Abstract
The ventral surface of the rostral medulla oblongata has been suspected since the 1960s of harboring central respiratory chemoreceptors, i.e. acid-activated neurons that regulate breathing to maintain arterial PCO2 constant. The key neurons a.k.a. retrotrapezoid nucleus (RTN) have now been identified. In this review we describe their transcriptome, developmental lineage and anatomical projections. We also review their contribution to CO2 homeostasis and to the regulation of breathing automaticity during sleep and wake. Finally we discuss several mechanisms that contribute to the activation of RTN neurons by CO2 in vivo: cell-autonomous effects of protons, paracrine effects of pH mediated by surrounding astrocytes and blood vessels, and excitatory inputs from other CO2-responsive CNS neurons.
Indexing terms: central respiratory chemoreception, hypercapnia, hypoxia, sleep apnea, brainstem
CO2, RTN and breathing
The CNS contains central respiratory chemoreceptors, i.e., interoceptors that sense PCO2 and activate breathing to maintain stability of arterial PCO2 (PaCO2). This fact has been established for many years but the biochemical nature and location of the chemoreceptors have long remained elusive [1–4]. This quest had to overcome two major challenges. The first was to identify the location of the chemosensitive cells within the huge network of neurons that regulate breathing and the other was to identify 6the relevant CO2/H+ detectors among countless proteins whose activity is pH-modulated.
The cellular and molecular basis for chemosensitivity – the types of sensory cells involved (neurons, astrocytes, blood vessels), their CNS location, and the molecular identity of the CO2/H+ detectors – are still actively debated. Here, we review the prevailing ideas. With regard to location, two diametrically opposing views have been championed. The first (distributed chemosensitivity theory) posits that the central respiratory chemoreflex results from the aggregate effects of acidification that is sensed throughout the pontomedullary respiratory pattern generator and myriad brain regions that control it [4]. According to the other viewpoint, the central chemoreflex results from effects of [H+] that are sensed at a single brain site located at the ventral surface of the medulla oblongata [1]. From a molecular standpoint, the prevailing view is that the CO2 is detected via the proxy of [H+] but some studies support an independent role for molecular CO2 and/or bicarbonate [4–7].
The focus of this review is the retrotrapezoid nucleus (RTN) because, at present, this structure can be viewed as a prototypical central respiratory chemoreceptor. RTN neurons reside at the ventral surface of the medulla oblongata, in a region suspected since the 1960s of harboring central respiratory chemoreceptors [1, 5, 8]. Their phenotype and early developmental lineage are known in great detail. RTN neurons are responsive to changes in PaCO2 in vivo and in vitro [9] and play a pivotal role in the regulation of breathing by CO2 [10, 11]. They have clear disease relevance [12]. Finally, the cellular and molecular mechanisms implicated in their CO2 sensitivity have been studied more extensively than for any other candidate respiratory chemoreceptors in the CNS (for information about other chemoreceptor candidates, see [4, 13]).
1. Definition of the retrotrapezoid nucleus (RTN)
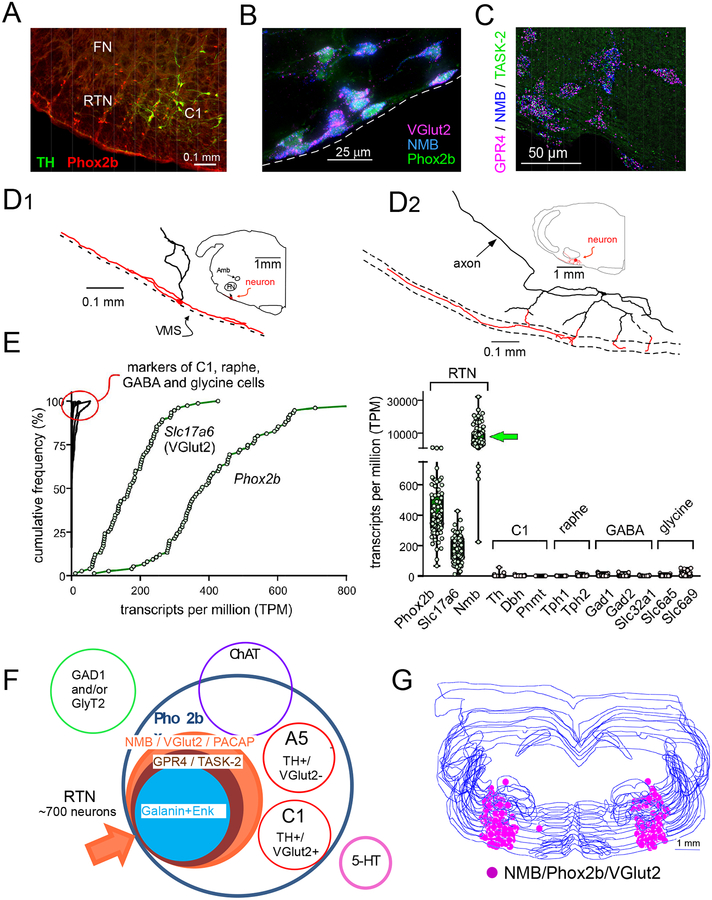
The RTN originally referred to neurons located below the facial motor nucleus that project to the rostral ventral respiratory group (rVRG) or, less restrictively, to the brain region that contains these neurons [14, 15]. Here we use the term RTN to describe a specific group of neurons located within this previously defined region that meet the following criteria. RTN neurons express VGlut2, Phox2b, NK1R and lack the defining markers of catecholaminergic, serotonergic, cholinergic, GABAergic and glycinergic neurons (Figure 1A,B,E,F); they number ~730 in mice and ~2000 in rats [16–22]. They are found at variable density under the entire extent of the facial motor nucleus (Figure 1G) and have long (>1 mm) dendrites within 200 microns of the ventral medullary surface that commonly extend to the medial edge of the trigeminal tract (Figure 1D1,D2). RTN neurons preferentially innervate the pontomedullary regions that generate the respiratory rhythm and pattern (caudal VRG, VRG, PreBötzinger and Bötzinger region, ventrolateral NTS, Kölliker-Fuse nucleus) [23].
Figure 1: location and phenotype of RTN neurons.
A. RTN identified in an adult Sprague-Dawley rat by the presence of Phox2b-immunoreactive nuclei and the absence of tyrosine hydroxylase (transverse section, left side). C1, tyrosine-hydroxylase-positive neurons that also express Phox2b. FN, facial motor nucleus (unlabeled).
B. Transverse section from Phox2b-EYFP mouse brain showing co-expression of Phox2b and mRNA transcripts for Neuromedin B (NMB) and VGlut2. NMB is absent from the C1 cells (not shown). Unpublished data from RL Stornetta.
C. Co-localization of mRNA transcripts for NMB, GPR4 and TASK-2 in RTN. GPR4 and TASK-2 are putative proton receptors.
D. Dendritic structure of two representative RTN neurons identified by unit recording in anesthetized rats and labeled juxtacellularly with biotinamide. Long dendrites (highlighted in red) confined to within 200 microns of the ventral medullary surface (dotted line) are a characteristic feature which suggests that RTN neurons may sense both CSF and ECF [H+].
E. Expression of transcripts for Phox2b, VGlut2 (Slc17a6) and Nmb in RTN neurons by single cell RNA-Seq; markers for C1, serotonergic, GABAergic and glycinergic neurons were virtually nonexistent.
F. Venn diagram depicting the biochemical features that distinguish the mouse RTN from neighboring neurons. RTN is defined as a cluster of neurons positive for NMB, VGlut2 and PACAP. GPR4 and TASK-2 transcripts are detectable in ~ 90% of these neurons.
G. Anatomical location of the mouse RTN defined as neurons (n = ~700) that co-express NMB, Phox2B and VGlut2; neurons plotted on a series of equidistant transverse sections (scale bar: 0.5mm).
Panel A modified from [5]; Panels C, E, F, G modified from [22]; Panel D modified from [9].
The RTN of mice has a distinctive developmental lineage that relies on transcription factors Egr2, Phox2b, Lbx1 and Atoh1 [16–20]. Phox2b is the only one that remains expressed in adulthood. RTN progenitors originate from the dB2 domain of rhombomere 5; these progenitors are Phox2b-positive, switch on Lbx1 at the post-mitotic stage, migrate ventrally and activate Atoh-1 expression once they reach the region of the facial motor nucleus [16–20].
All RTN neurons contain transcripts for PACAP and Neuromedin B and most (>80%) also contain mRNA coding for TASK-2 and GPR4, proteins that contribute to their pH sensitivity (Figure 1C,F) [22]. Large inter-individual differences in mRNA level have been observed among these neurons, e.g. those encoding neuromedin B, galanin, enkephalin and many receptors [22]. This variability may suggest the existence of distinct functional subgroups of RTN neurons, or differential adaptive regulation of specific genes under specific physiological circumstances.
2. Physiological role of the RTN
In this section we discuss the evidence that suggests the RTN is a major nodal point through which changes in brain PCO2 regulate breathing. We also examine how breathing is regulated by the RTN during sleep and wake and how this nucleus develops pre- and post-natally. Finally, we review the complementary role of the RTN and peripheral chemoreceptors in blood gas homeostasis and its pathophysiological implications (sleep disordered breathing, mutations affecting RTN development).
2.1. Methodology
In 2006, high levels of homeobox transcription factor Phox2b were identified in the RTN of the adult rat (Figure 1A) [24, 25]. Soon after, lentiviral vectors engineered with a Phox2b-responsive artificial promoter (PRSx8) were found to drive high levels of transgene expression in RTN neurons [26–28]. The use of these vectors and the availability of several transgenic mouse strains (e.g., Phox2b-EGFP, Phox2b-Cre) have been instrumental in unraveling the structure and function of the RTN [29].
