Abstract
Background
The early stages of lung cancer with ground‐glass opacity (GGO) pattern are detectable. However, it remains a challenge for physicians how best to treat GGO nodules as invasive tumors are occasionally found, even in pure GGO nodules. This study identified the invasiveness by the clinical features of the GGO nodules.
Methods
A retrospective review of patients with resected GGO nodules from August 2015 to February 2019 was performed. A total of 92 patients were enrolled and gender, age, tumor location, operation times, tumor size, histopathologic and radiological findings were analyzed.
Results
In this study, the sequential of GGO nodules invasiveness was significantly related to the tumor size and solid component. After regrouping the population into preinvasive and invasive groups, the invasiveness was significantly related to tumor size, solid component, tumor volume and maximal computed tomography (CT) value.
Conclusions
The invasiveness is difficult to evaluate according to the CT features only when the GGO nodules are less than 2 cm and consolidation/tumor ratio (C/T ratio) are less than 0.25. Tumor size and solid component are significant factors for predicting invasiveness. Part‐solid GGO nodules with a diameter greater than 1 cm require surgical consideration due to their high risk of invasiveness.
Keywords: Ground‐glass opacity, invasiveness, lung adenocarcinoma, surgical intervention
Key points
Significant findings of the study: Tumor size and solid component are significant factors for predicting invasiveness. In this study, the sequential development of GGO nodule invasiveness was significantly related to the tumor size and solid component.
What this study adds: This study determined that part‐solid GGO nodules with a diameter larger than 1 cm should be considered for surgery due to their high risk of invasiveness.
Introduction
Lung cancer is one of the most prevalent lethal diseases in the world,1 and with the increased use of computed tomography (CT) scanning for lung cancer screening, ground‐glass opacity (GGO) nodules are encountered more frequently.2 The GGO nodules result from a variety of differential diagnosis of benign and malignant diseases including inflammatory process, focal fibrosis, atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), and invasive adenocarcinoma (IA).3 For clinical physicians, it is a challenge to diagnose GGO nodules using the features of the CT images and thus results in an important issue on how best to manage the GGO nodules.
According to the National Comprehensive Cancer Network (NCCN) guidelines for lung cancer screening (Version 2.2019), pure GGO or part‐solid GGO with a solid component less than 5 mm should be closely followed‐up. For patients with AIS or MIA presenting with GGO nodules, an annual follow‐up CT scan may be reasonable and safe due to its 100% five‐year survival rate.4, 5 In patients diagnosed with IA, the five‐year survival rate is only 74.6%6 and surgical intervention as early as possible is critical. However, IA has been reported in the pure GGO or part‐solid GGO nodules with a solid component less than 5 mm,7, 8 which is determined as radiologic AIS or MIA, and that brings about an unsatisfactory outcome if the clinical physicians do not closely follow‐up IA patients.
Surgical timing is crucial for the GGO nodules, especially when invasiveness occurs. However, the optimal timing of surgical intervention for the GGO nodules is debatable due to their uncertainty.9, 10 The aim of this study was to identify the invasiveness of the GGO nodules by the clinical features of the CT scan and offer a valuable guide for clinical physicians to manage GGO nodules.
Methods
We performed a retrospective record and pathologic review from August 2015 to February 2019 at the Hsinchu Mackay Memorial Hospital, Taiwan. The study was approved by the Mackay Memorial Hospital Institutional Ethics Committee/Review Board (18MMHIS129).
Study population and design
From August 2015, patients with GGO nodules with a maximum diameter of 2 cm or less and consolidation/tumor ratio (C/T ratio) less than 0.25 were enrolled into the study. Through February 2019, we collected information on 99 patients who were considered to have clinically early stage lung cancer with pure or part‐solid GGO nodules which had been surgically removed. Among these patients, a total of seven were excluded. Six patients had synchronous lesions and one patient had a large mediastinal tumor which was resected during the same operation. A total of 92 patients were finally enrolled into the study. Medical records containing patient characteristics, features of GGO nodules, operative procedures, locations of the lesions, operative times, pathologic results, and radiologic findings were reviewed and analyzed.
Image analysis
All the images were taken using the multidetector CT system: Somatom Definition AS+ (Siemens Medical Solutions, Erlangen, Germany) with a section thickness of 1 mm using the lung window setting (window level, −500 HU; width, 1500 HU). The images were evaluated by five experienced radiologists. The pure GGO nodule was defined as a homogeneous hazy tumor that did not obscure the underlying structures or vessels, and the part‐solid GGO nodule was defined as a lesion which had solid component in a homogeneous background with increasing attenuation. The tumor size was measured in the maximal cross‐section diameter on the lung window setting.
