Abstract
Introduction:
High molecular weight kininogen (HK) and prekallikrein (PK) are proteins in the kallikrein/kinin system of the coagulation cascade. They play an important role in the contact activation system of the intrinsic coagulation pathway, renin-angiotensin activation, and inflammation. Hence these proteins have been posited to affect the occurrence of cardiovascular events and thus to be potential therapeutic targets. Previous case-control studies have provided inconsistent evidence for an association of HK and PK with cardiovascular disease.
Methods:
In the prospective population-based Atherosclerosis Risk in Communities(ARIC) Study, we used Cox proportional hazards regression models to investigate the association in 4195 middle-aged adults of plasma HK and PK concentrations in 1993–95 (linearly and in quartiles) with incident coronary heart disease, ischemic stroke, and heart failure through 2016.
Results:
Over a mean of 18 years follow-up, we identified incident cardiovascular events (coronary heart disease and ischemic stroke) in 618 participants and heart failure in 667. We observed no significant relation between HK or PK and cardiovascular disease or heart failure, before and after adjusting for several potential confounding variables.
Conclusions:
We found no compelling evidence to support an association of plasma HK or PK concentrations with incident CHD, ischemic stroke, or heart failure.
Keywords: Cardiovascular disease, Heart failure, High molecular weight kininogen, Ischemic stroke, Prekallikrein, Prospective study
1. Introduction
Globally, ischemic cardiovascular diseases (CVD) are the leading cause of mortality, responsible for at least one-third of all deaths in individuals over the age of 35 years [1]. The American Heart Association has projected that by 2035 up to 45% of the US population will have CVD [1]. Heart failure affects > 5.8 million people in the US and over 26 million people worldwide [2]. These cardiovascular conditions are associated with significant mortality, morbidity, and healthcare expenditures, and thus are an important public health concern [1,2]. The contact activation system and the kallikrein/kinin system play important roles in thrombosis and inflammation. Two key components of these systems are prekallikrein (PK) and high molecular weight kininogen (HK). HK acts as a co-factor in facilitating activation of Factor XII, thus contributing to the initiation of the intrinsic pathway of the coagulation cascade. Factor XIIa facilitates conversion of PK to plasma kallikrein, which in turn activates FXII, thereby forming a cyclical autoactivation chain-reaction. Activation of the intrinsic pathway leads to procoagulant and proinflammatory reactions potentially leading to coronary heart disease (CHD) and ischemic stroke [3]. Furthermore, plasma and tissue kallikrein cleave HK to liberate bradykinin. Bradykinin is a potent stimulator of nitric oxide production, prostacyclin production, and tissue plasminogen activator release [3]. A study in knockout mice found production of kinin to be protective against cardiovascular remodeling [4], suggesting links to heart failure [5]. Epidemiological evidence associating plasma HK or PK with cardiovascular outcomes is sparse. Three previous studies suggest positive associations of HK and PK with cardiovascular outcomes such as myocardial infarction [6,7], ischemic stroke [6] and venous thrombosis [8], although this association is debated [6,8,9]. To our knowledge, evidence on whether these proteins are associated with heart failure is lacking.
To better understand association of HK and PK with CHD, ischemic stroke and heart failure, we conducted a prospective population-based study. We investigated whether plasma HK and PK are associated positively with risk of incident CHD and ischemic stroke. We also investigated whether these peptides are associated inversely with incident heart failure, posited on a possible protective action of kinins against cardiovascular remodeling.
2. Methods
2.1. Study design and sample
The Atherosclerosis Risk in Communities (ARIC) study [10] enrolled 15,792 men and women aged 45 to 64 years in 1987–1989 (visit 1) from four US communities. ARIC followed-up participants in subsequent examinations in 1990–92 (visit 2), 1993–95 (visit 3), 1996–98 (visit 4), 2011–13 (visit 5), and 2016–2017 (visit 6), as well as through annual or semi-annual telephone contact. At each visit, risk factors were measured, and plasma was obtained and stored at −80 °C. The institutional review committees at each study center approved the methods, and ARIC staff obtained informed consent from each participant.