2.2. RTN drives breathing in proportion to arterial pH
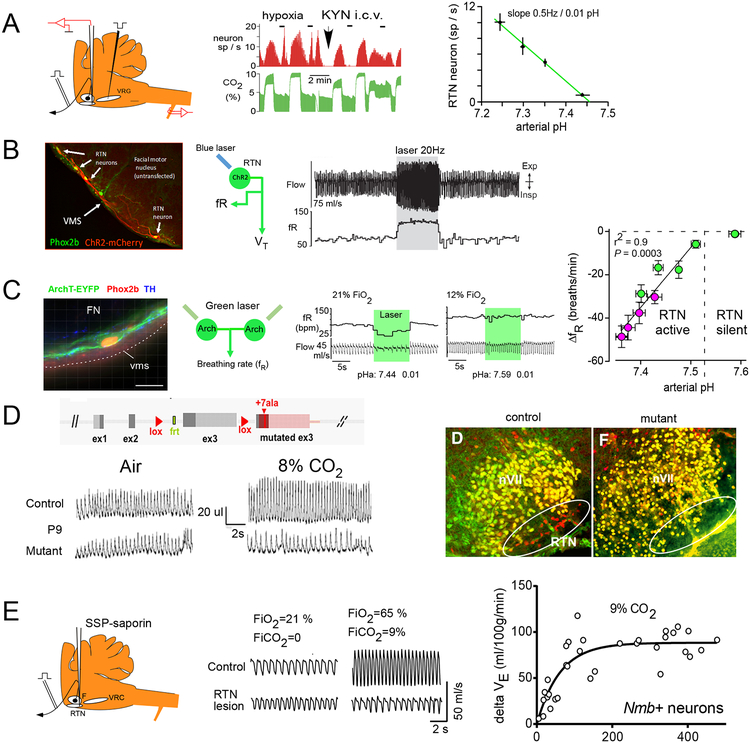
Under anesthesia, RTN neurons are typically silent when arterial pH (pHa) is above 7.4–7.5 and their activity increases sharply below that point (Figure 2A) [9, 30]. On average their discharge changes by 0.5 Hz per 0.01 pHa. In unanesthetized rats, selective optogenetic activation of RTN neurons produces a strong increase in breathing comparable to that evoked by hypercapnia (Figure 2B) [31]. This effect is primarily mediated by the release of glutamate because it is drastically reduced by deleting vesicular glutamate transporter-2 (VGlut2) from RTN neurons [32]. The ongoing respiratory drive contributed by RTN neurons in unanesthetized rats can be estimated by measuring the decrease in ventilation (VE) elicited by instantly silencing these neurons using inhibitory opsins (Figure 2C). By using this approach, the effect of RTN on breathing has the same relationship to arterial pH as their firing rate in anesthetized animals, i.e., it is proportional to pH between pHa 7.45 and 7.25, with a threshold of about pHa 7.5 (Figure 2C) [10]. These characteristics are consistent with what would be expected of neurons that mediate the stimulatory effect of CO2 on breathing. In sum, with or without anesthesia, lung ventilation appears roughly proportional to the mean level of activity of RTN neurons.
Figure 2: Evidence that RTN neurons mediate the central respiratory chemoreflex.
A. Left. RTN neurons are recorded below the facial motor nucleus and can be antidromically activated from (i.e., have axons that project to) the ventral respiratory column. Middle. The discharge of a representative single RTN neuron (red) tracks the end-expiratory CO2 concentration (proxy measure for PaCO2, green). RTN neurons also respond to hypoxia via a polysynaptic input from the carotid bodies. Intracerebral administration of the glutamate receptor antagonist kynurenate blocks the polysynaptic effect of hypoxia but has no effect on the response to CO2. This evidence is consistent with the notion that RTN neurons could be detecting local brain PCO2. Right, Linear relationship between the discharge rate of RTN neurons and arterial pH (anesthetized rat, kynurenic acid).
B. Left: RTN neurons transduced with channelrhodopsin2 (H134R) (ChR2) by local administration of PRSx8-mCherry-ChR2 lentiviral vector; after Abbott et al., 2009 ([26]. Right, breathing activation elicited by unilateral optogenetic activation of RTN in an unanesthetized rat and recorded via whole body plethysmography (unpublished example from PG Guyenet; see also [31]).
C. Evidence that RTN neurons drive breathing in proportion to arterial pH. Left, RTN neuron transduced with archaerhodopsin (vms, ventral medullary surface; scale bar: 20 μm). Middle, bilateral inhibition of RTN reduces breathing when arterial pH is 7.44 but not 7.59. pHa was manipulated by changing FiO2 or by reducing circulating bicarbonate with acetazolamide. Right, relationship between reduction of breathing frequency caused by bilateral inhibition of RTN neurons and pHa (red symbols, plasma acidified by administration of acetazolamide).
D. Top left, the Phox2b27alacki mouse expresses Phox2b27ala in the presence of Cre recombinase. The mutated protein prevents RTN from developing (right panels). Bottom left, Phox2b27alacki;;Egr-2Cre mice (P9) hypoventilate at rest and do not increase their breathing when exposed to high concentrations of CO2.
E. Left, local administration of SSP-saporin to kill RTN neurons in rats. Middle, unanesthetized rat with a ~90% reduction of RTN neurons hypoventilates at rest and does not respond to hypercapnia. Right, relationship between hypercapnic ventilatory reflex and number of surviving RTN neurons in rats with SSP-saporin lesions of RTN.
Panel A modified from [9]; Panel C modified from [10]; Panel D from [17];Panel E from [11].
2.3. State-dependence of the regulation of breathing by the RTN
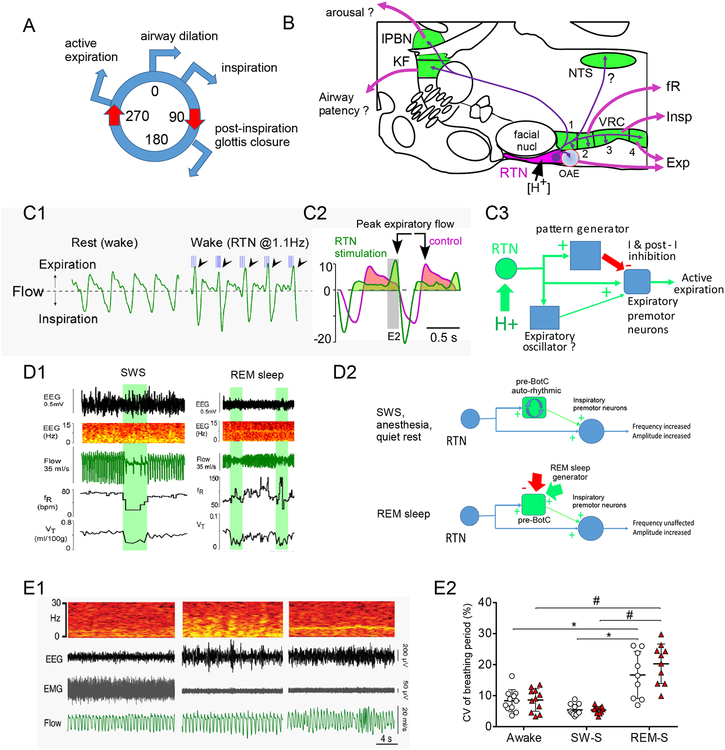
In quietly resting awake rats, optogenetic stimulation of RTN enhances alveolar ventilation by increasing respiratory frequency (fR) and inspiratory amplitude, and triggering active (a.k.a. abdominal) expiration (Figures 2B, 3A–C) [31]. Conversely, fR, inspiratory amplitude and active expiration are all decreased or eliminated by silencing RTN [10, 27].
Figure 3: How does RTN regulate breathing?
A. The breathing cycle. Airway dilation precedes activation of inspiratory muscles (diaphragm, intercostal) and is followed by a transient partial closure of the glottis which facilitates alveolar gas exchange. This is followed by expiration which is either passive, or active, i.e. caused by contraction of abdominal muscles.
B. RTN neurons predominantly innervate the lower brainstem regions that generate the respiratory rhythm and motor pattern (VRC and Kölliker-Fuse nucleus, lateral parabrachial region and nucleus of the solitary tract). The VRC is divided into 4 segments thought to control predominantly the breathing rhythm (Bötzinger and preBötzinger, segments 1 and 2), the activity of inspiratory muscles (rVRG, segment 3) or expiratory muscles (cVRG, segment 4).
C. C1. Evidence that RTN activates inspiratory amplitude, reduces post-inspiratory outward air flow and triggers abdominal expiration (unanesthetized rat; blue lines, 10ms light pulses to activate ChR2-transduced RTN neurons; arrows point to presumptive active expiration elicited by RTN stimulation). C2. Averaged response before and during RTN stimulation illustrating increase in inspiratory amplitude, slowed early expiratory flow suggesting glottis constriction and vigorous late expiratory flow denoting abdominal muscle contraction. C3. Interpretation. RTN may produce active expiration by directly activating abdominal premotor neurons during the late phase of the respiratory cycle and enhancing their inhibition during the other phases of the cycle. A more complicated alternative hypothesis states that active expiration is produced by an expiratory oscillator (neuronal phenotype unidentified) located near the RTN and innervated by the latter.
D. D1. RTN regulates breathing frequency only when the pattern generator is auto-rhythmic. Breathing automaticity during anesthesia, quiet rest and slow wave sleep (SWS) is probably generated by the autorhythmic properties of the preBötzinger complex. During SWS (left panel), bilateral optogenetic inhibition of RTN with Archaerhodopsin (green bars) profoundly reduces breathing frequency but this effect disappears during REM sleep (right panel). By contrast, breath amplitude (tidal volume, VT) is reduced during both SWS and REM. D2. Interpretation: RTN innervates both the preBötzinger complex and the region that contains inspiratory premotor neurons. During SWS, quiet rest or anesthesia, the preBötzinger complex is auto-rhythmic and its rate can be increased by the input from RTN. During REM sleep the preBötzinger complex is driven by powerful inputs from outside the pattern generator that override the effect of the RTN.
E. RTN lesion does not cause central sleep apnea in adult rats. E1. Example of breathing in one rat with almost complete destruction of RTN neurons. Sleep apnea was not detectable. E2. The coefficient of variation of the breathing frequency (a measure of breathing irregularity, hence of the presence of apneas) was unaffected by RTN lesion.