The tumor volume and attenuation were analyzed by one radiological technologist who was unaware of the clinical and pathologic reports of the patients. Prior to calculation, the tumor was marginated by drawing freehand a circular line with the computer mouse to cover an area as large as the whole tumor. The tumor volume, maximal diameter, and attenuation could then be calculated using Vitrea Enterprise Suite software (version 6.8).
Surgical procedure
Tumor localization was the first step for the patients who received the surgical resection. The patients were first referred to the radiological department for the CT‐guided localization with patent blue V dye (PBV 2.5%; Guerbet, Aulnay‐sous‐Bois, France) injection. The surgical procedures included wedge resection, segmentectomy, and lobectomy, which were chosen according to the tumor sizes, locations, and their characteristics. All surgical procedures were performed with single‐incisional video‐assisted thoracic surgery (VATS) by one thoracic surgeon.
Pathologic classification
All the specimens were examined by three experienced pathologists. The GGO nodule was classified as AAH, AIS, MIA, and IA according to the 2015 World Health Organization (WHO) classification of lung tumors and the final pathologic reports were reconfirmed by the other pathologist.
Statistical analysis
The Pearson chi‐square test or Fisher's exact test was used to compare categorical variables between groups. The statistical analysis of continuous variables was performed by Student's t‐test and one‐way analysis of variance (ANOVA). A two‐tailed P‐value of <0.05 was considered statistically significant. Analyses were carried out using the statistical package SPSS 17.0 (IBM, Armonk, New York, USA).
Results
Baseline characteristics
All 92 patients were classified into two groups: 59 patients in the pure GGO group and 33 patients in the part‐solid GGO group. The images of pure GGO nodules and part‐solid GGO nodules are shown in Fig 1a,b, respectively. All the consolidation/tumor ratios (C/T ratios) of part‐solid GGO nodules included in our study were less than 0.25. The clinical parameters and the features are summarized in Table 1. The characteristics of the patients were analyzed and there were no significant difference of gender, age, surgical procedure, and operative times between pure GGO and part‐solid GGO group. The mean tumor size was 0.78 cm and 1.04 cm in the pure GGO group and the part‐solid GGO group, respectively. The mean tumor size in the part‐solid GGO group was significantly greater than those in the pure GGO group (P = 0.003). In the histopathologic results, we found that there were 25 (42.4%) AAH, 32 (54.2%) AIS, and two (3.4%) MIA who presented with pure GGO nodules; in contrast, there were seven (21.2%) AAH, 18 (54.5%) AIS, seven (21.2%) MIA, and one (3.0%) IA who presented with part‐solid GGO nodules. The distribution of the adenocarcinoma subtypes showed significant difference in invasiveness between the pure GGO and part‐solid GGO group (P = 0.01).
Figure 1.
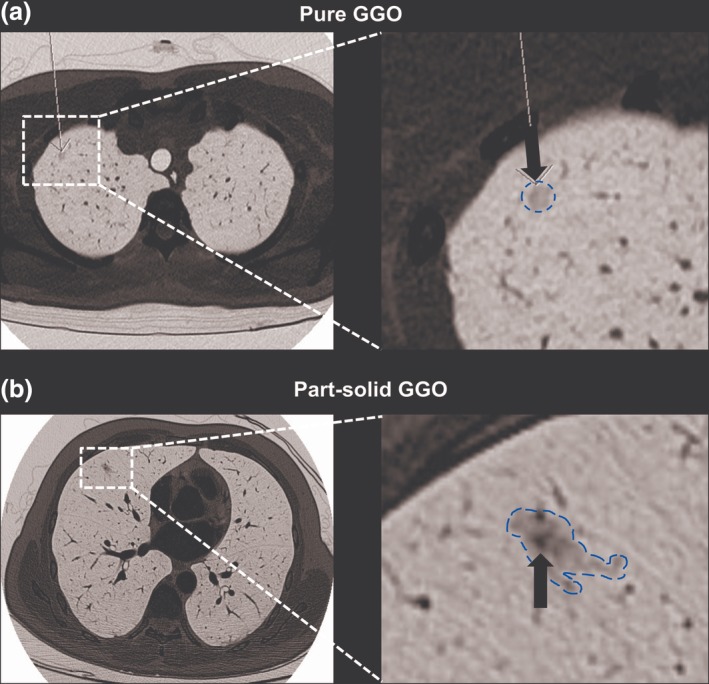
Ground‐glass opacity (GGO) nodules diagnosed by chest CT scan. (a) Pure GGO nodule. One 0.5 x 0.5 cm tiny GGO lesion was detected. Focusing on the GGO lesion, there was no solid component (arrow). Atypical adenomatous hyperplasia (AAH) was the definitive pathological diagnosis following resection. (b) Part‐solid GGO nodule. One 1.1 x 0.6 cm tiny GGO lesion was noted in the right middle lobe of lung. A solid component (arrow) in the GGO lesion was also noted. The final diagnosis after resection revealed minimally invasive adenocarcinoma (MIA).
