who appeared not to understand the task and did not suppress blinking in the blink suppression condition as compared to the baseline condition. A TS participant was excluded from the model comparison analysis because the first 30 seconds of the first task trial was not recorded on video. All participants rated discomfort and effort on separate trials.
3.1. Blink rate
The median number of blinks during a 60-s suppression trial was 2.0 for controls (interquartile range 0.8, 3.0) and 4.6 (3.1, 9.1) for TS subjects. During the 30 seconds in which they were told it was OK to blink, control subjects showed a median of 47.2 blinks per minute (40.0, 72.4) and TS subjects 46.4 (41.6, 54.4) blinks per minute. The groups differed significantly on blinking during the suppression condition (W = 42, p = 0.012) but not in the OK-to-blink period (W = 106, p = 0.712). The groups did not differ significantly on blink rates during the initial 2-minute baseline period (W = 77, p = 0.357), with baseline rates of 17.5 (11.5, 21.5) blinks per minute for controls and 25.5 (15.8, 31.5) for the TS group. We also computed a secondary measure, blink suppression efficacy, as a fraction of the baseline rate. Blink suppression efficacy was 91.6% (85.1%, 95.6%) for the control group and 79.2% (71.4%, 86.4%) for the TS group (W = 52, p = 0.038). In the TS group, the minimum blink suppression efficacy was 52%.
3.2. Tic suppression
By contrast, tic suppression efficacy in the TS group was less complete and more variable; median tic suppression was only 56% (range −29% to 100%). Blink suppression and tic suppression within TS subjects were not correlated (r = −0.13, p = 0.68).
3.3. Discomfort ratings
The raw discomfort ratings averaged across all trials for each subject did not differ significantly between groups (TS 3.2 ± 1.7, control 3.6 ± 1.0, p = 0.50). However, scaled discomfort ratings and blink timing varied substantially among subjects, supporting the individual-specific pathophysiological modeling approach. The model represented by Equations 3 and 5 of the appendix was applied to each subject and the average parameter estimates are summarized in Table 2.
The differences in the mean parameter values for a, b and r can be understood by their effect on the time–discomfort curve estimated for blink timing from one trial, as shown in Fig. 2.
Figure 2. Interpreting the different parameter values between TS and control subjects.
Discomfort during a suppression trial modeled using the average parameter values from Table 2 for both TS and control subjects, applied to a single set of observed blinks. The timing of blinks is shown by vertical black marks near the top of the graph, to help visualize the change in discomfort after a blink. Longer eye closures appear as thicker black marks.
3.4. Model comparison
In the LOOCV testing, the model that returned the highest correlation r with the reported discomfort was the individual mean model for 13 subjects, the blink timing model for 13 subjects, the sawtooth model for 2 subjects, and the box model for none. If all models were equally likely, the chance that any model would “win” in 13 or more subjects is .011 (binomial test). The correlations differed significantly (ANCOVA on mean z(r) values for each subject, with factors for model, diagnosis and their interaction; model significant at p<.001, diagnosis not significant, interaction p=.08). In post hoc tests (Tukey HSD), the box model performed worse than each of the others (p<.001 for each), while the individual mean (p=.029) and blink timing models (p=.005) were each superior to the sawtooth model but did not differ significantly from each other (p=.929).
3.5. Effort Ratings
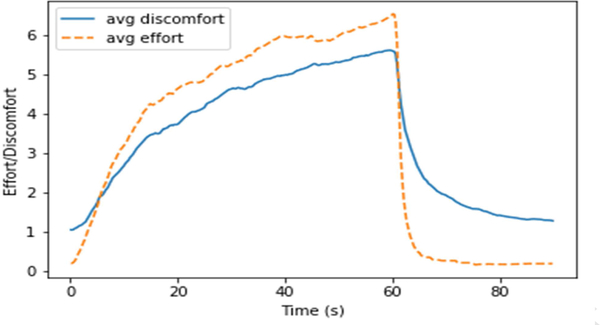
Finally, because we had collected both effort and discomfort ratings from each subject, we reviewed whether self-rated effort (to keep the eyes open) produced different signal than self-rated discomfort. The mean ratings across groups were nearly identical but for scaling (r=0.962; Figure 3). Across individuals the results were similar, with median r=0.921 (range 0.521–0.983, interquartile range 0.858–0.950). This result further supported our pilot study’s suggestion that self-rated effort and discomfort during blink suppression tasks did not differ significantly.
