Abstract
Background
Thymic carcinomas (TCs) are rare aggressive tumors with no standard first‐line treatment. This study was conducted to determine the optimal chemotherapy regimen for advanced TC.
Methods
This retrospective study included 67 patients treated for stage IV TC in 2006–2015. The primary endpoints were the objective response rate (ORR) and progression‐free survival (PFS) with different chemotherapy regimens. Multivariate Cox regression analysis was used to identify factors associated with PFS, including metastatic status, radiotherapy post‐chemotherapy, primary lesion resection before chemotherapy, and chemotherapy regimen.
Results
A total of 36 patients received a paclitaxel‐platinum regimen, 31 received a gemcitabine‐platinum regimen, 14 underwent primary lesion resection, and 33 underwent radiotherapy. ORR was 31% (11/36) and 29% (9/31) in the paclitaxel‐platinum and gemcitabine‐platinum groups, respectively (P = 0.890). Median PFS, one‐year PFS rate, and two‐year PFS rate were 7.0 months, 26%, and 6% with paclitaxel‐platinum treatment and 12 months, 48%, and 24% with gemcitabine‐platinum treatment (log‐rank P = 0.030). Median PFS, one‐year PFS rate, and two‐year PFS rate were 18.0 months, 57%, and 33% with surgical resection and 7.3 months, 31%, and 7% without resection (log‐rank P = 0.030). Median PFS, one‐year PFS rate, and two‐year PFS rate were 13.0 months, 52%, and 20% with radiotherapy and 4.3 months, 22%, and 7% without radiotherapy (log‐rank P = 0.001). In multivariate analysis, metastatic status (hazard ratio [HR], 0.33, P = 0.004), surgical resection (HR, 0.32; P = 0.004), and radiotherapy (HR, 0.32; P < 0.001) were associated with superior PFS.
Conclusions
Both gemcitabine‐platinum and paclitaxel‐platinum regimens were efficacious for advanced TC. Primary lesion resection and radiotherapy may also benefit selected patients.
Keywords: Chemotherapy, prognosis, radiation therapy, resection, thymic carcinoma
Introduction
Although thymic tumors are the most common primary tumors in the anterior mediastinum, overall they are rare. They occur at a rate of only 1.5 cases for every million people each year in the US.1, 2 Thymic carcinoma is very rare, accounting for less than 1% of all tumors in the thymus. The data of incidence and prevalence of TC was deficient in China. The five‐year survival rate of thymic carcinomas (TCs) was approximately 40%.3, 4 TCs are derived from the thymic epithelium and include several histopathologic subtypes, such as squamous cell carcinoma, basaloid carcinoma, mucoepidermoid carcinoma, lymphoepithelioma‐like carcinoma, clear cell carcinoma, sarcomatoid carcinoma, adenocarcinomas, and undifferentiated carcinoma.5 Although a number of staging systems exist, the Masaoka‐Koga system remains the most commonly used system for TC.1, 5 Currently, chemotherapy with or without radiotherapy is recommended for advanced TC.6 Well‐established prognostic factors for TC include the extent of resection,7 as well as the stage and histologic type of tumor.8 Whether radiotherapy or chemotherapy influences prognosis remains controversial.6, 7, 9 The low incidence of TC hinders the ability to conduct large randomized clinical trials. Consequently, systemic treatment decisions are often guided by limited data from prospective trials, retrospective studies, and case reports.10 There is no standard first‐line chemotherapy regimen for advanced TC.
Methods
The purpose of this study was to evaluate the optimal first‐line chemotherapy regimen in patients with advanced TC.
Patient eligibility
A total of 67 patients with stage IV (including IVa and IVb) TC admitted to Beijing Cancer Hospital between January 2006 and December 2015 were recruited. The study was approved by the Hospital's Institutional Review Board. All patients provided written informed consent before collection and use of their clinical information.
The enrollment criteria were as follows: (i) diagnosis of TC based on the 2004 World Health Organization criteria, which was established by experienced pathologists; (ii) clinical stages IVa or IVb, according to Masaoka‐Koga staging system11; and (iii) complete follow‐up data until the study end date.