Panel B from [21]; Panels C1 and D1 from [33]; Panel C2 from [107]; Panel E from [11].
These three breathing parameters are differentially regulated by the RTN according to the state of consciousness (Figure 3D1). Specifically, RTN regulates fR only under anesthesia, during quiet rest or slow wave sleep (SWS), i.e., when the brainstem respiratory pattern generator is auto-rhythmic and the inspiratory rhythm is presumably generated by the preBötzinger complex [33, 34]. During REM sleep or miscellaneous behaviors, RTN has no control over fR (Figure 3D1) [33]. During REM sleep, the timing and duration of the various phases of the breathing cycle are presumably defined by powerful drives that somehow override the effect of RTN neurons [33]. Unlike fR, RTN still controls inspiratory amplitude in REM sleep, suggesting that control of inspiratory amplitude is less state-dependent (Figure 3D1,D2) [33]. In short, RTN is a key regulator of breathing auto-rhythmicity, likely via facilitatory inputs to the rhythm-generating preBötzinger complex. RTN is less important to maintain breathing during REM sleep than during SWS because it controls breathing amplitude but not frequency. The brain regions that control the timing of inspiration during REM sleep are unknown.
2.4. Complementary control of breathing by the RTN and carotid bodies
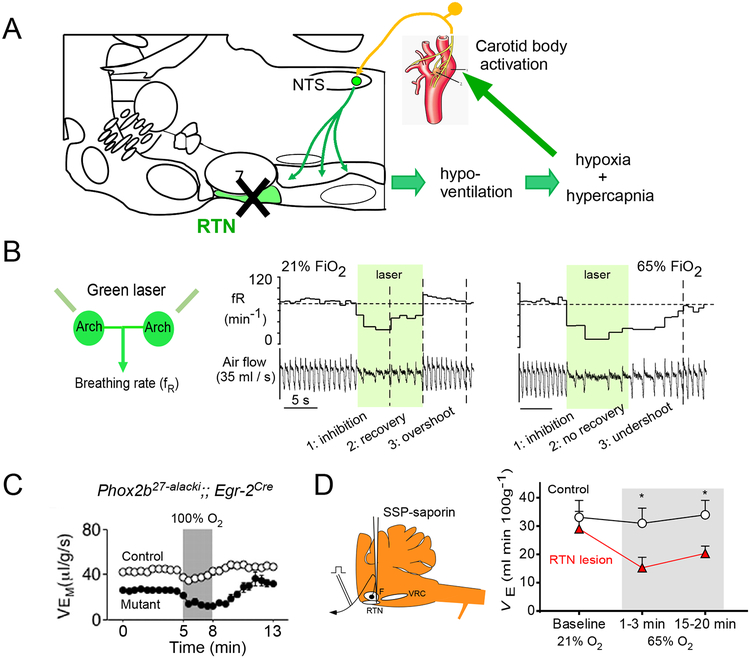
In quietly resting rats, ventilation (VE) is virtually unchanged during exposure to ambient gas mixtures varying in oxygen concentration between 65% (65% fraction inspired oxygen, FiO2; hyperoxia) and 15% FiO2 (hypoxia, PO2 equivalent to 2700 m altitude) [10]. During hypoxia-induced hyperventilation, pHa and, presumably brain ECF pH, become slightly more alkaline as excess CO2 is eliminated. Under these conditions, optogenetic inhibition of RTN produces virtually no breathing reduction, indicating that RTN is silent and breathing is driven primarily by the carotid bodies (Figure 2C). Conversely, at 65% FiO2, the activity of the carotid bodies is reduced, PaCO2 rises slightly and most of the drive to breathe originates from RTN neurons [10]. Also, in rats under normoxic ambient conditions, the hypoventilation elicited by opto-inhibition of RTN neurons wanes quickly because arterial hypoxia sets in, triggering an increase in respiratory drive from the carotid bodies (Figure 4B) [35]. In sum, the RTN and carotid bodies provide two sources of drive to the respiratory network that contribute unequally to breathing depending on FiO2 and, very likely, the physiological situation. When breathing is compromised, causing PaO2 (partial pressure of oxygen in arterial blood) to decrease and PaCO2 (partial pressure of CO2 in arterial blood) to rise concurrently, the two systems are recruited in parallel to maintain blood gas stability.
Figure 4: RTN regulates breathing in tandem with the carotid bodies.
A. Schematic interpretation. Massive RTN lesions using genetic manipulations affecting RTN neuron determination (Phox2b27alacki;;Egr-2cre mice) or with a neurotoxin in adult rats cause a ~20% reduction in resting ventilation relative to metabolism. The resulting hypercapnia and hypoxia sustains breathing primarily via carotid body stimulation.
B. Evidence for a dual control of breathing by RTN and the carotid bodies in intact unanesthetized rats. Left, experimental set-up: bilateral optogenetic inhibition of RTN and breathing measurements via plethysmography. Middle. If the rat breathes room air, breathing inhibition caused by RTN silencing is transient and overshoots after RTN neurons are no longer inhibited. Right, if the rat breathes an O2-enriched mixture to reduce the activity of the carotid bodies, the breathing inhibition is sustained and persists beyond the period of light application. The quick recovery and overshoot are therefore caused by the activation of the carotid bodies.
C. Evidence that breathing is maintained by a hypoxic drive after genetic lesion of RTN. Hyperoxia, which lowers the activity of the carotid bodies, reduces ventilation dramatically in the mutant mouse without RTN and only transiently in the control mouse.
D. Effect of hyperoxia and hypercapnia in adult rats with large RTN lesions. Hyperoxia (65% FiO2; 65/0 point on abscissa) dramatically reduces breathing in the lesioned rats and has virtually no effect in the intact rats.
Panel B from [35]; Panel C from [17]; Panel D modified from [11].
RTN has been relatively selectively ablated in mice using genetic manipulations affecting RTN neuron determination (Phox2b27alacki;;Egr2cre or Egr2-Lbx1FS mice) [17, 18] or using a neurotoxin in adult rats [11] -- with strikingly similar outcomes (Figure 2D,E). The loss of RTN neurons varies depending on the model (85% in Phox2b27alacki;;Egr2cre mice, 92% in adult rats, possibly 100% in the Egr2-Lbx1FS mice) and some collateral damage outside RTN has been documented in the Phox2b27alacki;;Egr2cre mice and in the rat model. In adult rats, RTN lesions cause hypoventilation, hypercapnia (+10 mmHg PaCO2,) and hypoxia [11]. Similar effects presumably occur in Phox2b27alacki;;Egr-2cre and Egr2-Lbx1FS mice, given their reduced resting VE [17, 18]. Stimulation of breathing by CO2 is massively decreased in all three models (by 100% at birth in both mouse models, and by >85% in adult rats) (Figure 2D,E). Also when tested, (Phox2b27alacki;;Egr-2cre mice and rat model), ventilation plummeted when the animals were exposed to hyperoxia, presumably because of a reduction in carotid body activity (Figure 4C,D) [11, 17]. In sum, in the absence of RTN, rodents hypoventilate but otherwise appear normal. Their breathing relies heavily on a hypoxic/hypercapnic drive, likely of carotid body origin (Figure 4A). In both Phox2b27alacki;;egr-2 cre and Egr2-Lbx1FS mice, born with few RTN neurons, the ability of CO2 to stimulate breathing is absent at birth but recovers to 40% of control value in adulthood, plausibly because of compensation by the carotid bodies. The long-term effects of RTN lesions in adult rodents have not been examined.
2.5. RTN and sleep apnea
Hypoxia-reoxygenation cycles are detrimental to the brain and the cardiovascular system [36]. Such events occur in a number of diseases, and are especially prevalent in some such as obstructive sleep apnea and congestive heart failure [37, 38]. More extreme hypoventilation or respiratory arrest is a major sign of other much rarer diseases such as congenital central hypoventilation syndrome (CCHS), and is thought to be involved in sudden infant death syndrome (SIDS) and possibly sudden death in epilepsy (SUDEP) [39, 40]. Periodic breathing, the rapid crescendo-decrescendo alternation of hyperventilation and apnea during sleep, is usually attributed to exacerbation of the hypoxic ventilatory reflex, which results in ventilation overshoot, excessive PaCO2 reduction and transient suppression of the respiratory drive contributed by central chemoreceptors [37]. The problem occurs, for instance, at altitude because of low ambient PO2, and in advanced stages of congestive heart failure [37]. Periodic RTN inactivity likely contributes to periodic breathing because acute hypoxia silences this nucleus (Figure 2C) and acetazolamide, a drug given to reduce sleep-disordered breathing at altitude because it acidifies the plasma, activates RTN even during hypoxia (Figure 2C)[10].
CCHS, mentioned earlier, is a genetic developmental disease that is characterized by hypoventilation during sleep (central sleep apnea) and reduced central respiratory chemoreflex [18, 39, 41, 42]. It is generally caused by Phox2b mutations or, more rarely, by frameshift mutation of the transcription factor Lbx1. A Phox2b mutation that causes both severe sleep apnea and complete loss of the chemoreflex in humans (Phox2b27ala/+) prevents RTN development in mice [43, 44]. This observation suggested that CCHS patients might also lack RTN at birth and that the loss of this nucleus could account for the sleep apnea as well as the loss of the chemoreflex. This theory however seems only partly correct, at best. First, whether the RTN (or an RTN-like structure) is absent in CCHS remains to be clarified. This question is a testable one, since the existence of an RTN-like structure has been documented in humans and macaques [45, 46]. Second, while selective loss of the RTN (Phox2b27alacki;; Egr-2cre mice, Egr2-Lbx1FS mice or toxin-treated adult rats) does indeed greatly reduce CO2-stimulated breathing, the hypoventilation is comparable across all states of vigilance, and sleep apnea is not detectable in normoxia [11, 17]. Therefore, additional defects besides the probable, but still unconfirmed, absence of the RTN likely underlie the sleep apnea in CCHS. Given that the carotid bodies compensate for the loss of RTN in rodents [11, 35], the severe hypoventilation experienced by CCHS patients during SWS could be caused by a reduction of both homeostatic breathing reflexes – i.e., the central mechanisms for CO2 regulation and peripheral mechanisms for O2 regulation [47]. Phox2b is indeed required for the development of the carotid bodies, their sensory innervation and the NTS, where their afferents terminate [48]. Preliminary evidence from our laboratory suggests that near complete lesions of RTN in rats in which the carotid bodies had been previously removed causes fatal hypoventilation.