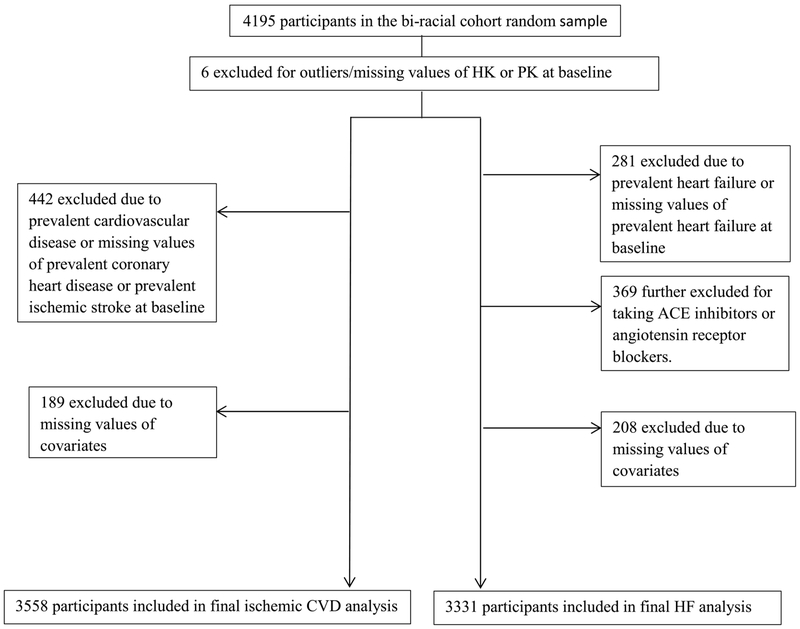
The sample for this prospective study of the association of plasma HK and PK with incident cardiovascular outcomes was a simple random sample (sub-cohort) of 4195 participants attending ARIC visit 3, who were selected for a case-cohort study of venous thromboembolism.9 Plasma HK and PK were measured in stored visit 3 (1993–95) samples, and the bi-racial sub-cohort of 4195 participants was followed from 1993–95 (baseline) through 2016 for incident ischemic CVD (CHD, ischemic stroke) and heart failure events. Fig. 1 summarizes participant selection and exclusions. For incident ischemic CVD, we excluded from the 4195 sub-cohort participants 6 with outlying/missing values of HK and PK, 442 due to prevalent CVD (present at visit 1 or incident by visit 3) or missing prevalent CVD information at visit 1, and 189 for missing values of covariates used in analysis, yielding a final analytic sample 3558. Similarly for heart failure, out of the 4195 participants, we excluded 6 with outlying HK or PK, 281 for having prevalent heart failure (prevalent at visit 1 or incident hospital discharge with ICD-9-CM code 428 by visit 3) or missing prevalent heart failure information at visit 1, 369 who were taking angiotensin convertase inhibitors or angiotensin receptor blockers (medications that might interfere with a HK or PK effect through the bradykinin pathway, potentially affecting heart failure risk) [3,4], and 208 for missing values of covariates used. Our final analytic sample for analyzing incident heart failure was 3331 participants.
Fig. 1.
Participant selection for analysis of high molecular weight kininogen and prekallikrein with ischemic cardiovascular disease (left side) and heart failure (right side).
2.2. Outcome identification
We followed participants from 1993–95 through 2016 for incident events. ARIC staff contacted participants annually or semi-annually by telephone and identified hospitalizations and deaths. The staff then retrieved and abstracted hospital records and death certificates. Outcomes were incident CHD (definite fatal CHD or definite or probable myocardial infarction by ARIC criteria) [11], incident ischemic stroke (definite or probable ischemic stroke by ARIC criteria) [12], and hospitalized heart failure defined by an ICD-9-CM discharge code of 428.xx in any position [13]. In the main analysis, incident CVD was defined by the first occurrence of either CHD or ischemic stroke.
2.3. Measurement of biomarkers at ARIC visit 3
In 2018, the Laboratory for Clinical Biochemistry Research at the University of Vermont thawed plasma samples for measurement of HK and PK [9]; HK was measured upon third thaw and PK upon fourth thaw following storage at visit 3. To investigate the effect of thawing on plasma levels of HK and PK, fresh samples were measured over four consecutive thaws. Plasma levels of HK declined 16% across repeated thaws (mean value for one to four thaws in the 6 independent samples: 29.4, 26.2, 23.5, and 24.8 μg/mL). We proceeded with analysis assuming this effect to be random across the entire sub-cohort. Plasma levels of PK were stable over multiple thaws (mean value for one to four thaws in 4 independent samples: 18.8, 20.6, 18.4, and 20.1 μg/mL). Using a single reagent lot, the laboratory measured PK employing ELISA test using monoclonal antibodies from LifeSpan Biosciences (Seattle, WA) with an average coefficient of variation of 8.9% in the sub-cohort. HK was measured using Simple Step ELISA® from Abcam (Eugene, OR). HK required a high dilution (1:40,000) and produced an average coefficient of variation of 16.9%. To assess reproducibility of assay measures, some samples had been split and stored at visit 3 as pairs. Reliability estimates (correlation coefficients) were measured in 93 such pairs in the sub-cohort. For HK measurement, the intraclass correlation coefficient was r = 0.56. For PK measurement, the intraclass correlation coefficient was r = 0.49, and Pearson correlation coefficient was r = 0.60.