Response evaluation and statistical analysis
The Response Evaluation Criteria in Solid Tumors (RECIST 1.1) system was used to evaluate tumor response. Objective response rate (ORR) was defined as the sum of the complete response (CR) and partial response (PR). Progression‐free survival (PFS) was defined as the length of time from the first cycle of chemotherapy to the time of documented tumor progression or death.
The primary endpoints of the study were the ORR and PFS of different chemotherapy regimens. Baseline clinical characteristics and ORRs of the two regimens were compared using Pearson's chi‐square test or Fisher's exact test. The following factors were analyzed to determine their effects on PFS: age, sex, metastatic status, radiotherapy after chemotherapy, resection of primary lesion before chemotherapy, type of chemotherapy regimen, and number of cycles of chemotherapy. The Kaplan‐Meier method was used to estimate median PFS, PFS rates at one year, and two years. Univariate analysis was conducted using the log‐rank test to identify prognostic factors for PFS. Significant factors (P < 0.05) in the univariate analysis were included in the multivariate Cox proportional hazards model. All P‐values were two‐sided, and P < 0.05 was considered significant. All statistical analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
A total of 67 patients with Masaoka‐Koga stage IV TC were enrolled in this study. Their clinical characteristics are summarized in Table 1. There were 42 men and 25 women, with a mean age at diagnosis of 53 years (range, 14–75 years). At the time of diagnosis, seven patients (10.4%) had stage IVa TC (only pleural or pericardial dissemination) and 60 patients (89.6%) had stage IVb TC. Among those patients with stage IVb disease, 18 had only lymph node metastasis, whereas 42 had distant metastasis involving one organ (n = 32) or more than one organ (n = 10). The extra‐lymphatic sites of metastasis included bone (19 patients), lungs (16 patients), liver (14 patients), and adrenal glands (three patients). Twenty‐seven patients had both lymph node and distant metastases. All patients received four to six cycles of chemotherapy.
Table 1.
Baseline patient, tumor and treatment characteristics
| Factor | Total (n = 67) | GEM (n = 31) | PTX (n = 36) | P‐value |
|---|---|---|---|---|
| Age, mean ± SD | 53.0 ± 12.6 | 51.9 ± 14.3 | 53.9 ± 10.9 | 0.50† |
| Sex, n (%) | 0.77‡ | |||
| Male | 42 (62.7) | 20 (64.5) | 22 (61.1) | |
| Female | 25 (37.3) | 11 (35.5) | 14 (38.9) | |
| Age, n (%) | 0.53‡ | |||
| <65 | 52 (77.6) | 23 (74.2) | 29 (80.6) | |
| ≥65 | 15 (22.4) | 8(25.8) | 7 (19.4) | |
| Pathological type | 0.001‡ | |||
| squamous carcinoma | 64 | 31 | 33 | |
| Adenocarcinoma | 2 | 0 | 2 | |
| Neuroendocrine carcinoma | 1 | 0 | 1 | |
| Metastasis, n (%) | 0.022‡ | |||
| Lymph node | 18 (26.9) | 12 (38.7) | 6 (16.7) | |
| Distant | 42 (62.7) | 14 (45.2) | 28 (77.8) | |
| None | 7 (10.4) | 5 (16.1) | 2 (5.6) | |
| Masaoka‐Koga stage, n (%) | 0.24§ | |||
| IVa | 7 (10.4) | 5 (16.1) | 2 (5.6) | |
| IVb | 60 (89.6) | 26 (83.9) | 34 (94.4) | |
| Surgery, n (%) | 0.36‡ | |||
| No | 53 (79.1) | 23 (74.2) | 30 (83.3) | |
| Yes | 14 (20.9) | 8 (25.8) | 6 (16.7) | |
| Radiotherapy, n (%) | 0.020‡ | |||
| No | 34 (50.7) | 11 (35.5) | 23 (63.9) | |
| Yes | 33 (49.3) | 20 (64.5) | 13 (36.1) |
Student's t‐test.
Pearson's chi‐square test.
Fisher's exact test.
GEM, gemcitabine‐based chemotherapy; PTX, paclitaxel‐based chemotherapy; SD, standard deviation.