Hypercapnia also causes arousal from sleep, a life-saving reflex that contributes to restoring breathing after an apnea. CO2-induced arousal is facilitated by serotonin and largely mediated via neurons located in the external-lateral parabrachial nucleus [49–51]. A contribution of RTN to CO2 arousal via excitatory projections to this brain region is suspected but not yet documented.
2.6. The embryonic and postnatal RTN
In vitro (neonatal brainstem-spinal cord preparation), the ventral medullary surface contains neurons that have a biphasic pre- and post-inspiratory discharge (“pre-inspiratory” for short) and were originally called the parafacial respiratory group (pfRG) [52]. pfRG neurons likely excite the pre-Bötzinger complex (postulated inspiratory rhythm generator, see Figure 3B for location) [34] thereby contributing to the timing of phrenic motor neuronal discharges. Emphasizing their post-inspiratory burst, other investigators posited that the pfRG might also drive the activity of expiratory muscles [53]. The “pre-inspiratory” neurons located under the facial motor nucleus are depolarized by [H+] and express VGlut2 and Phox2b [54]. These particular pfRG neurons are undoubtedly neonatal RTN neurons, unlike similarly biphasic neurons located elsewhere in the lower brainstem. The prenatal precursors of the mouse RTN (positive for VGlut2, Phox2b and NK1R and dependent on Egr2 and Atoh1 for their development) originally called the “embryonic parafacial oscillator” also have a rhythmic “pfRG-like” discharge pattern in a brainstem /spinal cord preparation [55]. These neurons are connected by gap junctions and their group discharge is also driven by [H+] [55].
2.7. RTN and the parafacial expiratory oscillator
Expiratory muscles are recruited for enhanced breathing during exercise or when chemoreceptors are strongly activated [27, 56]. The “parafacial region” (region surrounding the facial motor nucleus) contributes to the generation of this motor outflow, called active or abdominal expiration [57–59]. This property was attributed to the presence in this region of an expiratory “oscillator” which is distinct from, but coupled to, the inspiratory oscillator otherwise known as the pre-Bötzinger complex [34, 57]. This expiratory oscillator may reside at the ventrolateral edge of the parafacial region (pFl) [60]. However, pFl contains numerous RTN somata and a profusion of RTN dendrites (Figure 1D,G)[22]. Furthermore, active expiration is elicited by optogenetic activation of RTN neurons and eliminated by their pharmacogenetic inhibition [27, 31]. Finally, the only demonstrated oscillating circuit located within the parafacial region is the perinatal RTN; its burst discharge does not occur during the correct (late expiratory) phase to drive expiratory muscles and its oscillating properties are not known to outlast the perinatal period (see preceding section) [54, 55].
In short, RTN drives active expiration along with the other components of breathing that enhance alveolar ventilation but the downstream connectome is speculative. RTN may provide CO2 sensitivity to an expiratory oscillator made of currently unidentified neurons that are interspersed with a lateral group of RTN neurons. Alternately, the oscillator could be a subset of RTN neurons. Finally, it is possible that there is no oscillator at all within the parafacial region, but rather merely high-threshold RTN neurons providing a tonic CO2-modulated drive to downstream rhythmically active components of the respiratory pattern generator that drive expiratory pre-motoneurons.
3. Four mechanisms contribute to the response of RTN neurons to CO2.
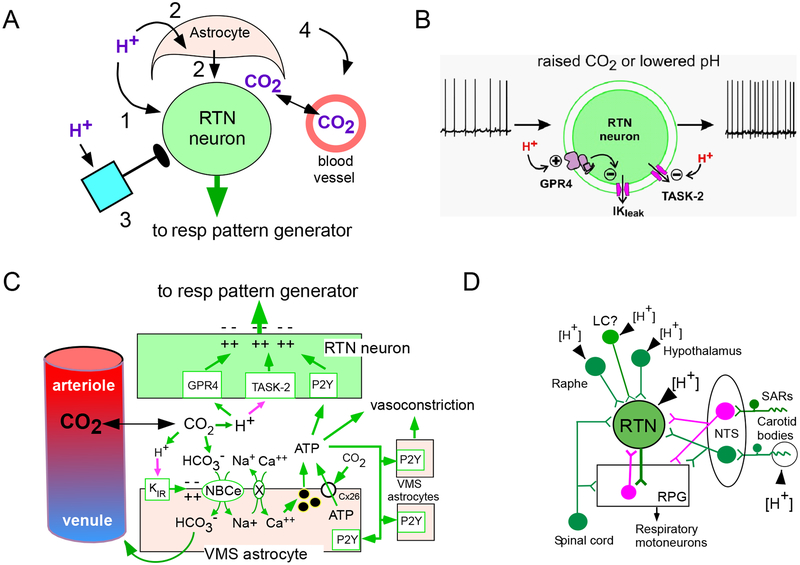
The experimental results discussed in the previous section show that RTN neurons mediate the central chemoreflex. They do not provide evidence that RTN neurons are proton or CO2 sensors. Although a consensus has emerged that RTN neurons are indeed responsive to local changes in PCO2, three mechanisms illustrated in Figure 5A–C (key figure) are currently proposed. Arguments for and against each theory will be presented. In addition, the activation of RTN by hypercapnia is also partly caused by a fourth mechanism, i.e., excitatory inputs from other CO2-responsive neurons (Figure 5D).
Figure 5 (Key Figure): Mechanisms that underlie the CO2 sensitivity of RTN neurons (key figure).
A. Four classes of mechanisms contribute to the CO2 sensitivity of RTN neurons in vivo: cell autonomous sensitivity to acid (pathway 1), paracrine activation via astrocytes (pathway 2), inputs from neurons that are directly or indirectly responsive to acid (pathway 3) and acid–induced vascular contraction (leading to CO2 retention and parenchymal acidification, pathway 4).
B. Cell-autonomous acid sensitivity theory. Acid-sensitivity requires two proton receptors TASK-2 and GPR4 expressed by RTN neurons. Neuronal depolarization is caused by acid-induced closure of TASK-2 and by closure of an unidentified leak potassium channel operated by GPR4.
C. Astrocyte-dependent theories. Acidification depolarizes astrocytes and activates an electrogenic sodium-bicarbonate exchanger (NBCe) causing intracellular [Na+] to rise and increasing intracellular [Ca++] via Na/Ca exchange. The rise in intracellular calcium causes vesicular release of ATP. ATP is presumed to directly activate RTN neurons via P2Y receptors. ATP may also trigger vasoconstriction, CO2 retention and further extracellular acidification. ATP release from astrocytes through carbamylated connexin26 has also been evoked. Acid-induced vasoconstriction in the RTN region requires ATP release but the notion that astrocytes are the source of this ATP, as drawn, is speculative.
D. Synaptic inputs as a source of CO2-dependent activation of RTN neurons. Information regarding inputs to RTN neurons is incomplete. RTN neurons receive an excitatory polysynaptic input from the carotid bodies, organs that sense arterial acidification in an O2-dependent manner. RTN is also activated by serotonin, orexin and noradrenaline, transmitters whose release may originate from CNS neurons that are directly (intrinsically pH-activated) or indirectly activated by hypercapnia in vivo (via arousal, stress etc.). RTN also receives inhibitory mono- or polysynaptic input (in magenta) from neurons that are activated by hypercapnia, e.g. inhibitory interneurons activated by lung inflation receptors (SARs) or the respiratory pattern generator.
Panels A and C modified from [107]; Panels B and D modified from [21].
3.1. Cell autonomous pH-sensitivity hypothesis
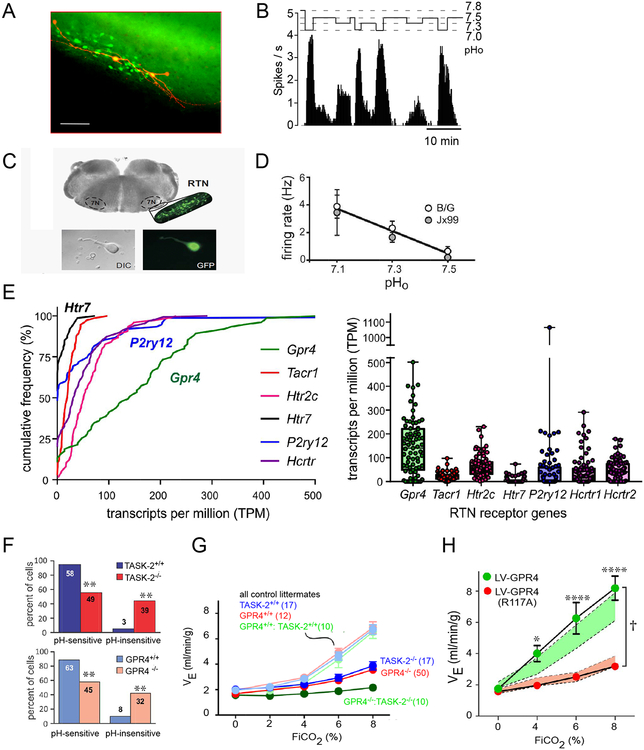
The pH-sensitivity of RTN neurons is at least partly an intrinsic property requiring expression of two putative pH sensors, the two-pore domain potassium channel TASK-2 and the G-protein coupled receptor GPR4 (Figure 5A,B) [5]. The key evidence is as follows. RTN neurons from neonatal mice respond to acidification in brainstem slices when synaptic input is reduced (TTX, low-Ca/ high-Mg, blockers of glutamate receptors) and, importantly, after acute dissociation (Figure 6A–D) [9, 54, 61]. TASK-2 mediates a pH-dependent background potassium current that decreases in amplitude with acidification through the physiological pH range [62–64], and GPR4 stimulates pH-dependent cyclic AMP accumulation with pH50 of 7.4 [21, 65, 66]. TASK-2 and GPR4 transcripts are detectable histologically in >80% of RTN neurons (Figure 1C) and in ~90% by single-cell transcriptomics (Figure 6E) [22]. GPR4 is the most highly transcribed GPCR in the RTN (Figure 6E) [22]. The pH sensitivity of RTN neurons in slices is absent or greatly reduced in TASK-2 or GPR4 KO mice (Figure 6F) [65, 67]. Incubation of brainstem slices from wild-type mice with a small molecule GPR4 receptor antagonist reduced the proportion of pH-sensitive RTN neurons [65, 68] and acute systemic treatment with NE 52-QQ57, another antagonist of GPR4 (but not of TASK-2), depressed CO2-stimulated breathing in conscious rats and mice (by ~15%−25%) [69]. Moreover, mice in which either gene has been knocked out have a 65% reduction of their central respiratory chemoreflex and the double KO mice have virtually no reflex (95% reduction; Figure 6G) [21, 65, 70]. Finally, reintroducing GPR4 selectively into RTN neurons of GPR4 KO mice restores the respiratory chemoreflex (Figure 6H) and the ability of hypercapnia to elicit Fos expression in RTN neurons [65].