Figure 3. Average discomfort and effort ratings across trials.
Self-reported discomfort and effort is plotted across all blink suppression trials. The ratings were averaged across all subjects’ individual trials to create an average curve for both across the “Don’t Blink” and “OK to Blink” trials. There was no significant difference found between the two curves (Pearson’s r = 0.962).
4. Discussion
This report describes novel methods to impute momentary discomfort associated with the urge to blink during blink suppression, based on the actual observed timing of blinks and the individual subject’s rating of discomfort. We show that both new methods more accurately predict discomfort than simpler models that do not account for individual subject sensitivity (or the timing of blinks). This result raises our expectation of finding regions with a pertinent BOLD signal when individual response characteristics are taken into account, but of course, that expectation remains to be tested. The highly similar ratings for discomfort and effort suggest that these two theoretically distinct domains are unlikely in practice to identify different regions when applied to fMRI data.
Our data provide some interesting observations beyond these methodological results. The 15-fold ratio of the mean blink rates during the blink suppression and recovery phases indicates that overall, subjects successfully suppressed the urge to blink. However, motor response differed between groups. Subjects with TS averaged an additional 2.6 blinks during each suppression task than did controls, who often went the whole 60 seconds without blinking. By contrast, the number of blinks between the two groups during the rest period did not differ significantly. The group difference in blink suppression is consistent with the hypothesis that people with TS may exhibit a motor inhibition deficit compared to controls when engaged in real-world suppression of an uncomfortable urge to move. This finding is interesting given that deficits in inhibition have long been hypothesized in TS but have not been reliably identified in most standard psychological tests (Morand-Beaulieu et al., 2017). Conceivably, inhibiting longstanding (overlearned) motor responses to sensory discomfort differs in important ways from inhibiting button presses as directed by an experimenter (Jackson, Parkinson, Kim, Schüermann, & Eickhoff, 2011). While tic subjects were less effective at suppressing blinks than controls, the correlation between blink suppression and tic suppression within TS subjects was not significant.
This result may suggest that although blink suppression and tic suppression both reflect somatomotor inhibition, the two may not overlap completely. We examined the correlation between other clinical variables as well and found that although YGTSS, a measure for tic severity over the past week, was strongly related to observed baseline tic frequency (rs = 0.768, p = 8.37×10−4), neither was strongly correlated to blink suppression (rs = 0.0832, p = 0.768 for tic frequency and rs =0.145, p = 0.606 for YGTSS).
Blink frequency in the initial baseline period was not significantly higher in the TS group. Previous investigations have examined the resting blink rate in TS at rest or during various tasks. Some found an increased spontaneous blink rate in TS (Bonnet 1982; Tulen et al., 1999; Tharp et al., 2015), whereas another did not (Karson et al., 1985). Nevertheless, we show decreased suppression of the blink rate in TS during intentional suppression.
Additionally, subjects in the TS group experienced discomfort from blink suppression in a quantitatively different manner than did controls. Specifically, in the blink timing model, the TS group had higher baseline discomfort, smaller excursion from baseline to peak during the blink suppression block, and slower return to baseline during the recovery block (see Table 2 and Fig. 2). However, these differences come from the scaled data, and raw baseline discomfort scores were not higher in the TS group. This may reflect the fact that reported discomfort (both within and across subjects) was also more variable in the TS group, as reflected by the quantitative model fit to the data. Thus these group differences in reported discomfort should be interpreted with caution.