Patients were followed until 30 July 2018, with a median follow‐up period of 27.8 months (range, 4.5–88.7 months). By the time of last follow‐up, 58 patients had experienced progressive disease (PD) and 29 patients had died. The mortality rate was 43.2% (29/67).
Chemotherapy
All patients received either a paclitaxel‐platinum regimen (n = 36; 53.7%) or a gemcitabine‐platinum regimen (n = 31; 46.3%) as their first‐line chemotherapy regimen. The paclitaxel‐based group patients received paclitaxel (175 mg/m2) plus cisplatin (75 mg/m2, day 1), carboplatin (area under the curve = 5, day 1), or nedaplatin (80 mg/m2, day 1) every three weeks for a maximum of six cycles. The gemcitabine‐based group patients were treated with gemcitabine (1250 mg/m2, days 1 and 8) plus cisplatin (75 mg/m2, day 1), carboplatin (area under the curve = 5, day 1), or nedaplatin (80 mg/m2, day 1) every three weeks for a maximum of six cycles. The distribution, PR, PD/SD (stable disease), and ORR of each regimen are shown in Table S1. No patient experienced a CR. ORR was not significantly different between the paclitaxel‐platinum and gemcitabine‐platinum regimens (30.6% and 29.0%, respectively; P = 0.890).
Figure 1 shows the effects of the two chemotherapy regimens on PFS. The median PFS, one‐year PFS rate, and two‐year PFS rate were 7.0 (95% confidence interval [CI], 4.0–8.0) months, 26%, and 6%, respectively, in the paclitaxel‐platinum group and 12 months (95% CI, 11.2–24.0), 48%, and 24% in the gemcitabine‐platinum group. PFS was superior with the gemcitabine‐platinum regimen (log‐rank P = 0.030).
Figure 1.
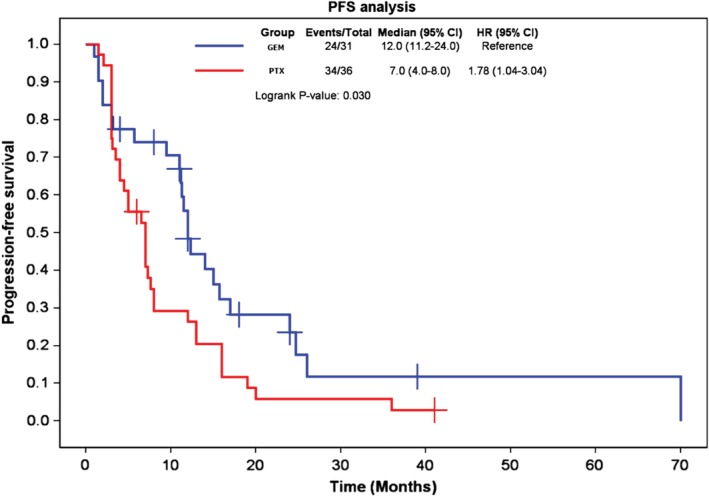
Kaplan‐Meier curves comparing progression‐free survival with the two chemotherapy regimens. GEM, gemcitabine‐based chemotherapy; PTX, paclitaxel‐based chemotherapy.
Surgical resection
Only 14 patients (20.9%) underwent surgical resection before chemotherapy because resection was difficult to achieve. In four of these patients, the tumor was completely resected; in the other 10 patients, only palliative surgery was performed. Figure 2 shows the effects of surgical resection on PFS. The median PFS, one‐year PFS rate, and two‐year PFS rate were 18.0 months (95% CI, 12.0–36.0), 57%, and 33%, respectively, for patients who underwent resection and 7.3 months (95% CI, 5.0–11.3), 31%, and 7% for those who did not. PFS was superior with surgery (log‐rank P = 0.030).
Figure 2.