Figure 6: The cell-autonomous acid-sensitivity theory.
A. Intracellular fills (biotinamide) of two RTN neurons recorded in a transverse brain slice (neonatal Phox2b-EGFP JX99 mouse; scale bar: 50 μm). Note the extensive superficial dendrites.
B. Effect of acidification on the discharge rate of a single RTN neuron (neonatal Phox2b-EGFP JX99 mouse).
C. Dissociation and isolation of RTN neurons from the Phox2b-EGFP JX99 mouse;
D. Relationship between mean discharge rate and bath pH. RTN neurons were isolated from two different transgenic strains of mice.
E. Expression of select G protein-coupled receptor transcripts in RTN neurons by single cell RNASeq. The most highly expressed receptor is GPR4, which was observed in ~90% of cells (see y-intercept). Of other receptors implicated in pH sensitivity of RTN neurons, the most highly expressed purinergic receptor is P2Y12 (P2ry12, in ~40% of cells). The 5HT7 receptor (Htr7, detectable in only ~25% of cells) is found at lower levels than the 5HT2C receptor (Htr2c), which was universally present. Other receptors of note in RTN neurons may convey pH or arousal-dependent signals (NK1R, Tacr1; and orexin receptors, Hcrtr1 and Hcrtr2).
F. A substantial population of pH-insensitive RTN neurons is evident in recordings from brain slices from either TASK2−/− or GPR4−/− mice.
G. The central respiratory chemoreflex of TASK2−/− and GPR4−/− mice (breathing response to CO2 in hyperoxia) is ~65% reduced relative to control mice. The reflex of the double KO mice is virtually absent.
H. Normal chemoreflex of GPR4−/− mice is restored by re-expressing wild-type GPR4, but not a signaling-deficient GPR4(R117A) mutant, in RTN neurons with a lentiviral vector.
Panel A from [21]; Panel B from [29]; Panels C and D modified from [61]; Panel F modified from [65] [67]; Panels G and H modified from [65].
This evidence has limitations. TASK-2 is not exclusively expressed by RTN neurons [70] and low levels of GPR4 may be expressed by the brain vascular endothelium and subsets of serotonergic neurons [22, 65, 69]. Thus, the reduction of CO2-stimulated breathing observed with global deletion of TASK-2 or GPR4 cannot be unequivocally attributed only to their absence in RTN neurons. Because some GPR4 is expressed by endothelial cells, the reduced CO2-stimulated breathing in GPR4-deleted mice could conceivably result from changes in blood flow (Hosford et al., 2018). However, this interpretation overlooks that the central chemoreflex of GPR4−/− mice is restored by reintroducing the gene selectively into RTN neurons (figure 6H) [65]. Moreover, although both TASK-2 and GPR4 are clearly sensitive to pH changes, it is formally possible that their contributions to RTN neuron chemoreceptor function reflect some other modulatory action. For example, TASK-2 is reportedly inhibited by G protein βγ subunits [71], leaving it potentially susceptible to receptor stimulation by other CO2-dependent signaling molecules; likewise, GPR4 could have a yet-unidentified endogenous ligand that is released onto RTN neurons in a CO2-dependent manner. GPR4 deletion could conceivably reduce the general excitability of RTN neurons, and reintroducing this receptor selectively into RTN neurons of GPR4 KO mice may have restored the chemoreflex by increasing cellular excitability and responsiveness to other CO2-dependent inputs [65]. Also, while arguably supportive of a role of GPR4 in RTN, the pharmacological evidence is incomplete and not fully consistent. The GPR4 antagonist used by Kumar et al. was not tested in vivo [65] and NE 52-QQ57 was ineffective when applied directly to the ventral surface of the medulla of anesthetized rats, albeit without a corresponding positive control [69].
In sum, the contribution of TASK-2 and GPR4 to the CO2-sensitivity of RTN neurons in vitro and the central respiratory chemoreflex is generally well supported. Alternative interpretations are plausible, but they require discounting the proven pH sensitivity of both TASK-2 and GPR4 in favor of some other modulatory effect of these proteins, which at the moment remains speculative.
3.2. The paracrine hypothesis
The paracrine hypothesis holds that astrocytes that sense molecular CO2 or [H+] are responsible for the activation of the respiratory pattern generator by hypercapnia [72, 73]. Such astrocytes are present not only in the RTN, but throughout the respiratory network [72], and in particular within the respiratory rhythm generator [74]. In essence, this hypothesis upholds the concept that the central chemoreflex results from widely distributed effects of protons (or CO2) on the respiratory pattern generator [4] and contrasts with the view that the RTN is a privileged site for CO2 chemoreception.
One version of the paracrine hypothesis suggests that astrocytes detect molecular CO2; in this conception, CO2 carbamylates connexin-26 expressed by astrocytes causing hemi-channel opening, ATP release and subsequent neuronal activation via purinergic receptors [6, 75]. This interpretation is seemingly at odds with prior evidence that breathing is driven by extracellular [H+] [2] but both mechanisms could conceivably co-exist. The best documented astrocytic paracrine mechanism considers that CO2 operates via changes in pH (Figure 5C). The central feature is the electrogenic sodium-bicarbonate exchanger (NBCe1), whose activity is assumed to increase by CO2-induced intracellular acidification [76] or when the membrane of astrocytes is depolarized by CO2 via inhibition of a pH-sensitive inwardly-rectifying K channel [77]. NBCe1-mediated Na entry would then stimulate sodium-calcium exchange, and the rise in intracellular calcium would cause ATP release via exocytosis [76]. ATP would then activate neighboring neurons, notably RTN, via P2Y-type purinergic receptors [73, 78]. Currently, evidence that knocking out NBCe1 in brainstem astrocytes attenuates the central respiratory chemoreflex is not available. Also, the theory relies heavily on the observation that ChR2-driven astrocyte depolarization activates breathing [73]. This procedure releases potassium which activates surrounding neurons or glia [79].
The exocytotic release of transmitters by astrocytes, including ATP, is attenuated when these cells are transduced with dominant negative SNARE protein (dnSNARE) or tetanus toxin light chain (TeLC). In the pre-Bötzinger complex (rhythm generator), this procedure reduces modestly the resting respiratory frequency of conscious rats (11%) [74]. Bilateral overexpression of a potent ectonucleotidase (TMPAP) by astrocytes had the same effect, whereas activation of astrocytes transduced with a Gq-coupled DREAAD (receptor activated by administration of clozapine N-oxide) stimulated the resting breathing rate (+23%)[74]. These results show that RTN is not the only region of the breathing network that is regulated by astrocytes. However, expression of dnSNARE or TeLC by astrocytes also depressed (20–50%) the ventilatory response to every stimulus tested (hypoxia, hypercapnia and exercise) and reduced hypoxia-induced sighing [74]. The uniformly depressant effect of dnSNARE and TeLC transduction on all ventilatory responses tested suggests that astrocytes may facilitate synaptic transmission within the preBötzinger complex regardless of the modality and source of these inputs. A similar non-specific facilitation could explain the contribution of RTN astrocytes to the CO2 responses of RTN neurons.
In summary, brainstem astrocytes are capable of activating various components of the respiratory pattern generator including RTN and the pre-Bötzinger complex by releasing ATP and possibly other transmitters. Whether this glial mechanism selectively mediates the effects of [H+] or broadly regulates synaptic connectivity remains to be seen. At present, the astrocyte theory does not satisfactorily explain, in our view, why highly selective RTN lesions eliminate the central chemoreflex at birth [17, 18] unless the astrocytic mechanism develops postnatally. This is conceivable because the intensity of the hypercapnic ventilatory reflex increases markedly in rodents during the first two weeks of post-natal life and recovers to 40% of control in adult mice with genetically programmed absence of RTN [17, 18, 80]. However, this interpretation still does not explain the massive reduction of the central chemoreflex caused by RTN lesions in the adult rat nor the elimination of the reflex in adult TASK-2/GPR4 double knock-out mice [11, 21].