4.1. Comparisons
While a previous study by (Berman, Horovitz, Morel, & Hallett, 2012) examined the fMRI data for a signal that matched a hypothesized urge model or mean urge across subjects, our analysis can account for responses to the timing of each subject’s blinks in the scanner and his or her individual response characteristics. Berman and colleagues did perform an analysis of their BOLD data on an individual subject basis, but their approach identified signal temporally coinciding with blinks, not urge (Berman, Horovitz, Morel, & Hallett, 2012; Berman personal correspondence to KJB, 2/21/2018). Our subject-specific and blink-dependent models had a significantly higher correlation to urge than did the two simpler models proposed by Berman, Mazzone, and colleagues.
Brandt and colleagues (2016) also examined the timing of urge and related movement to tics in subjects with TS. However, they only used blink timing and suppression with controls, and tic timing and suppression with TS subjects, while we aimed to compare the same task (blink suppression) across groups. Our results supported their finding that an increase in urge intensity is temporally related to blinks, increasing until a blink is executed and immediately decreasing afterward. Another interesting result from their study was that all their healthy control subjects, but only two with TS, reported an increase in their self-reported experience of urge intensity when they were required to monitor and rate their current urge. We asked subjects to rate urge intensity continuously, so we cannot verify that finding, but in future studies it may be interesting to investigate the subject’s attention to urge, as one interpretation may be that patients with TS pay more attention to their urges in daily life. However, tic frequency in TS decreases with self-monitoring of tic and urge occurrence (King, Scahill, Findley, & Cohen, 1999), though by contrast, watching oneself tic increases tic frequency (Brandt, Lynn, Obst, Brass, & Münchau, 2015). It is possible that our subjects may have heightened discomfort ratings because of increased attention to their discomfort, but this result would be carried through all trials and thus should not confound the comparisons reported above.
In perhaps the first attempt to image neural correlates of the urge to blink, Sandor and colleagues reported pilot data on two other approaches to elicit urge to blink in the scanner. One focused on the few seconds just before the first blink versus the few seconds after a 5-s eyes closed rest condition, and the other compared cued blinking at twice (control) or half (urge) the subject’s observed baseline blink rate (Sandor, Silveira, Mikulis, & Crawley, 1998). A block design was used to analyze each comparison. More recently, Abi-Jaoude et al. used fMRI to study increased failure of blink suppression in healthy subjects over repeated trials (Abi-Jaoude, Segura, Cho, Crawley, & Sandor, 2018). Although their study design differed somewhat from ours, we did not see in either the TS or control group the increase in escape blink frequency over repeated trials that formed the basis of their analyses. Additionally, the current blink suppression tasks analyzed in this report were done on a separate day before the fMRI, so this should limit the effect of suppressing blinks or tics for extended periods of time.
4.2. Limitations
This study relies on self-report for discomfort, a subjective experience that people may report differently. However, we believe this is a reasonable proxy and will apply it to BOLD signal from the fMRI data described above with the goal of identifying a rater-independent, objective, neuroimaging measure of discomfort. This study does have a sample size of only 15 TS subjects and 15 controls and we did not exclude potentially confounding factors such as OCD and ADHD. Their prevalence in TS makes exclusion difficult in a study of this size. Another potential limitation is the use of discomfort as a proxy for urge. We used discomfort because it seemed an easier term to understand and rate than “urge,” and the urge to blink, one would think, would correspond very closely to discomfort. We had wanted to separate urge into two dimensions, discomfort and effort; however, these did not differ significantly. As noted above, group differences on the scaled discomfort ratings should be interpreted with caution. Finally, any study of blinking in tic patients raises the question of whether blinking tics affect the analysis. However, in this sample only three of the subjects with TS reported blinking tics in the past week or had a blinking tic observed on exam. Furthermore, the two groups did not differ in terms of blink rate during the recovery period. If blinking tics were prominent enough to overcome blink suppression, one would expect that they would also increase the number of blinks when the subjects were free to blink during the ‘OK to blink’ period. The most likely explanation for these observations is that blinking tics did not substantially affect our results.