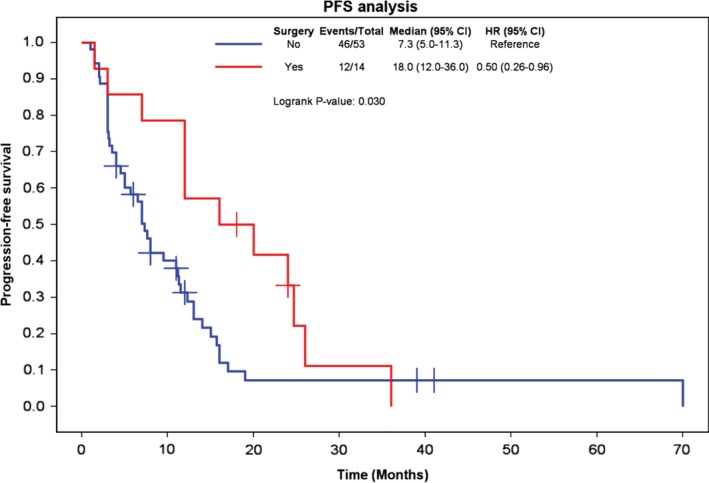
Kaplan‐Meier curves comparing progression‐free survival in patients who underwent surgical resection or not.
Radiotherapy
A total of 33 patients (49.3%) received radiotherapy after chemotherapy. Figure 3 shows the effect of radiotherapy on PFS. The median PFS, one‐year PFS rate, and two‐year PFS rate were 13.0 months (95% CI, 11.3–17.0), 52%, and 20%, respectively, in patients who received radiotherapy and 4.3 months (95% CI, 3.0–7.3), 22%, and 7% in those who did not. PFS was superior with radiotherapy (log‐rank P = 0.001).
Figure 3.
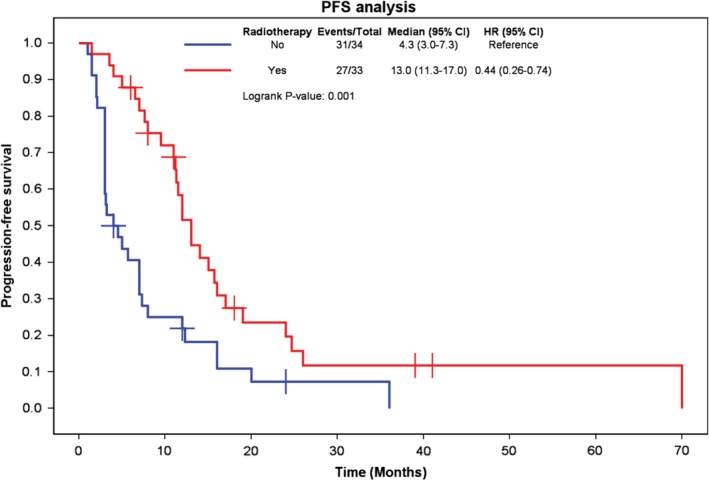
Kaplan‐Meier curves comparing progression‐free survival in patients who did or did not receive radiotherapy.
Tumor stage
Figure S1 shows the effect of tumor stage on PFS. The median PFS, one‐year PFS rate, and two‐year PFS rate were 26.0 months (95% CI, 12.3–36.0), 86%, and 51%, respectively, in patients with stage IVa TC and 7.6 months (95% CI, 6.5–12.0), 31%, and 9% in patients with stage IVb disease. PFS was not significantly different between these two groups (P = 0.080).
Tumor metastasis
Figure 4 shows the PFS curves of patients with different metastasis status at the time of diagnosis. Median PFS was 12.0 months (95% CI, 6.6–17.3) in patients with only lymph node metastasis and 5.7 months (95% CI, 3.4–7.9) in those with distant metastasis. PFS was superior in patients with only lymph node metastasis (log‐rank P = 0.003).
Figure 4.