2.4. Measurement of other risk factors
Risk factors were mostly measured at ARIC visit 3. Participants reported race, smoking status, alcohol consumption, and use of antihypertensive, diabetic, or hormonal replacement therapy (HRT) medications. They also brought in all medications used in the past two weeks, which were transcribed. ARIC staff measured sitting blood pressure thrice after a 5-minute rest via a random-zero sphygmomanometer, and we used the mean of the last two measures. Staff measured weight and height, to calculate body mass index. We defined diabetes as a fasting blood glucose of 126 mg/dL or higher, non-fasting blood glucose of 200 mg/dL or higher, a self-reported physician diagnosis of diabetes, or use of antidiabetic medication in the past 2 weeks. Enzymatic methods were used to measure total cholesterol [14] and high density lipoprotein (HDL) cholesterol [15]. Prevalent atrial fibrillation was determined by a 12-lead ECG at visits 1, 2, or 3 or a hospital discharge code between visits 1 and 3 with ICD9 codes 427.31 and 427.32. Estimated glomerular filtration rate (eGFR), unavailable at ARIC visit 3, was measured at visit 2 from creatinine using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) algorithm [16].
2.5. Statistical methods
We used SAS 9.4 (Statistical Analysis System, SAS Institute, Cary, NC) for analysis. We evaluated potential confounding variables by examining means or frequencies of risk factors across quartiles of HK and PK. We performed Cox proportional hazards regressions, to estimate the hazard ratio (HR) of ischemic CVD (CHD and ischemic stroke) and heart failure, in relation to HK or PK concentrations (testing both quartiles and continuous measures of HK or PK). The proportional hazards assumption was checked by plotting of log(−log) survival curves and testing the interaction between the exposures and time. Follow-up for CVD and heart failure began at ARIC visit 3 and went until the date of occurrence, loss to follow-up, death, or else December 31, 2016. We also examined associations with CHD and ischemic stroke individually, with censoring for incidence of the other outcome, if the other outcome occurred first [17]. For each outcome, model 1 adjusted for age, race, and sex; model 2 adjusted for age, race (white, black), sex-HRT (women currently using, women not using, women with missing HRT use, men), body mass index, diabetes (yes, no), smoking and drinking status (current, former, never), systolic blood pressure, antihypertensive medications (yes, no), eGFR, total cholesterol, HDL cholesterol, and history of atrial fibrillation (yes, no). We additionally adjusted for prevalent CHD in analyses for heart failure. We chose p < 0.05 as the threshold for statistical significance.
3. Results
At baseline, the mean plasma value of HK was 23.6 μg/mL (SD, 6.4), and the mean PK was 15.1 μg/mL (SD, 9.1). As shown in Table 1 for the cohort followed for ischemic CVD (and Supplementary Table 1 for the cohort followed for heart failure), higher HK and PK quartiles were associated with higher total cholesterol, being a woman, and higher diabetes prevalence; additionally, higher PK but not HK was associated with higher HDL cholesterol.
Table 1.
Baseline participant characteristics in relation to quartiles of high molecular weight kininogen and prekallikrein in those followed for incident ischemic cardiovascular outcomes, ARIC, 1993–1995.