4.3. Future directions
The next step will be to apply this model to fMRI data from the same subjects. All of the above blink suppression tasks were completed outside of the scanner, but the same subjects later completed one blink suppression task repeated twice in an MRI scanner as well, rating their discomfort only at the end of each completed scan block consisting of 5 suppression trials. We expected that continuous rating during the fMRI trials would change the nature of the task and that this change would be reflected in the BOLD activity. Rather, we can combine the timing of the recorded blinks inside the scanner with the pathophysiological model created for each subject during the suppression tasks outside of the scanner. The degree to which each voxel’s time–signal curve matches the predicted time–discomfort curve, convolved with an individually estimated, model-free 16-s hemodynamic response period, will be computed using a general linear model to create a statistical image of urge-related voxels. The timing of individual tics observed on the video recordings can be entered separately to remove tic-related signal (and analyzed separately for signal related to tics and failure of tic inhibition).
Many candidate regions have already been identified by previous studies. Direct current stimulation over the inferior frontal gyrus improved blink suppression (Yaniv & Lavidor, 2017). Blink suppression activates the ventrolateral prefrontal cortex in healthy controls (Berman et al., 2012; van der Salm et al., 2018). The insula and mid-cingulate cortex have been described as important to the urge-for-action model proposed by Jackson and colleagues (Jackson et al., 2011). The insula has also figured in other blink suppression studies (Lerner et al., 2009; Berman et al., 2012). We will also investigate regions thought to be relevant to tic suppression, including the supplementary motor area (SMA), anterior cingulate cortex and the caudate. The SMA is a prime region of interest because it is consistently associated with tic generation and control (Ganos, Roessner, & Münchau, 2013) and direct electrical stimulation of the SMA caused the anticipation of movement or the ‘urge’ to execute a movement, without the motor activity itself (Fried et al., 1991). Similarly, fMRI studies by Wang et al. (2011) have shown that the anterior cingulate cortex and caudate are more active during effective tic suppression. We expect these findings will correlate across subjects with blink suppression success, and possibly with tic suppression success in subjects with TS. Our new models may allow for a more individualized approach and reflect a more accurate neuronal signal with less noise than would the previous one-size-fits-all models.
Table 1.
Study 2 Subject Characteristics
| TS (n=15) | Controls (n=15) | |
|---|---|---|
| Sex | ||
| M | 6 | 6 |
| F | 8 | 8 |
| Race | ||
| Caucasian | 12 | 13 |
| African-American | 2 | 2 |
| Other | 1 | 0 |
| Age | 27.7 ± 3.8 | 26.1 ± 2.2 |
| OCD | 9 | 0 |
| ADHD | 3 | 1 |
| YGTSS | 10.3 ± 12.9 | - |
| PUTS | 22.2 ± 4.4 | - |
| TS DCI Score | 55.1 ± 15.3 | - |
| Y-BOCS | 12.4 ± 6.7 | - |
| Baseline Tic Frequency (per min) | 5.3 ± 4.3 | - |
Acknowledgements
The authors thank Dr. Aleksandra Królak of the Department of Medical Electronics, Institute of Electronics, Lodz University of Technology, Łódź, Poland, for kindly sharing her C++ code for eyeblink detection from a live video feed (Królak & Strumiłło, 2012).