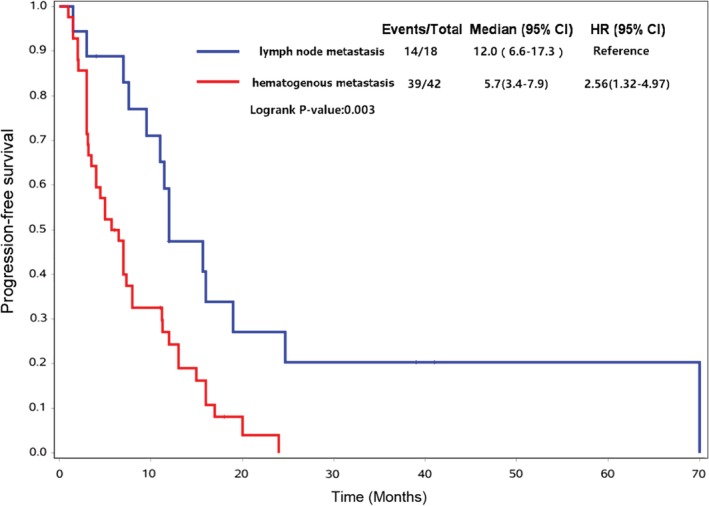
Kaplan‐Meier curves comparing progression‐free survival of patients with only lymph node metastasis and patients with hematogenous metastasis.
Multivariate analysis
In stepwise multivariate Cox regression analysis, surgical resection (hazard ratio [HR], 0.32; P = 0.005), radiotherapy after chemotherapy (HR, 0.32, P < 0.001), and metastatic status (HR, 2.63; P = 0.004) were independent prognostic factors for PFS (Table 2). The type of chemotherapy regimen was not significantly associated with PFS (P = 0.225). Age, sex, and number of cycles of chemotherapy were also not significantly associated with PFS.
Table 2.
Evaluating influencing factors on progression‐free survial (PFS) by stepwise multivariate Cox regression mode
| Variable | Regression coefficient | Standard error | P‐value | Hazard ratio | Lower HR | Upper HR |
|---|---|---|---|---|---|---|
| Surgery (yes vs. no) | −1.1228 | 0.3942 | 0.0049 | 0.32 | 0.15 | 0.70 |
| Radiotherapy (yes vs. no) | −1.2327 | 0.3156 | 0.0001 | 0.32 | 0.16 | 0.54 |
| Metastasis (distant vs. lymphatic only) | 0.9684 | 0.3557 | 0.0044 | 2.63 | 1.31 | 5.29 |
| Chemotherapy regimen (PTX vs. GEM) | −1.4336 | 1.1812 | 0.2249 | 0.24 | 0.02 | 2.41 |
Discussion
In this study, which is one of the largest to evaluate chemotherapeutic efficacy and prognostic factors for advanced TC, we identified metastatic status, surgical resection before chemotherapy, and radiotherapy after chemotherapy as independent predictors of PFS.
Whether Masaoka‐Koga stage is an independent prognostic factor for advanced TC remains unclear. Engels and Pfeiffer4 Kelly12 and Masaoka et al.,13 considered Masaoka‐Koga stage to be a prognostic factor, whereas Cardillo et al.14 and Hosaka et al.15 did not. Although stage was not identified as an independent factor for PFS in our multivariate regression analysis, the PFS of patients with stage IVa TC was 26.0 months, which was much longer than the PFS of patients with stage IVb disease (7.6 months). We also found that distant metastasis was an independent prognostic factor for poor PFS; patients with lymph node metastasis alone had a superior PFS.
Many studies have confirmed surgical resection as the most important factor in the treatment of TC. Kondo and Monden reported five‐year survival rates of 67%, 30%, and 24% for total resection, subtotal resection, and inoperable treatment, respectively.3 Indeed, Yano et al. reported that resectability was the only prognostic factor for TC.16 In this study, we also demonstrated that PFS was superior in patients who underwent surgical resection (either total or palliative), compared with those who had inoperable tumors. Because of the highly aggressive behavior and frequent distant metastases seen with TC, it is difficult to achieve complete resection, especially for advanced tumors. Detterbeck et al. reported a low rate (25%) of R0 resection for stage IV thymomas and TCs.8 In the current study, only 14 (20.9%) of our patients underwent surgical resection, including total and palliative resections.
It has been previously reported that patients with advanced TC may benefit from radiotherapy.17, 18 Radiotherapy has long been established as an important component of therapy for this disease. Our results confirm its beneficial effects, as PFS was superior in patients who received radiotherapy after chemotherapy, compared with those who received no radiotherapy.