| Characteristic | HK quartiles (μg/mL) | PK quartiles (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Range (quartiles) | 2.08–19.25 | 19.26–22.81 | 22.82–26.98 | 26.98–58.57 | 2.07–10.78 | 10.79–12.96 | 12.97–15.90 | 15.90–95.79 |
| Count (n) | 889 | 890 | 890 | 889 | 889 | 890 | 890 | 889 |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age (yrs) | 60 (6) | 60 (6) | 60 (6) | 60 (6) | 60 (6) | 60 (6) | 60 (6) | 60 (6) |
| Body mass index (kg/m2) | 28.1 (5.8) | 28.5 (5.3) | 28.7 (5.6) | 28.9 (5.6) | 28 (5.5) | 28.4 (5.5) | 28.6 (5.5) | 29.0 (5.7) |
| eGFR (mL/min per 1.73 m2) | 97 (14) | 96 (15) | 97 (15) | 97 (15) | 98 (15) | 96 (15) | 97 (15) | 97 (14) |
| Total Cholesterol (mg/dL) | 198 (37) | 205 (37) | 212 (38) | 216 (39) | 200 (37) | 204 (37) | 211 (38) | 216 (40) |
| HDL-cholesterol (mg/dL) | 53 (18) | 53 (19) | 53 (18) | 52 (18) | 51 (18) | 51 (17) | 54 (18) | 55 (19) |
| Systolic blood pressure (mmHg) | 124 (20) | 124 (18) | 125 (18) | 123 (18) | 125 (20) | 124 (18) | 124 (18) | 124 (19) |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Sex (female) | 429 (48) | 487 (55) | 526 (59) | 606 (68) | 352 (40) | 414 (47) | 574 (65) | 708 (80) |
| Current HRT, females (yes) | 130 (30) | 151 (31) | 170 (32) | 199 (33) | 71 (20) | 98 (24) | 165 (29) | 316 (45) |
| (No) | 219 (51) | 252 (52) | 253 (48) | 294 (48) | 205 (58) | 229 (55) | 307 (53) | 277 (39) |
| (Missing) | 80 (19) | 84 (17) | 103 (20) | 113 (19) | 76 (22) | 87 (21) | 102 (18) | 115 (16) |
| Race (White) | 685 (77) | 706 (79) | 704 (79) | 699 (79) | 619 (70) | 702 (79) | 738 (83) | 735 (83) |
| Smoking (current) | 172 (19) | 145 (16) | 159 (18) | 141 (16) | 203 (23) | 172 (19) | 144 (16) | 98 (11) |
| (Former) | 351 (40) | 371 (42) | 350 (39) | 345 (39) | 361 (41) | 371 (42) | 341 (38) | 344 (39) |
| (Never) | 366 (41) | 374 (42) | 381 (43) | 403 (45) | 325 (37) | 347 (39) | 405 (46) | 447 (50) |
| Alcohol consumption (current) | 473 (53) | 493 (55) | 475 (53) | 455 (51) | 440 (49) | 483 (54) | 493 (55) | 480 (54) |
| (Former) | 185 (21) | 198 (22) | 193 (22) | 193 (22) | 233 (26) | 200 (23) | 166 (19) | 170 (19) |
| (Never) | 231 (26) | 199 (22) | 222 (25) | 241 (27) | 216 (24) | 207 (23) | 231 (26) | 239 (27) |
| Diabetes mellitus | 100 (11) | 106 (12) | 143 (16) | 183 (21) | 112 (13) | 129 (15) | 135 (15) | 156 (18) |
| Hypertension medication | 296 (33) | 283 (32) | 299 (34) | 351 (40) | 304 (34) | 265 (30) | 317 (36) | 343 (39) |
| Prevalent atrial fibrillation | 10 (1.1) | 12 (1.4) | 6 (0.7) | 5 (0.6) | 8 (0.9) | 9 (1.0) | 11 (1.2) | 5 (0.6) |
ARIC, Atherosclerosis Risk in Communities; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HK, high molecular weight kininogen; HRT, hormone replacement therapy; PK, prekallikrein; SD, standard deviation.
Over a mean of 18 years of follow-up (maximum 23 years), 618 participants developed incident ischemic CVD (402 CHD first, 216 ischemic stroke first), and 667 developed incident heart failure. As shown in Table 2, there was little evidence for associations of plasma HK or PK with incident ischemic CVD in Model 1 (adjusted for age, race, and sex) or after adjusting for other potential confounders in Model 2. The HRs were mostly close to the null value, without evidence of a trend, for both biomarkers expressed as quartiles and as continuous variables. There also was little evidence for associations of HK or PK individually with either incident CHD (Supplemental Table 2) or incident ischemic stroke (Supplemental Table 3). Similarly, there were no material associations of HK or PK with heart failure in either model (Table 3).
Table 2.