3.3. The synaptic input hypothesis
According to this hypothesis the activation of RTN neurons by hypercapnia is mediated by synaptic inputs from CO2-activated neurons located elsewhere (Figure 5A,D). This hypothesis also has considerable support. RTN neurons are activated by stimulation of the carotid bodies, organs whose response to hypercapnia, albeit hypoxia-dependent, is indisputable [81, 82]. Administration of glutamate receptor antagonists into RTN decreases breathing and attenuates the central respiratory chemoreflex, implying that RTN neurons could be driven from elsewhere [15]. The neuropil surrounding RTN neurons contains orexinergic, noradrenergic and serotonergic terminals, and RTN neurons express receptors for these transmitters (Figure 6E); in slices, NE, serotonin, orexin, substance P or TRH activate RTN neurons as much as a severe (0.4) pH change [83–89]. Subsets of CNS noradrenergic and serotonergic neurons respond to hypercapnia in anesthetized rats [90] and brain slices [91, 92]. Finally, lesions of orexinergic, noradrenergic or serotonergic neurons or administration of the appropriate receptor antagonists attenuate the central respiratory chemoreflex [84, 93–96]. According to Wu et al., the CO2 response of RTN neurons in mouse brain slices (postnatal 13–20 days) or in neuronal co-culture, is predominantly driven by serotonin release and subsequent activation of 5-HT7 receptors [97]. The evidence is primarily pharmacological. The interpretation assumes that most RTN neurons express the 5HT7 receptor (but see Fig. 6E), and that the antagonists are selective for that receptor and do not non-specifically reduce the general excitability of RTN neurons. In addition, the ability of 5HT7 receptor antagonists to reduce RTN activity and CO2-stimulated breathing in vivo was not established. Finally, the theory does not fully explain why chemogenetic inhibition of the particular serotonergic neurons believed to innervate RTN (r3/r5-derived subgroup) produced only a 15% reduction of CO2-stimulated breathing [92].
In short, RTN neurons receive inputs from neurons that are activated by CO2. The contribution of these inputs to the overall response of RTN neurons to CO2 in vivo is yet to be determined.
3.4. The microvasculature hypothesis
Brain hypercapnia typically produces vasodilation. If this were to happen in a region where CO2 sensors are located, the resulting rise in blood flow would wash out locally produced CO2 and reduce the ability of the chemoresponsive neurons (or glia) to detect changes in arterial PaCO2 [98]. A specialization within the RTN may prevent this potentially countervailing effect of CO2; unlike in the cortex, acidification constricts arterioles in the RTN [99]. This vasoconstriction is reduced by application of ATP-receptor antagonists. The source of this ATP is unidentified. If astrocytes are the source of it (as depicted in Figure 5C), vascular constriction and subsequent CO2 retention by the brain parenchyma may also partly explain why optogenetic depolarization of RTN astrocytes ultimately activates RTN neurons and breathing [73].
Concluding remarks and future perspectives
RTN neurons can be objectively distinguished from surrounding neurons by their expression profile and cell lineage. RTN neurons are demonstrably activated by acidification/hypercapnia regardless of the preparation. Despite its small size, this nucleus is phenotypically heterogeneous and may contain neurons with different connectomes and functions.
RTN is a major nodal point for the control of breathing by CO2. RTN lesion or simultaneous deletion of TASK-2 and GPR4 reduces the central respiratory chemoreflex to an extent that no other type of lesion or pharmacological treatment has heretofore produced. Based on this evidence the postulated acid sensitivity of the respiratory pattern generator must contribute very little to the central chemoreflex. The effectiveness of RTN lesions is compatible with the notion that many other types of CNS neurons contribute to the respiratory chemoreflex [4]. Those neurons may enhance this reflex by potentiating the effect of RTN on its downstream targets or by directly exciting RTN neurons. Subsets of serotonergic neurons likely do both [92, 97].
RTN controls alveolar ventilation by regulating breathing frequency, inspiratory amplitude and active expiration. This occurs via axonal projections to all segments of the respiratory pattern generator but details are few and the persisting uncertainties regarding where and how the respiratory rhythm and pattern are generated in mammals [34, 100, 101] further hinder understanding how RTN regulates this network. There could be several functional subgroups of RTN; for example, separate populations of RTN neurons may control expiratory muscles, breathing frequency, and/or inspiratory amplitude. The possibility that the parafacial expiratory oscillator [34] might be a subset of RTN neurons that drive expiratory premotor neurons deserves scrutiny.
Multiple mechanisms may underlie the response of RTN neurons to CO2 in vivo. None is demonstrated without reservation and there is no consensus regarding their relative importance. Theories include a cell-autonomous pH sensitivity, paracrine effects of [H+] mediated by astrocytes and blood vessels, and excitatory inputs (mono- or polysynaptic) from the carotid bodies and other CNS CO2-responsive neurons. The intrinsic pH sensitivity of RTN neurons requires two membrane proteins, TASK-2 and GPR4 that have proven proton-sensing properties in heterologous expression systems. These putative proton detectors are expressed relatively selectively in RTN neurons, with GPR4 found at extremely high levels. Nevertheless, although their genetic deletion (in a double knock-out line) disrupts RTN neuronal pH sensitivity and eliminates the chemoreflex, one cannot assert that these deficits are entirely due to the intrinsic pH sensitivity of the proteins or their expression in RTN. The astrocyte-based theory is appealing but questions remain on whether it can adequately explain the special importance of RTN to the chemoreflex. Acid-sensitive astrocytes are reportedly present throughout the brainstem; the reason why these astrocytes do not confer significant acid sensitivity to the pattern generator in the absence of the RTN needs to be clarified. Also, although a molecular basis for serotonergic neuron pH sensitivity has not been identified, their response to acid seems to be an intrinsic property [97]. RTN receives excitatory as well as inhibitory inputs from other CO2 responsive neurons [15, 81, 92, 97, 102]. This fact precludes any definitive statement as to whether the sum total of these CO2-modulated inputs causes a notable activation of RTN and breathing in an intact unanesthetized mammal. Finally, a CO2-induced arteriolar constriction, possibly peculiar to the RTN, may prevent increases in local blood flow when blood PCO2 rises thereby insuring that the chemoreceptor neurons more faithfully sample PaCO2. This theory is based on a single study [99] and needs independent confirmation. The origin of the ATP that mediates this vasoconstriction is unknown. If derived from astrocytes, the postulated paracrine effect of acid on RTN and other neurons could partly result from astrocyte-mediated vasoconstriction, an effect whose physiological relevance in an intact animal should be tested.
Which of the four proposed mechanisms, if any, contributes most to the regulation of breathing under normal physiological circumstances (rest, sleep, moderate exercise) is yet to be determined. Although the central chemoreflex is important, and has been the main focus of this review, it should be noted that blood gas homeostasis relies on several additional mechanisms, including, at a minimum, the peripheral chemoreflex, a “central command” that drives breathing in proportion to exercise, inputs from peripheral sensors that gauge the metabolic activity of tissues, thermoregulation and, possibly, central oxygen sensors [56, 103–105]. Acute increases in brain and arterial PCO2 of the magnitude used to elicit the central respiratory chemoreflex in experimental situations occur only under pathological conditions (e.g. airway blockade, central apnea), and they also elicit symptoms (aversion, air hunger, dyspnea, panic) that reflect the activation of brain circuits involved in arousal, anxiety and stress. These symptoms are not elicited by the very small PaCO2 changes associated with normal breathing fluctuations. The contribution of noradrenergic, orexinergic and, perhaps, subsets of serotonin neurons, to the overall central respiratory chemoreflex could be related to CO2-induced arousal or to the affective consequences of CO2 administration.
The postnatal development of the RTN is of theoretical and practical interest. Early childhood is associated with breathing instability and pathologies such as neonatal sleep apnea and sudden death which are likely caused, or exacerbated, by disruption of the metabolic control of breathing [106]. Pharmacological activation of RTN neurons could in theory reduce central apneas. Acetazolamide appears to work at least partly this way [10]. Unfortunately, perinatal and adult RTN neurons have been studied in entirely different preparations and the early development of central chemosensitivity in vivo is usually gauged by changes in the chemoreflex [80]. This reflex is not a reliable measure of the properties of the pH sensors, per se [80]. It is therefore impossible yet to state whether and how much the discharge pattern and other properties of RTN neurons change during early postnatal life.
Blood gas homeostasis depends on interactive feedback mechanisms mediated by both the RTN and carotid bodies. After carotid body ablation, RTN maintains ventilation at near normal levels, at the expense of a slight increase in steady-state PaCO2. Conversely, hypoxic stimulation of the carotid bodies attenuates the hypoventilation that would otherwise be caused by the absence of the RTN.
The congenital absence of RTN is a likely but unproven cause of the reduced CO2 sensitivity of CCHS patients. In rodents at least, lesions of RTN do not cause sleep apnea. This particular CCHS sign likely denotes additional brain lesions, possibly of the sensors (e.g. carotid bodies) and brainstem circuits responsible for the HVR.
Finally, among the most interesting open questions to be addressed is what else besides CO2 regulates the activity of RTN neurons. For instance, does this nucleus mediate the hyperpnea of exercise? Is it involved in matching O2 delivery with consumption? Does it receive information from temperature sensors as originally postulated [1] or from “metabotropic” afferents that sample the metabolic rate of various bodily tissues? Indeed, the RTN could well be an integrative nexus that relays many interoceptive signals besides pH information onward to the respiratory pattern generator for homeostatic and state-dependent control of breathing.
Outstanding Questions Box.
Is the RTN the principal central respiratory chemoreceptor, the nodal point of the central chemoreflex or both?
Four mechanisms have been proposed to account for the activation of RTN by CO2. Which one, if any, contributes most to PaCO2 stability under physiological conditions (e.g. rest and moderate exercise)?
Do astrocytes regulate RTN neurons directly or via their effects on local blood flow and tissue CO2 retention?
RTN neurons signal via glutamate and express several neuropeptides (galanin, PACAP, Nmb). What is the role of these peptides?
How is the excitability of RTN neurons regulated at the cellular level? What is the contribution of voltage-dependent channels, intracellular messengers and membrane receptors?
How many functional subtypes of RTN neurons are there?
What are the synaptic inputs to RTN neurons? What regulates the RTN besides CO2?
Is the parafacial “expiratory oscillator” a subset of RTN neurons or a distinct cell group that is driven by RTN?
Two circumstances trigger active (abdominal) expiration: hypercapnia and exercise. RTN mediates active expiration caused by hypercapnia. Does it also mediate the hyperpnea of exercise?
Does RTN mediate the arousal or aversive effects of hypercapnia?
Does RTN contribute to blood pressure regulation? Hypercapnia increases sympathetic tone, which prevents arterial pressure drop from the peripheral vasodilation effect of CO2. Is RTN involved in this reflex?
Is RTN hypoplastic or absent in CCHS? One particular Phox2b mutation (Phox2b27ala/+) prevents RTN development in mice. Is this so in humans? Do other Phox2b mutations cause milder or worse deficits?