There is currently no standard first‐line chemotherapy regimen for advanced TC. Cisplatin‐ and adriamycin‐based regimens have been recommended by some authors, with response rates ranging from 42% to 50% and median PFS durations ranging from six to eight months.19, 20 In the latest version of National Comprehensive Cancer Network guidelines, a regimen of paclitaxel and carboplatin was recommended as first‐line chemotherapy for TC. This recommendation was based on a phase II study that reported an ORR of 21.7% and a median PFS of 5.0 months with paclitaxel‐carboplatin in patients with advanced TC.21 Retrospective studies reported ORRs of 22% to 38% and median PFS durations of five to nine months with this regimen.22, 23, 24 Our findings of an ORR of 30.6% and median PFS of seven months with the paclitaxel‐platinum regimen are consistent with prior results.
The literature is unclear regarding the efficacy of gemcitabine‐platinum regimens in TC. Only one study demonstrated that the regimen was effective in advanced TC, producing an ORR of 61.5% and a median PFS of 14.5 months.25 In the current study, the ORR of the gemcitabine‐platinum regimen was 29.03%, which was similar to that of the paclitaxel‐platinum regimen. In univariate analysis (log‐rank test), the median PFS of gemcitabine‐platinum regimen was 12 months, which was superior to that of the paclitaxel‐platinum regimen (seven months), but in multivariate analysis, the type of chemotherapy regimen was not associated with PFS. The discrepancy between univariate and multivariate analysis results likely reflects the differences in tumor stage and radiotherapy between the paclitaxel‐platinum and gemcitabine‐platinum groups. Despite the lack of clear superiority of the gemcitabine‐platinum regimen, our results indicate that it is an effective regimen for advanced TC, providing clinicians with another treatment option. Large‐scale prospective trials are warranted to determine the optimal chemotherapy regimen for TC.
The current study is limited by its retrospective nature and small sample size. However, because of the low incidence of TC, it is difficult to develop a large, prospective clinical study.
In conclusion, both gemcitabine‐platinum and paclitaxel‐platinum regimens demonstrated promising efficacy in advanced TC. Resection of the primary lesion before chemotherapy and radiotherapy after chemotherapy may be beneficial options for selected patients.
Disclosure
No authors report any conflict of interest.
Supporting information
Figure S1. Kaplan‐Meier curves comparing progression‐free survival of patients with stage IVa or stage IVb tumors.
Table S1. Distribution and response rates of the two chemotherapy regimens.
Acknowledgments
Supported by National Key Research and Development Program of China (2016YFC0904900).
Xue Yang, Minglei Zhuo, Anhui Shi contributed equally to this work.
References
- 1. Proceedings of the First International Conference on Thymic Malignancies. August 20‐21, 2009. Bethesda, Maryland, USA. J Thorac Oncol 2010; 5 (10 Suppl. 4): S259–370. PMID: 21275152. [PubMed] [Google Scholar]
- 2. Detterbeck FC, Parsons AM. Management of stage I and II thymoma. Thorac Surg Clin 2011; 21: 59–67, vi‐vii. [DOI] [PubMed] [Google Scholar]
- 3. Scorsetti M, Leo F, Trama A et al Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016; 99: 332–50. [DOI] [PubMed] [Google Scholar]
- 4. Engels EA, Pfeiffer RM. Malignant thymoma in the United States: Demographic patterns in incidence and associations with subsequent malignancies. Int J Cancer 2003; 105 (4): 546–51. [DOI] [PubMed] [Google Scholar]
- 5. Kondo K, Monden Y. Therapy for thymic epithelial tumors: A clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003; 76 (3): 878–84; discussion 884‐5. [DOI] [PubMed] [Google Scholar]
- 6. Weksler B, Dhupar R, Parikh V, Nason KS, Pennathur A, Ferson PF. Thymic carcinoma: A multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013; 95 (1): 299–303. [DOI] [PubMed] [Google Scholar]