Hazard ratios (95% confidence intervals) of incident ischemic cardiovascular disease (coronary heart disease or ischemic stroke) by quartiles or SD increment of high molecular weight kininogen or prekallikrein, ARIC, 1993–95 through 2016.
| Quartiles | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Per SDa increment | |
| High molecular weight kininogen, μg/mL | 2.08–19.25 | 19.26–22.81 | 22.82–26.98 | 26.98–58.57 | |
| n at risk | 889 | 890 | 890 | 889 | |
| Incident CVD, n | 145 | 156 | 170 | 147 | |
| Model 1 HR (95% CI) | 1 (Reference) | 1.14 (0.91–1.43) | 1.30 (1.04–1.63) | 1.15 (0.91–1.45) | 1.04 (0.97–1.13) |
| Model 2 HR (95% CI) | 1 (Reference) | 1.15 (0.91–1.44) | 1.21 (0.96–1.51) | 0.99 (0.78–1.25) | 0.99 (0.91–1.08) |
| Prekallikrein, μg/mL | 2.07–10.78 | 10.79–12.96 | 12.97–15.90 | 15.90–95.79 | |
| n at risk | 889 | 890 | 890 | 889 | |
| Incident CVD, n | 178 | 151 | 143 | 146 | |
| Model 1 HR (95% CI) | 1 (Reference) | 0.91 (0.73–1.13) | 0.94 (0.75–1.17) | 1.05 (0.83–1.32) | 1.02 (0.94–1.11) |
| Model 2 HR (95% CI) | 1 (Reference) | 0.90 (0.72–1.12) | 0.88 (0.70–1.10) | 0.96 (0.76–1.22) | 1.02 (0.94–1.11) |
Model 1: Adjusted for age, race, and sex.
Model 2: Adjusted for age, race, sex-hormone replacement therapy, body mass index, diabetes, smoking and drinking status, systolic blood pressure, antihypertensive medications, estimated glomerular filtration rate, total cholesterol, high density lipoprotein cholesterol, and previous history of atrial fibrillation.
ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; CVD, cardiovascular disease; HR, hazard ratio; SD, standard deviation.
SD denotes standard deviation, which for high molecular weight kininogen was 6.4 μg/ml and for prekallikrein was 9.2 μg/ml.
Table 3.
Hazard ratios (95% confidence intervals) of incident heart failure by quartiles or SD increment of high molecular weight kininogen or prekallikrein, ARIC, 1993–95 through 2016.
| Quartiles | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Per SDa increment | |
| High molecular weight kininogen, μg/mL | 2.08–19.24 | 19.25–22.80 | 22.81–26.83 | 26.84–57.47 | |
| n at risk | 832 | 833 | 833 | 833 | |
| Incident HF, n | 157 | 168 | 176 | 166 | |
| Model 1 HR (95% CI) | 1 (Reference) | 1.10 (0.89–1.37) | 1.21 (0.98–1.50) | 1.17 (0.94–1.46) | 1.05 (0.97–1.13) |
| Model 2 HR (95% CI) | 1 (Reference) | 1.09 (0.87–1.35) | 1.12 (0.90–1.40) | 1.00 (0.80–1.25) | 0.99 (0.92–1.07) |
| Prekallikrein, μg/mL | 4.36–10.69 | 10.70–12.84 | 12.85–15.81 | 15.82–95.79 | |
| n at risk | 832 | 834 | 832 | 833 | |
| Incident HF, n | 175 | 177 | 156 | 159 | |
| Model 1 HR (95% CI) | 1 (Reference) | 1.07 (0.87–1.32) | 0.99 (0.79–1.23) | 1.01 (0.80–1.27) | 1.02 (0.95–1.10) |
| Model 2 HR (95% CI) | 1 (Reference) | 1.05 (0.85–1.29) | 1.01 (0.81–1.27) | 0.95 (0.75–1.20) | 1.05 (0.97–1.14) |
Model 1: Adjusted for age, race, and sex.
Model 2: Adjusted for age, race, sex-hormone replacement therapy, body mass index, diabetes, smoking and drinking status, systolic blood pressure, antihypertensive medications, estimated glomerular filtration rate, total cholesterol, high density lipoprotein cholesterol, and previous history of atrial fibrillation and coronary heart disease.
ARIC, Atherosclerosis Risk in Communities; CI, confidence interval; HF, heart failure; HR, hazard ratio; SD, standard deviation.
SD denotes standard deviation, which for high molecular weight kininogen was 6.4 μg/mL and for prekallikrein was 9.1 μg/mL.