Funding
This research was supported by the Tourette Association of America (research grants to Cheryl Richards, Kevin Black, Bradley Schlaggar), by the McDonnell Center for Systems Neuroscience at Washington University (research grant to Cheryl Richards) and by the U.S. National Institutes of Health (NIH): Clinical and Translational Science Award (CTSA) Grant (UL1 TR000448) and the Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant (P30 CA091842). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix. A. Initial data visualization and model construction
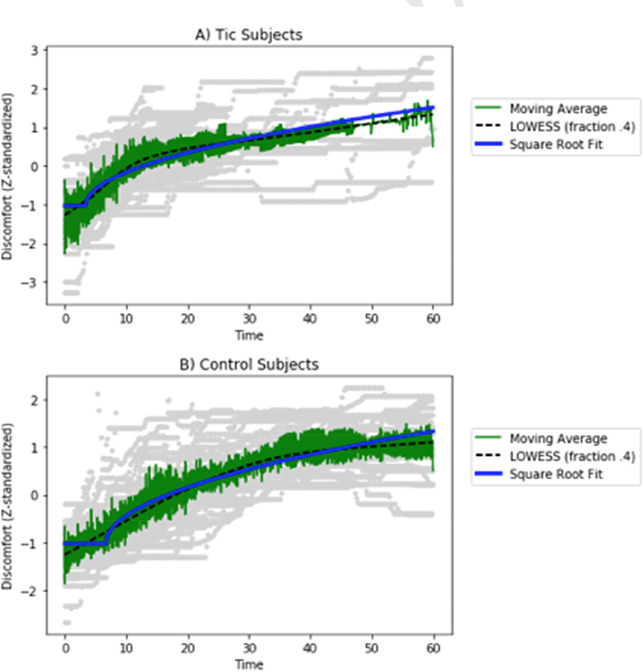
In order to investigate the relationship between discomfort intensity and blink occurrence uninterrupted by a blink, we used Python and Matplotlib to plot the discomfort of each subject prior to the first blink in each trial (Hunter, 2007). There was substantial variance within and across subjects, but the central tendency was an increasing curve that appeared to flatten out over time. We initially used a logarithmic functional form, y(t) = alog(t + c) + b, which reasonably described the averaged data from either TS or control subjects. However, the log function initially gave unreliable estimates for the model parameters, so we eventually abandoned it for a delayed square root function,
| Eq. (A.1) |
Figure A.3. Discomfort during successful blink suppression.
Discomfort is plotted across all blink suppression trials, beginning at the start of each “don’t blink” trial and extending to the first blink. The functional form was fit to the data and compared to a LOWESS (locally weighted scatterplot smoothing) fit to the data. Individual subject data is plotted in gray, while the mean is shown in green and LOWESS in black. (A) Data from tic subjects; (B) data from control subjects.
We then wanted to examine the effect of eye closure on discomfort. To do so we plotted the discomfort in between blinks (more than 2 seconds apart) from Study 2. We examined whether the discomfort would dip after a blink and how long it would take to return back to the predicted discomfort. The difference in reported discomfort and expected discomfort (based on the square root fit to the data before the first blink and the observed discomfort when the blink started) was then plotted using the equation
| Eq. (A.2) |
where D(t) represents the subject’s discomfort ratings at time t, y(t) is the delayed square root function (Eq. A.1) fit to the subject’s data prior to the first blink, t1 indicates the time point at which the first blink occurred, and t2 the time at which the second blink occurred. The observed relief in discomfort showed substantial variation, but a tendency to a small, transient dip in discomfort after eye closure, as hypothesized, which we modeled as a parabola passing through (0,0) with vertex at (h, k), h > 0, k < 0.
Together, these data and assumptions led to the following model for discomfort during the “don’t-blink” blocks. For a given time point ti in the interval [0,60s],
| Eq. (A.3) |
where y(t) is from Eq. 1, fj is the fraction of each quarter-second time bin j during which the eyes were actually closed, kHij(t) represents the sum of the expected drop in discomfort from each recent 0.25s eye closure,
| Eq. (A.4) |
and Δij = ti − tj. Most of the fj terms are zero, simplifying the calculation somewhat.
We modeled discomfort during the 30-second “OK to blink” block that immediately followed the 60 seconds of blink suppression based on the mean subject data, which suggested approximately exponential decay, dD/dt = −r(D − b), with discomfort decay rate constant r. We added a factor to account for the presumed modification by eye closure in the preceding time frame, resulting in this difference equation for t in the interval (60 s, 90 s):
| Eq. (A.5) |
where fi−1 is the fraction of time the subject’s eyes were closed in the previous interval.