- 7. Marx A, Chan JK, Coindre JM. The 2015 World Health Organization classification of tumors of the thymus: Continuity and changes. J Thorac Oncol 2015; 10 (10): 1383–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu JX, Chen HQ, Shao LD et al Long‐term follow‐up and prognostic factors for advanced thymic carcinoma. Medicine 2014; 93 (28): e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hishida T, Nomura S, Yano M et al Long‐term outcome and prognostic factors of surgically treated thymic carcinoma: Results of 306 cases from a Japanese Nationwide Database Study. Eur J Cardiothorac Surg 2016; 49 (3): 835–41. [DOI] [PubMed] [Google Scholar]
- 10. Detterbeck F, Youssef S, Ruffini E, Okumura M et al A review of prognostic factors in thymic malignancies. J Thorac Oncol 2011; 6 (7 Suppl 3): S1698–704. [DOI] [PubMed] [Google Scholar]
- 11. Litvak AM, Woo K, Hayes S et al Clinical characteristics and outcomes for patients with thymic carcinoma: Evaluation of Masaoka staging. J Thorac Oncol 2014; 9 (12): 1810–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kelly RJ. Systemic treatment of advanced thymic malignancies. Am Soc Clin Oncol Educ Book 2014; 34: e367–73. [DOI] [PubMed] [Google Scholar]
- 13. Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow‐up study of thymomas with special reference to their clinical stages. Cancer 1981; 48 (11): 2485–92. [DOI] [PubMed] [Google Scholar]
- 14. Cardillo G, Carleo F, Giunti R et al Predictors of survival in patients with locally advanced thymoma and thymic carcinoma (Masaoka stages III and IVa). Eur J Cardiothorac Surg 2010; 37 (4): 819–23. [DOI] [PubMed] [Google Scholar]
- 15. Hosaka Y, Tsuchida M, Toyabe S, Umezu H, Eimoto T, Hayashi J. Masaoka stage and histologic grade predict prognosis in patients with thymic carcinoma. Ann Thorac Surg 2010; 89 (3): 912–7. [DOI] [PubMed] [Google Scholar]
- 16. Ogawa K, Toita T, Uno T et al Treatment and prognosis of thymic carcinoma: A retrospective analysis of 40 cases. Cancer 2002; 94 (12): 3115–9. [DOI] [PubMed] [Google Scholar]
- 17. Tseng YL, Wang ST, Wu MH, Lin MY, Lai WW, Cheng FF. Thymic carcinoma: Involvement of great vessels indicates poor prognosis. Ann Thorac Surg 2003; 76 (4): 1041–5. [DOI] [PubMed] [Google Scholar]
- 18. Yano M, Sasaki H, Yokoyama T et al Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol 2008; 3 (3): 265–9. [DOI] [PubMed] [Google Scholar]
- 19. Nonaka T, Tamaki Y, Higuchi K et al The role of radiotherapy for thymic carcinoma. Jpn J Clin Oncol 2004; 34 (12): 722–6. [DOI] [PubMed] [Google Scholar]
- 20. Eng TY, Thomas CR Jr. Radiation therapy in the management of thymic tumors. Semin Thorac Cardiovasc Surg 2005; 17 (1): 32–40. [DOI] [PubMed] [Google Scholar]
- 21. Agatsuma T, Koizumi T, Kanda S et al Combination chemotherapy with doxorubicin, vincristine, cyclophosphamide, and platinum compounds for advanced thymic carcinoma. J Thorac Oncol 2011; 6 (12): 2130–4. [DOI] [PubMed] [Google Scholar]
- 22. Yoh K, Goto K, Ishii G et al Weekly chemotherapy with cisplatin, vincristine, doxorubicin, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer 2003; 98 (5): 926–31. [DOI] [PubMed] [Google Scholar]
- 23. Lemma GL, Lee JW, Aisner SC. Phase II study of carboplatin and paclitaxel in advanced thymoma and thymic carcinoma. J Clin Oncol 2011; 29 (15): 2060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu JP, Hao XZ, Zhang XR, Yang S, Shi YK. Efficacy and safety of the combination of paclitaxel and platinum in advanced thymic carcinoma. Thorac Cancer 2016; 7 (2): 222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furugen M, Sekine I, Tsuta K et al Combination chemotherapy with carboplatin and paclitaxel for advanced thymic cancer. Jpn J Clin Oncol 2011; 41 (8): 1013–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan‐Meier curves comparing progression‐free survival of patients with stage IVa or stage IVb tumors.
Table S1. Distribution and response rates of the two chemotherapy regimens.