Is RTN a druggable target? RTN activation produces a breathing response that is indistinguishable from that of CO2. Could pharmacological activation of RTN during sleep find some use in treating neonatal apnea, central sleep apnea, mountain sickness and opioid-induced hypoventilation?
Highlights.
The retrotrapezoid nucleus (RTN) is the most completely characterized cluster of central respiratory chemoreceptors.
RTN is exquisitely responsive to CO2 in vivo and drives breathing in proportion to blood acidity or PCO2 by increasing breathing frequency and the contraction of inspiratory and expiratory muscles. RTN lesions virtually eliminate the breathing stimulation caused by brain acidification.
RTN neurons maintain breathing automaticity during slow wave sleep.
RTN neurons regulate breathing in conjunction with the carotid bodies.
RTN development depends on transcription factors Egr2, Phox2b, Lbx1 and Atoh1.
Selected mutations of Phox2b or Lbx1 prevent RTN development in mice and cause congenital central hypoventilation syndrome.
RTN activation by CO2 relies on their intrinsic pH-sensitivity (via proton sensors GPR4 and TASK-2), on astrocyte-dependent paracrine effects of pH and on input from other CO2-responsive neurons. The relative contribution of these three mechanisms is unsettled.
RTN may be an integrative nexus that relays pH and other interoceptive signals to the respiratory pattern generator for homeostatic and state-dependent control of breathing.
Acknowledgements:
This work is supported by grants from the National Institutes of Health (HL28785, HL074011) and the Congenital Central Hypoventilation Syndrome Foundation to PGG and by grants from the National Institutes of Health HL108609 and Pilot Grant Award from the CCHS Family Network to DAB.
Glossary:
- Central respiratory chemoreceptor
CNS neuron activated by acidification of the surrounding neuropil and capable of stimulating breathing. Such neuron may be intrinsically acid-sensitive or respond to signals released by surrounding astrocytes
- Central respiratory chemoreflex
breathing stimulation elicited by a rise in the partial pressure of CO2 (PCO2) within the brain. This reflex is measured by increasing the fraction CO2 in the inspired gas mixture (FiCO2 to between 3 and 10%). In order to reduce the contribution of the carotid bodies to the hypercapnic ventilatory response, FiO2 is simultaneously raised (65–90%) or these organs are surgically removed
- HCVR
hypercapnic ventilatory reflex; breathing stimulation elicited by increasing FiCO2. The HCVR results from the combined activation of central respiratory chemoreceptors and the carotid bodies
- HVR
hypoxic ventilatory reflex: breathing stimulation elicited by hypoxia (reduced fraction oxygen in inspired air, FiO2). The HVR is primarily caused by the activation of the carotid bodies
- NTS
nucleus tractus solitarius. Sensory nucleus that receives input from the internal organs including carotid bodies, lungs and airways
- Parafacial
adjective. Refers to any brain region that surrounds the facial motor nucleus
- pFRG
parafacial respiratory group. Depending on the author, this term refers to the neonatal RTN or both to RTN (as defined in this review) and other parafacial respiratory neurons
- Phox2b
Paired-like homeobox 2b. Transcription factor required for development of visceral afferents and selected lower brainstem neurons, including RTN
- Respiratory neuron
term describing any neuron whose discharge is phase-locked with the respiratory outflow. Such neurons may or may not play a role in breathing
- Sleep apnea
breathing cessation during sleep
- Ve
minute ventilation; product of tidal volume (volume of air inhaled during a single breath) × fR
- VRC
ventral respiratory column: neuronal network located in the ventrolateral part of the medulla oblongata from the facial motor nucleus to the spino-medullary junction. The VRC generates the respiratory rhythm and pattern and consists of four segments, from rostral to caudal: Bötzinger region (controls the breathing rate and the circulation), preBötzinger complex (rhythm generating region), rostral VRG (subdivision containing inspiratory premotor neurons) and caudal VRG (region that controls the activity of the abdominal muscles implicated in active expiration)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Loeschcke HH (1982) Central chemosensitivity and the reaction theory. J. Physiol 332, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappenheimer JR et al. (1965) Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol 208, 436–450. [DOI] [PubMed] [Google Scholar]
- 3.Haldane JS and Priestley JG (1905) The regulation of the lung-ventilation. J. Physiol 32 (3–4), 225–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nattie E and Li A (2012) Central chemoreceptors: locations and functions. Compr. Physiol 2 (1), 221–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guyenet PG and Bayliss DA (2015) Neural Control of Breathing and CO2 Homeostasis. Neuron 87 (5), 946–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meigh L et al. (2013) CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 22:e01213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncalves CM and Mulkey DK (2018) Bicarbonate directly modulates activity of chemosensitive neurons in the retrotrapezoid nucleus. The Journal of physiology 596 (17), 4033–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell RA et al. (1963) Respiratory responses mediated through superficial chemosensitive areas on the medulla. J. Appl. Physiol 18, 523–533. [DOI] [PubMed] [Google Scholar]
- 9.Mulkey DK et al. (2004) Respiratory control by ventral surface chemoreceptor neurons in rats. Nat. Neurosci 7 (12), 1360–1369. [DOI] [PubMed] [Google Scholar]
- 10.Basting TM et al. (2015) Hypoxia Silences Retrotrapezoid Nucleus Respiratory Chemoreceptors via Alkalosis. J. Neurosci 35 (2), 527–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza G et al. (2018) Breathing regulation and blood gas homeostasis after near complete lesions of the retrotrapezoid nucleus in adult rats. J. Physiol 596 (13), 2521–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goridis C and Brunet JF (2010) Central chemoreception: lessons from mouse and human genetics. Respir. Physiol. Neurobiol 173 (3), 312–21. [DOI] [PubMed] [Google Scholar]
- 13.Hodges MR and Richerson GB (2010) Medullary serotonin neurons and their roles in central respiratory chemoreception. Respir. Physiol. Neurobiol 173 (3), 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JC et al. (1989) Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J. Comp. Neurol 281 (1), 69–96. [DOI] [PubMed] [Google Scholar]
- 15.Nattie EE and Li A (1995) Rat retrotrapezoid nucleus iono- and metabotropic glutamate receptors and the control of breathing. J. Appl. Physiol 78 (1), 153–163. [DOI] [PubMed] [Google Scholar]
- 16.Ruffault PL et al. (2015) The retrotrapezoid nucleus neurons expressing Phox2b and Atoh-1 are essential for the respiratory response to CO2. eLife 4, doi: 10.7554/eLife.07051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanantsoa N et al. (2011) Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J. Neurosci 31 (36), 12880–12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Miranda LR et al. (2018) Mutation in LBX1/Lbx1 precludes transcription factor cooperativity and causes congenital hypoventilation in humans and mice. Proceedings of the National Academy of Sciences of the United States of America 115 (51), 13021–13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubreuil V et al. (2009) Defective respiratory rhythmogenesis and loss of central chemosensitivity in phox2b mutants targeting retrotrapezoid nucleus neurons. J. Neurosci 29 (47), 14836–14846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang WH et al. (2012) Atoh1 governs the migration of postmitotic neurons that shape respiratory effectiveness at birth and chemoresponsiveness in adulthood. Neuron 75 (5), 799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyenet PG et al. (2016) Proton detection and breathing regulation by the retrotrapezoid nucleus. J. Physiol 594 (6), 1529–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y et al. (2017) Neuromedin B Expression Defines the Mouse Retrotrapezoid Nucleus. J. Neurosci 37 (48), 11744–11757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bochorishvili G et al. (2012) Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. J. Comp. Neurol 520 (5), 1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stornetta RL et al. (2006) Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J. Neurosci 26 (40), 10305–10314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pattyn A et al. (1997) Expression and interactions of the two closely related homeobox genes Phox2a and Phox2b during neurogenesis. Development 124 (20), 4065–4075. [DOI] [PubMed] [Google Scholar]
- 26.Abbott SB et al. (2009) Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J. Neurosci 29 (18), 5806–5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marina N et al. (2010) Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J. Neurosci 30 (37), 12466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang DY et al. (2001) A high-efficiency synthetic promoter that drives transgene expression selectively in noradrenergic neurons. Human Gene Therapy 12 (14), 1731–1740. [DOI] [PubMed] [Google Scholar]
- 29.Lazarenko RM et al. (2009) Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J. Comp. Neurol 517 (1), 69–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guyenet PG et al. (2005) Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J. Neurosci 25 (39), 8938–8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbott SB et al. (2011) Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J. Neurosci 31 (45), 16410–16422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holloway BB et al. (2015) The retrotrapezoid nucleus stimulates breathing by releasing glutamate in adult conscious mice. Eur J Neurosci 42 (6), 2271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burke PG et al. (2015) State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593 (13), 2909–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Negro CA et al. (2018) Breathing matters. Nat. Rev. Neurosci 19 (6), 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Basting TM et al. (2016) Is plasticity within the retrotrapezoid nucleus responsible for the recovery of the PCO2 set-point after carotid body denervation in rats? J. Physiol 594 (12), 3371–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harper RM et al. (2013) Sleep-disordered breathing: effects on brain structure and function. Respir. Physiol Neurobiol 188 (3), 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Javaheri S and Dempsey JA (2013) Central sleep apnea. Compr. Physiol 3 (1), 141–163. [DOI] [PubMed] [Google Scholar]
- 38.Dempsey JA et al. (2010) Pathophysiology of sleep apnea. Physiol Rev 90 (1), 47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weese-Mayer DE et al. (2010) An official ATS clinical policy statement: Congenital central hypoventilation syndrome: genetic basis, diagnosis, and management. Am J Respir Crit Care Med 181 (6), 626–644. [DOI] [PubMed] [Google Scholar]
- 40.Garcia AJ III et al. (2013) The physiological determinants of Sudden Infant Death Syndrome. Respir. Physiol Neurobiol 189, 288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carroll MS et al. (2014) Residual chemosensitivity to ventilatory challenges in genotyped congenital central hypoventilation syndrome. J Appl Physiol (1985) 116 (4), 439–50. [DOI] [PubMed] [Google Scholar]