Together, Equations A.3 and A.5 form the model we fit to the data.
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abi-Jaoude E, Segura B, Cho SS, Crawley A, & Sandor P (2018). The neural correlates of self-regulatory fatigability during inhibitory control of eye blinking. J Neuropsychiatry Clin Neurosci, 30(4), 325–333. 10.1176/appi.neuropsych.17070140 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Tic Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM5). American Psychiatric Publishing Inc; 10.1176/appi.books.9780890425596.316088 [DOI] [Google Scholar]
- Barkley RA (1990). Attention Deficit Disorders In Handbook of Developmental Psychopathology (pp. 65–75). Springer US; 10.1007/978-1-4615-7142-1_6 [DOI] [Google Scholar]
- Belluscio BA, Tinaz S, & Hallett M (2011). Similarities and differences between normal urges and the urge to tic. Cogn Neurosci, 2, 245–6. [DOI] [PubMed] [Google Scholar]
- Benjamin CL, O’Neil KA, Crawley SA, Beidas RS, Coles M, & Kendall PC (2010). Patterns and predictors of subjective units of distress in anxious youth. Behav Cogn Psychother, 38, 497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman BD, Horovitz SG, Morel B, & Hallett M (2012). Neural correlates of blink suppression and the buildup of a natural bodily urge. Neuroimage, 59, 1441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JK, Koller JM, & Black KJ (2017). TicTimer software for measuring tic suppression. F1000Research, 6, 1560 10.12688/f1000research.12327.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss J, Cohen DJ, & Freedman DX (1980). Sensory experiences of Gilles de la Tourette syndrome. Arch Gen Psychiatry, 37, 1343–1347. 10.1001/archpsyc.1980.01780250029002 [DOI] [PubMed] [Google Scholar]
- Bonnett KA. Neurobiological dissection of Tourette syndrome: a neurochemical focus on a human neuroanatomical model In: Friedhoff AJ, Chase TN, editors. Gilles de la Tourette Syndrome. New York: Raven Press; (1982). p. 77–82 [PubMed] [Google Scholar]
- Brandt VC, Beck C, Sajin V, Baaske MK, Bäumer T, Beste C, … Münchau A (2016). Temporal relationship between premonitory urges and tics in Gilles de la Tourette syndrome. Cortex, 77, 24–37. [DOI] [PubMed] [Google Scholar]
- Brandt VC, Lynn MT, Obst M, Brass M, & Münchau A (2015). Visual feedback of own tics increases tic frequency in patients with Tourette’s syndrome. Cogn Neurosci, 6, 1–7. 10.1080/17588928.2014.954990 [DOI] [PubMed] [Google Scholar]
- Bretthorst GL (1988). Bayesian Spectrum Analysis and Parameter Estimation. New York: Springer-Verlag; Retrieved from http://bayes.wustl.edu/ [Google Scholar]
- Bretthorst GL, & Marutyan K (2016). Bayesian Data-Analysis Toolbox, v. 4.22 (software). Available at http://bayesiananalysis.wustl.edu.