To potentially replicate a previous study’s positive finding [8], we also tested the association of the highest decile of HK versus < 90th percentile with incident ischemic stroke. The hazard ratio of developing ischemic stroke was 0.85 (95% CI 0.52–1.38) after adjusting for Model 1 covariates and 0.76 (95% CI 0.47–1.24) in Model 2.
4. Discussion
In this large prospective population-based study, we found no significant positive linear association between baseline plasma HK or PK values and incident CVD. Furthermore, we did not find a negative linear association between HK or PK and incident heart failure. Overall, we found no compelling evidence to support any association between these biomarkers and incident cardiovascular outcomes.
There is little prior epidemiological evidence on the association of plasma concentrations of HK or PK with CVD outcomes, and it is limited to case-control studies. Siegerink et al. reported no significant association of PK antigen level with risk of myocardial infarction or ischemic stroke in a population-based case-control study of young women [18]. In the same study, there was a borderline association of very high levels of HK with increased occurrence of ischemic stroke (odds ratio 1.82, 95% CI = 1.00–3.29 for the highest decile of HK versus < 90th percentile), but no association of HK with myocardial infarction [8]. We were unable to reproduce this positive association between the highest decile of HK and incident ischemic stroke. Merlo et al. reported that elevated PK was significantly associated with prevalent myocardial infarction after adjustment for cholesterol concentration and other confounders (odds ratio 5.5, 95% CI = 1.3–22.5 for the highest versus lowest quartile of PK), but HK concentration was not associated with myocardial infarction [6]. However, blood was taken after CVD occurrence in these case-control studies, albeit after acute phase of the event, thus leaving the potential for reverse-causation. Some studies have focused instead on plasma kallikrein-C1 inhibitor complex, a marker of the activity level of plasma kallikrein. In the Northwick Park Heart Study II cohort of men, Govers-Riemslag et al. found kallikrein-C1 inhibitor complex not associated with CHD but inversely associated with total stroke (p = 0.02), but without a graded relation, with odds ratios across tertiles of the complex being 1 (reference), 0.29 (95% CI = 0.12–0.72), and 0.67 (95% CI = 0.30–1.52). However, the middle tertile had only 8 stroke events. [19]. Subsequently, the population-based case-control study of young women reported a positive association of kallikrein-C1 inhibitor complex and other markers of the intrinsic coagulation activation with stroke, but no association with myocardial infarction [22]. Other prospective clinical studies found mostly no associations of kallikrein-C1 inhibitor complex with recurrent myocardial infarction [20] or adverse outcomes after hospitalized chest pain [21]. ARIC differs from most previous reports by studying long-term stored plasma samples in a population-based cohort of middle-aged men and women followed for several decades for CVD occurrence.
The KNG1 gene produces HK [3]. Due to limited statistical power, we did not perform analyses using any genetic markers. The Cardio-Gram consortium GWAS, which included 22,233 CHD cases and 64,762 controls did not find rs710446, a missense variant in KNG1, significantly associated with CHD (OR = 0.97, p = 0.06) [23,24]. Three other KNG1 SNPs, rs5030062, rs2304456 and rs5029980, have been independently associated with HK levels [25,26]. Of them, rs5030062, tags rs710446 (r2 = 0.96 in European individuals). A recently published study did not find these 3 SNPs associated with ischemic stroke (n = 182 patients) or myocardial infarction (n = 216 patients) compared with 630 healthy controls (OR range: 0.87–0.95 for myocardial infarction and 0.77–1.12 for ischemic stroke, p > 0.05 for all) [26]. Based on these findings, we postulate that the pathway mediated by HK is not important for the pathogenesis of CVD.
Despite rationale provided in the Introduction for an inverse association of HK and PK with heart failure, we did not find any evidence for an association. To our knowledge, there are no prior studies assessing this relation. Future studies could investigate this association in heart failure with preserved versus reduced ejection fraction.
Our results should be considered in light of several limitations. The plasma samples were stored at −80 °C and had been thawed multiple times. Laboratory variability for both PK and especially HK was high. Our pilot studies suggested that HK concentrations in the citrate plasma dropped with every freeze-thaw cycle. This measurement error would likely be similar across the range of HK, as all samples were handled similarly during freeze-thaw cycles; therefore, our HRs might be unbiased. In healthy volunteers, the standard normal mean plasma value for HK is about 70 μg/mL and for PK is about 50 μg/mL. In our study sample, the observed mean plasma values of these biomarkers were much lower. This could be due to deterioration of the frozen ARIC samples over time, to age differences in prior groups studied, or to the commercial assays chosen for the study. However, the measurement error again should be similar across HK levels. Another major limitation is that we had only single baseline measures of HK and PK, and participants’ values likely changed over the long follow-up period. This would most likely be a non-differential error (i.e., unrelated to CVD outcomes). Similarly, potential confounding variables were also measured only once at baseline, but could vary with time, leading to residual confounding.