- 42.Amiel J et al. (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat. Genet 33 (4), 459–461. [DOI] [PubMed] [Google Scholar]
- 43.Goridis C et al. (2010) Phox2b, congenital central hypoventilation syndrome and the control of respiration. Semin. Cell Dev. Biol 21 (8), 814–822. [DOI] [PubMed] [Google Scholar]
- 44.Dubreuil V et al. (2008) A human mutation in Phox2b causes lack of CO2 chemosensitivity, fatal central apnoea and specific loss of parafacial neurons. Proc. Natl. Acad. Sci. USA 105, 1067–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudzinski E and Kapur RP (2010) PHOX2B immunolocalization of the candidate human retrotrapezoid nucleus. Pediatr. Dev. Pathol 13 (4), 291–299. [DOI] [PubMed] [Google Scholar]
- 46.Levy J et al. (2019) Tridimensional mapping of Phox2b expressing neurons in the brainstem of adult Macaca fascicularis and identification of the retrotrapezoid nucleus. J Comp Neurol [DOI] [PubMed] [Google Scholar]
- 47.Perez IA and Keens TG (2013) Peripheral chemoreceptors in congenital central hypoventilation syndrome. Respir. Physiol. Neurobiol 185 (1), 186–93. [DOI] [PubMed] [Google Scholar]
- 48.Dauger S et al. (2003) Phox2b controls the development of peripheral chemoreceptors and afferent visceral pathways. Development 130 (26), 6635–6642. [DOI] [PubMed] [Google Scholar]
- 49.Kaur S et al. (2017) A Genetically Defined Circuit for Arousal from Sleep during Hypercapnia. Neuron 96 (5), 1153–1167 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchanan GF et al. (2015) 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol 114 (1), 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buchanan GF and Richerson GB (2010) Central serotonin neurons are required for arousal to CO2. Proc. Natl. Acad. Sci. USA 107 (37), 16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onimaru H and Homma I (2003) A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J. Neurosci 23 (4), 1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wittmeier S et al. (2008) Pacemakers handshake synchronization mechanism of mammalian respiratory rhythmogenesis. Proc. Natl. Acad. Sci. USA 105 (46), 18000–18005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Onimaru H et al. (2014) Cytoarchitecture and CO(2) sensitivity of Phox2b-positive Parafacial neurons in the newborn rat medulla. Prog. Brain Res 209, 57–71. [DOI] [PubMed] [Google Scholar]
- 55.Thoby-Brisson M et al. (2009) Genetic identification of an embryonic parafacial oscillator coupling to the preBotzinger complex. Nat. Neurosci 12 (8), 1028–1035. [DOI] [PubMed] [Google Scholar]
- 56.Forster HV et al. (2012) Control of breathing during exercise. Compr. Physiol 2 (1), 743–77. [DOI] [PubMed] [Google Scholar]
- 57.Janczewski WA and Feldman JL (2006) Distinct rhythm generators for inspiration and expiration in the juvenile rat. J. Physiol 570, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pagliardini S et al. (2011) Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J. Neurosci 31 (8), 2895–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pisanski A and Pagliardini S (2018) The parafacial respiratory group and the control of active expiration. Resp. Physiol. Neurobiol 10.1016/j.resp.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Huckstepp RT et al. (2016) Interactions between respiratory oscillators in adult rats. eLife 5 ( 10.7554/eLife.14203). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang S et al. (2013) Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J. Neurosci 33 (18), 7756–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bayliss DA et al. (2015) The role of pH-sensitive TASK channels in central respiratory chemoreception. Pflugers Arch 467 (5), 917–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reyes R et al. (1998) Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem 273 (47), 30863–30869. [DOI] [PubMed] [Google Scholar]
- 64.Lesage F and Barhanin J (2011) Molecular physiology of pH-sensitive background K(2P) channels. Physiology (Bethesda.) 26 (6), 424–437. [DOI] [PubMed] [Google Scholar]
- 65.Kumar NN et al. (2015) Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348 (6240), 1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ludwig MG et al. (2003) Proton-sensing G-protein-coupled receptors. Nature 425 (6953), 93–98. [DOI] [PubMed] [Google Scholar]
- 67.Wang S et al. (2013) TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J. Neurosci 33 (41), 16033–16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong L et al. (2017) Acidosis Activates Endoplasmic Reticulum Stress Pathways through GPR4 in Human Vascular Endothelial Cells. Int J Mol Sci 18 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hosford PS et al. (2018) CNS distribution, signalling properties and central effects of G-protein coupled receptor 4. Neuropharmacology 138, 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gestreau C et al. (2010) Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc. Natl. Acad. Sci. U.S.A 107 (5), 2325–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cid LP et al. (2013) TASK-2: a K2P K(+) channel with complex regulation and diverse physiological functions. Front. Physiol 4, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gourine AV and Kasparov S (2011) Astrocytes as brain interoceptors. Exp. Physiol 96 (4), 411–416. [DOI] [PubMed] [Google Scholar]
- 73.Gourine AV et al. (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329 (5991), 571–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sheikhbahaei S et al. (2018) Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9 (1), 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huckstepp RT et al. (2010) Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol 588, 3901–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turovsky E et al. (2016) Mechanisms of CO2/H+ Sensitivity of Astrocytes. J. Neurosci 36 (42), 10750–10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wenker IC et al. (2010) Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J. Neurophysiol 104 (6), 3042–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasymov V et al. (2013) Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J. Neurosci 33 (2), 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Octeau JC et al. (2019) Transient, Consequential Increases in Extracellular Potassium Ions Accompany Channelrhodopsin2 Excitation. Cell reports 27 (8), 2249–2261.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Putnam RW et al. (2005) Neonatal maturation of the hypercapnic ventilatory response and central neural CO2 chemosensitivity. Respiratory physiology & neurobiology 149 (1–3), 165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takakura AC et al. (2006) Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J. Physiol 572 (Pt 2), 503–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar P and Prabhakar NR (2012) Peripheral chemoreceptors: function and plasticity of the carotid body. Compr. Physiol 2 (1), 141–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rosin DL et al. (2006) Afferent and efferent connections of the rat retrotrapezoid nucleus. J. Comp. Neurol 499, 64–89. [DOI] [PubMed] [Google Scholar]
- 84.Kuo FS et al. (2016) In vitro characterization of noradrenergic modulation of chemosensitive neurons in the retrotrapezoid nucleus. J. Neurophysiol 116 (3), 1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sobrinho CR et al. (2016) Cholinergic control of ventral surface chemoreceptors involves Gq/inositol 1,4,5-trisphosphate-mediated inhibition of KCNQ channels. J. Physiol 594 (2), 407–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hawryluk JM et al. (2012) KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J. Neurosci 32 (47), 16943–16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mulkey DK et al. (2007) Serotonergic neurons activate chemosensitive retrotrapezoid nucleus neurons by a pH-independent mechanism. J. Neurosci 27 (51), 14128–14138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y et al. (2016) Nalcn Is a “Leak” Sodium Channel That Regulates Excitability of Brainstem Chemosensory Neurons and Breathing. J. Neurosci 36 (31), 8174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazarenko RM et al. (2011) Orexin A activates retrotrapezoid neurons in mice. Respir. Physiol. Neurobiol 175 (2), 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elam M et al. (1981) Hypercapnia and Hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic sympathetic nerve. Brain Res 222, 373–381. [DOI] [PubMed] [Google Scholar]
- 91.Pineda J and Aghajanian GK (1997) Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neurosci 77 (3), 723–743. [DOI] [PubMed] [Google Scholar]
- 92.Brust RD et al. (2014) Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell reports 9 (6), 2152–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li A and Nattie E (2010) Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J. Physiol 588 (Pt 15), 2935–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez JA et al. (2009) Deletion of TASK1 and TASK3 channels disrupts intrinsic excitability but does not abolish glucose or pH responses of orexin/hypocretin neurons. Eur. J. Neurosci 30 (1), 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Biancardi V et al. (2008) Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch 455 (6), 1119–1128. [DOI] [PubMed] [Google Scholar]
- 96.Oliveira LM et al. (2016) alpha1- and alpha2-adrenergic receptors in the retrotrapezoid nucleus differentially regulate breathing in anesthetized adult rats. J. Neurophysiol 116 (3), 1036–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Y et al. (2019) Chemosensitivity of Phox2b-expressing retrotrapezoid neurons is mediated in part by input from 5-HT neurons. J. Physiol In Press, 10.1113/JP277052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie A et al. (2006) Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J. Physiol 577, 319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hawkins VE et al. (2017) Purinergic regulation of vascular tone in the retrotrapezoid nucleus is specialized to support the drive to breathe. eLife 6, 10.7554/eLife.25232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lindsey BG et al. (2012) Computational models and emergent properties of respiratory neural networks. Compr. Physiol 2 (3), 1619–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baertsch NA et al. (2019) A spatially dynamic network underlies the generation of inspiratory behaviors. Proc. Natl. Acad. Sci. U. S. A 116 (15), 7493–7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takakura AC et al. (2007) GABAergic pump cells of solitary tract nucleus innervate retrotrapezoid nucleus chemoreceptors. J. Neurophysiol 98 (1), 374–381. [DOI] [PubMed] [Google Scholar]
- 103.Eldridge FL et al. (1981) Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211 (4484), 844–846. [DOI] [PubMed] [Google Scholar]
- 104.Rajani V et al. (2018) Release of ATP by pre-Botzinger complex astrocytes contributes to the hypoxic ventilatory response via a Ca(2+) -dependent P2Y1 receptor mechanism. J. Physiol 596 (15), 3245–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gourine AV and Funk GD (2017) On the existence of a central respiratory oxygen sensor. J Appl Physiol (1985) 123 (5), 1344–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Darnall RA (2010) The role of CO(2) and central chemoreception in the control of breathing in the fetus and the neonate. Respiratory physiology & neurobiology 173 (3), 201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guyenet PG et al. (2018) Interdependent feedback regulation of breathing by the carotid bodies and the retrotrapezoid nucleus. J. Physiol 596, 3029–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]