- Bullen JG, & Hemsley DR (1983). Sensory experience as a trigger in Gilles de la Tourette’s syndrome. J Behav Ther Exp Psychiatry, 14, 197–201. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Black KJ, Hallett M, & Voon V (2017). Neurobiology of the premonitory urge in Tourette’s syndrome: Pathophysiology and treatment implications. J Neuropsychiatry Clin Neurosci, 29, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claudio Torres E, Black WK, Richards C, & Black KJ (2014). A preliminary study of quantitative temporal modeling of the urge to blink during blink suppression. F1000Posters 2014, 5:659 (poster). Retrieved from https://f1000research.com/posters/1095804 [Google Scholar]
- Constantino JN (2013). Social Responsiveness Scale In Encyclopedia of Autism Spectrum Disorders (pp. 2919–2929). New York: Springer; 10.1007/978-1-4419-1698-3_296 [DOI] [Google Scholar]
- Crossley E, & Cavanna AE (2013). Sensory phenomena: clinical correlates and impact on quality of life in adult patients with Tourette syndrome. Psychiatry Res, 209(3), 705–710. 10.1016/j.psychres.2013.04.019 [DOI] [PubMed] [Google Scholar]
- Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, & Spencer DD (1991). Functional organization of human supplementary motor cortex studied by electrical stimulation. The Journal of Neuroscience, 11(11), 3656–3666. 10.1523/jneurosci.11-11-03656.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganos C, Roessner V, & Münchau A (2013). The functional anatomy of Gilles de la Tourette syndrome. Neuroscience & Biobehavioral Reviews, 37(6), 1050–1062. 10.1016/j.neubiorev.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Goodman WK (1989). The Yale-Brown Obsessive Compulsive Scale. Archives of General Psychiatry, 46(11), 1012–1016. 10.1001/archpsyc.1989.01810110054008 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 42(2), 377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JD (2007). Matplotlib: A 2D graphics environment. Computing In Science & Engineering, 9(3), 90–95. 10.1109/MCSE.2007.55 [DOI] [Google Scholar]
- Jackson SR, Parkinson A, Kim SY, Schüermann M, & Eickhoff SB (2011). On the functional anatomy of the urge-for-action. Cogn Neurosci, 2, 227–243. 10.1080/17588928.2011.604717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J (1997). Tourette syndrome. Phenomenology and classification of tics. Neurol Clin, 15, 267–275. [DOI] [PubMed] [Google Scholar]
- Karson CN, Kaufmann CA, Shapiro AK, & Shapiro E (1985). Eye-Blink Rate in Touretteʼs Syndrome. The Journal of Nervous and Mental Disease, 173(9), 566–569. 10.1097/00005053-198509000-00008 [DOI] [PubMed] [Google Scholar]
- King RA, Scahill L, Findley D, & Cohen DJ (1999). Psychosocial and behavioral treatments In Tourette’s Syndrome—Tics, Obsessions, Compulsions: Developmental Psychopathology and Clinical Care (pp. 338–359). John Wiley and Sons. [Google Scholar]
- Królak A, & Strumiłło P (2012). Eye-blink detection system for humancomputer interaction. Universal Access in the Information Society, 11(4), 409–419. 10.1007/s10209-0110256-6 [DOI] [Google Scholar]
- Kwak C, Dat VK, & Jankovic J (2003). Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord, 18, 1530–1533. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, & Cohen DJ (1989). The Yale Global Tic Severity Scale: Initial testing of a clinician-rated scale of tic severity. Journal of the American Academy of Child & Adolescent Psychiatry, 28(4), 566–573. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Walker DE, & Cohen DJ (1993). Premonitory urges in Tourette’s syndrome. Am J Psychiatry, 150, 98–102. [DOI] [PubMed] [Google Scholar]
- Lerner A, Bagic A, Hanakawa T, Boudreau EA, Pagan F, Mari Z, … Hallett M (2009). Involvement of insula and cingulate cortices in control and suppression of natural urges. Cereb Cortex, 19, 218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone L, Yu S, Blair C, Gunter BC, Wang Z, Marsh R, & Peterson BS (2010). An fMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am J Psychiatry, 167, 341–349. 10.1176/appi.ajp.2009.08121831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel EC, do Rosário-Campos MC, Prado HS, do Valle R, Rauch SL, Coffey BJ, … Leckman JF (2000). Sensory phenomena in obsessive-compulsive disorder and Tourette’s disorder. J Clin Psychiatry, 61(2), 150–156; quiz 157. [DOI] [PubMed] [Google Scholar]