Our study results concur with most previous studies, suggesting no material association of HK or PK with CVD, CHD or heart failure. We also found that neither HK nor PK was associated with venous thromboembolism in ARIC [9]. Future well-designed prospective studies could be considered to further address whether the concentrations of factors in the contact activation and the kallikrein/kinin systems are associated with cardiovascular events. As Schmaier noted, interest in HK and PK has risen due to possibility to target thrombosis independent of hemostasis [3]. This could be especially useful to address increase in contact activation due to several pathophysiologic biologic surfaces and medical devices [3]. As newer studies in murine models show possible association of peptides in the kallikrein/kinin system with other outcomes such as endotoxemia [27] and Alzheimer’s dementia [28], there is a need to conduct carefully planned prospective population-based human studies to validate related hypotheses.
In conclusion, we found no compelling evidence to support an association of plasma HK or PK concentrations with incident CHD, ischemic stroke, or heart failure.
Supplementary Material
Acknowledgements
The authors thank the participants and staff of the ARIC study for their important contributions and Elaine Cornell for assistance with laboratory measurements.
Funding sources
The National Heart, Lung, and Blood Institute (NHLBI) provided support for laboratory assays via R01 HL059367 and for the Atherosclerosis Risk in Communities Study via contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I.
Abbreviations:
- ARIC
Atherosclerosis Risk in Communities
- CHD
coronary heart disease
- CKD-EPI
Chronic Kidney Disease Epidemiology Collaboration
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- HK
high molecular weight kininogen
- HR
hazard ratio
- HRT
hormonal replacement therapy
- PK
prekallikrein
Footnotes
Ethics approval
The institutional review boards of the collaborating institutions approved the study.
Declaration of competing interest
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2019.08.009.
References
- [1].Cherepanov D, Bentley TGK, Hsiao W, et al. , Real-world cardiovascular disease burden in patients with atherosclerotic cardiovascular disease: a comprehensive systematic literature review, Curr. Med. Res. Opin 34 (2018) 459–473. [DOI] [PubMed] [Google Scholar]
- [2].Roger VL, Epidemiology of heart failure, Circ. Res 113 (2013) 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schmaier AH, The contact activation and kallikrein/kinin systems: pathophysiologic and physiologic activities, J. Thromb. Haemost 14 (2016) 28–39. [DOI] [PubMed] [Google Scholar]
- [4].Liu YH, Yang XP, Mehta D, et al. , Role of kinins in chronic heart failure and in the therapeutic effect of ACE inhibitors in kininogen-deficient rats, Am. J. Physiol. Heart Circ. Physiol 278 (2000) H507–H514. [DOI] [PubMed] [Google Scholar]
- [5].Rastogi A, Novak E, Platts AE, et al. , Epidemiology, pathophysiology and clinical outcomes for heart failure patients with a mid-range ejection fraction, Eur. J. Heart Fail. 19 (2017) 1597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Merlo C, Wuillemin WA, Redondo M, et al. , Elevated levels of plasma prekallikrein, high molecular weight kininogen and factor XI in coronary heart disease, Atherosclerosis 161 (2002) 261–267. [DOI] [PubMed] [Google Scholar]
- [7].Siegerink B, Rosendaal FR, Algra A, High-molecular-weight kininogen and the risk of a myocardial infarction and ischemic stroke in young women: the RATIO case–control study, J. Thromb. Haemost 10 (2012) 2409–2412. [DOI] [PubMed] [Google Scholar]
- [8].Gallimore MJ, Harris SL, Jones DW, et al. , Plasma levels of factor XII, prekallikrein and high molecular weight kininogen in normal blood donors and patients having suffered venous thrombosis, Thromb. Res 114 (2004) 91–96. [DOI] [PubMed] [Google Scholar]
- [9].Folsom AR, Tang W, Basu S, et al. , Plasma concentrations of high molecular weight kininogen and prekallikrein and venous thromboembolism incidence in the general population, Thromb. Haemost 119 (2019) 834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].The ARIC Investigators, The Atherosclerosis Risk in Communities (ARIC) study: design and objectives, Am. J. Epidemiol 129 (1989) 687–702. [PubMed] [Google Scholar]
- [11].White AD, Folsom AR, Chambless LE, et al. , Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience, J. Clin. Epidemiol 49 (1996) 223–233. [DOI] [PubMed] [Google Scholar]
- [12].Rosamond WD, Folsom AR, Chambless LE, et al. , Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort, Stroke 30 (1999) 736–743. [DOI] [PubMed] [Google Scholar]
- [13].Loehr LR, Rosamond WD, Chang PP, et al. , Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study), Am. J. Cardiol 101 (2008) 1016–1022. [DOI] [PubMed] [Google Scholar]
- [14].Siedel J, Hagele E, Ziegenhorn J, et al. , Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency, Clin. Chem 29 (1983) 1075–1080. [PubMed] [Google Scholar]
- [15].Warnick GR, Benderson J, Albers JJ, Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol, Clin. Chem 28 (1982) 1379–1388. [PubMed] [Google Scholar]
- [16].Inker LA, Schmid CH, Tighiouart H, et al. , Estimating glomerular filtration rate from serum creatinine and cystatin C, N. Engl. J. Med 367 (2012) 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Seo WK, Yong HS, Koh SB, et al. , Correlation of coronary artery atherosclerosis with atherosclerosis of the intracranial cerebral artery and the extracranial carotid artery, Eur. Neurol 59 (2008) 292–298. [DOI] [PubMed] [Google Scholar]
- [18].Siegerink B, Rosendaal FR, Algra A, Antigen levels of coagulation factor XII, coagulation factor XI and prekallikrein, and the risk of myocardial infarction and ischemic stroke in young women, J. Thromb. Haemost 12 (2014) 606–613. [DOI] [PubMed] [Google Scholar]
- [19].Govers-Riemslag JW, Smid M, Cooper JA, Bauer KA, Rosenberg RD, Hack CE, Hamulyak K, Spronk HM, Miller GJ, ten Cate H, The plasma kallikrein-kinin system and risk of cardiovascular disease in men, J. Thromb. Haemost 5 (2007) 1896–1903. [DOI] [PubMed] [Google Scholar]
- [20].Konings J, Govers-Riemslag JW, Spronk HM, Waltenberger JL, ten Cate H, Activation of the contact system in patients with a first acute myocardial infarction, Thromb. Res 132 (2013) 138–142. [DOI] [PubMed] [Google Scholar]
- [21].Pönitz V, Govers-Riemslag JW, Brügger-Andersen T, ten Cate H, Nilsen DW, Inhibitor complexes of the plasma kallikrein-kinin system and outcome prediction in patients following admission for chest pain, J. Thromb. Haemost 7 (2009) 1231–1233. [DOI] [PubMed] [Google Scholar]
- [22].Siegerink B, Govers-Riemslag JW, Rosendaal FR, Ten Cate H, Algra A, Intrinsic coagulation activation and the risk of arterial thrombosis in young women: results from the Risk of Arterial Thrombosis in relation to Oral contraceptives (RATIO) case-control study, Circulation 122 (2010) 1854–1861. [DOI] [PubMed] [Google Scholar]
- [23].Schunkert H, König IR, Kathiresan S, et al. , Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease, Nat. Genet 43 (2011) 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tang W, Schwienbacher C, Lopez LM, et al. , Genetic associations for activated partial thromboplastin time and prothrombin time, their gene expression profiles, and risk of coronary artery disease, Am. J. Hum. Genet 91 (2012) 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Suhre K, Arnold M, Bhagwat AM, et al. , Connecting genetic risk to disease end points through the human blood plasma proteome, Nat. Commun 8 (2017) 14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rohmann JL, Haan HG, Algra A, et al. , Genetic determinants of activity and antigen levels of contact system factors, J. Thromb. Haemost 17 (2019) 157–168. [DOI] [PubMed] [Google Scholar]
- [27].Yang A, Xie Z, Wang B, et al. , An essential role of high-molecular-weight kininogen in endotoxemia, J. Exp. Med 214 (2017) 2649–2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yamamoto-Imoto H, Zamolodchikov D, Chen ZL, et al. , A novel detection method of cleaved plasma high-molecular-weight kininogen reveals its correlation with Alzheimer’s pathology and cognitive impairment, Alzheimers Dement (Amst) 10 (2018) 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.