- Morand-Beaulieu S, Grot S, Lavoie J, Leclerc JB, Luck D, & Lavoie ME (2017). The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci Biobehav Rev, 80, 240–262. 10.1016/j.neubiorev.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Reese HE, Scahill L, Peterson AL, Crowe K, Woods DW, Piacentini J, … Wilhelm S (2014). The premonitory urge to tic: measurement, characteristics, and correlates in older adolescents and adults. Behav Ther, 45(2), 177–186. 10.1016/j.beth.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MM, Banerjee S, Kurlan R, Cohen DJ, Leckman JF, McMahon W, … van de Wetering BJM (1999). The Tourette Syndrome Diagnostic Confidence Index: Development and clinical associations. Neurology, 53(9), 2108–2108. 10.1212/wnl.53.9.2108 [DOI] [PubMed] [Google Scholar]
- van der Salm SM, van der Meer JN, Cath DC, Groot PF, van der Werf YD, Brouwers E, … Tijssen MA (2018). Distinctive tics suppression network in Gilles de la Tourette syndrome distinguished from suppression of natural urges using multimodal imaging. NeuroImage: Clinical, 20, 783–792. doi: 10.1016/j.nicl.2018.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandor P, Silveira JM, Mikulis D, & Crawley A (1998). fMRI strategies for detecting brain activation related to premonitory urge in patients with Tourette syndrome [abstract]. NeuroImage, 7(4), S923 10.1016/S1053-8119(18)31756-7 [DOI] [Google Scholar]
- Specht MW, Woods DW, Nicotra CM, Kelly LM, Ricketts EJ, Conelea CA, … Walkup JT (2013). Effects of tic suppression: ability to suppress, rebound, negative reinforcement, and habituation to the premonitory urge. Behav Res Ther, 51, 24–30. [DOI] [PubMed] [Google Scholar]
- Steinberg T, Harush A, Barnea M, Dar R, Piacentini J, Woods D, … Apter A (2013). Tic-related cognition sensory phenomena, and anxiety in children and adolescents with Tourette syndrome. Comprehensive Psychiatry, 54(5), 462–466. 10.1016/j.comppsych.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Tharp JA, Wendelken C, Mathews CA, Marco EJ, Schreier H, & Bunge SA (2015). Tourette Syndrome: Complementary Insights from Measures of Cognitive Control, Eyeblink Rate, and Pupil Diameter. Frontiers in Psychiatry, 6. doi: 10.3389/fpsyt.2015.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulen JH, Azzolini M, Vries JA, Groeneveld WH, Passchier J, & Van De Wetering BJM. (1999). Quantitative study of spontaneous eye blinks and eye tics in Gilles de la Tourettes syndrome. Journal of Neurology, Neurosurgery & Psychiatry, 67(6), 800–802. doi: 10.1136/jnnp.67.6.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdellen CW, Keijsers GP, Cath DC, & Hoogduin CA (2004). Exposure with response prevention versus habit reversal in Tourette’s syndrome: a controlled study. Behav Res Ther, 42, 501–511. 10.1016/S0005-7967(03)00154-2 [DOI] [PubMed] [Google Scholar]
- Wang Z, Maia TV, Marsh R, Colibazzi T, Gerber A, & Peterson BS (2011). The Neural Circuits That Generate Tics in Tourettes Syndrome. American Journal of Psychiatry, 168(12), 1326–1337. 10.1176/appi.ajp.2011.09111692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DW, Piacentini JC, Chang SW, Deckersbach T, Ginsburg GS, Peterson AL, … Wilhelm S (2008). Assessment Strategies for Tourette Syndrome In Managing Tourette Syndrome (pp. 19–26). Oxford University Press; 10.1093/med:psych/9780195341287.003.0002 [DOI] [Google Scholar]
- Woods DW, Piacentini J, Himle MB, & Chang S (2005). Premonitory Urge for Tics Scale (PUTS). Journal of Developmental & Behavioral Pediatrics, 26(6), 397–403. 10.1097/00004703-200512000-00001 [DOI] [PubMed] [Google Scholar]
- Yaniv A, & Lavidor M (2017). Without blinking an eye: Proactive motor control enhancement. Journal of Cognitive Enhancement, 2(1), 97–105. 10.1007/s41465-017-0060-1 [DOI] [Google Scholar]