Table 1.
Comparison of the pure GGO and part‐solid GGO nodules with different characteristics
| Parameters | Pure GGO (n = 59) | Part‐solid GGO (n = 33) | P‐value |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 21 (35.6) | 17 (51.5) | 0.137 |
| Female | 38 (64.4) | 16 (48.5) | |
| Age (years), median (range) | 49 (28–72) | 54 (33–88) | 0.173 |
| Operation, n (%) | |||
| Wedge resection | 48 (81.4) | 25 (75.8) | 0.725 |
| Segmentectomy | 8 (13.6) | 5 (15.2) | |
| Lobectomy | 3 (5.1) | 3 (9.1) | |
| Operative times (minute), mean (range) | |||
| Wedge resection | 65 (29–129) | 57 (21–83) | 0.093 |
| Segmentectomy | 154 (76–265) | 199 (145–290) | 0.218 |
| Lobectomy | 228 (164–281) | 278 (245–323) | 0.300 |
| Tumor size (cm), mean (range) | 0.78 (0.4–1.8) | 1.04 (0.3–1.9) | 0.003 |
| Histopathology, n (%) | |||
| AAH | 25 (42.4) | 7 (21.2) | 0.010 |
| AIS | 32 (54.2) | 18 (54.5) | |
| MIA | 2 (3.4) | 7 (21.2) | |
| IA | 0 (0) | 1 (3.0) | |
GGO, ground‐glass opacity; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma.
Characteristics of the different histopathologic subtypes
The histopathologic subtypes of the GGO nodules were diagnosed and the histopathologic images of AAH (Fig 2a), AIS (Fig 2b), MIA (Fig 2c), and IA (Fig 2d) were shown. Moreover, the tumor volume and attenuation values of the GGO nodules, which were calculated using Vitrea Enterprise Suite software were selected for invasiveness evaluation (Fig 3). As detailed in Table 2, the patient's numbers of four subtypes were 32 patients in AAH (34.8%), 50 in AIS (54.3%), nine in MIA (9.8%), and one patient in IA (1.1%). We evaluated the gender, age, tumor size, tumor location, and CT findings in these four histopathologic subtypes. A significant difference was noted in the average tumor size (0.74 cm in AAH, 0.88 cm in AIS, 1.20 cm in MIA and 1.9 cm in IA, P = 0.009).
Figure 2.
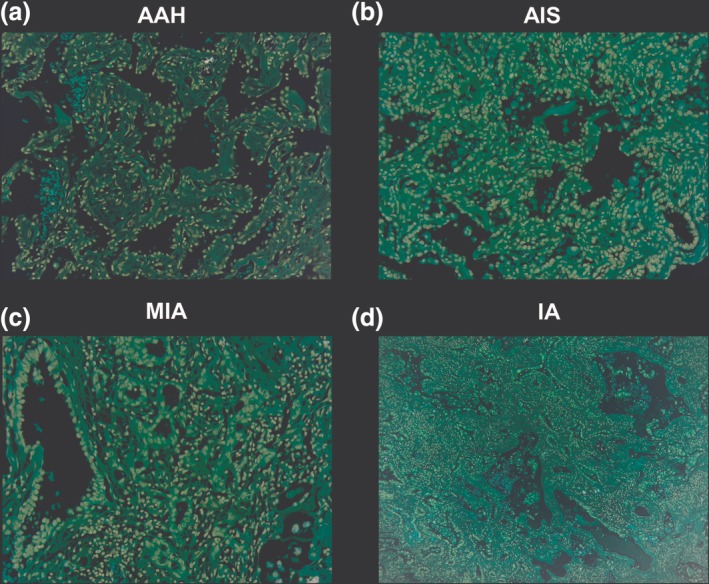
Demonstration of the histopathologic images of the early lung adenocarcinoma subtypes. (a) Atypical adenomatous hyperplasia (AAH). The tumor cells show a purely lepidic pattern without stoma invasion (150x); (c) Minimally invasive adenocarcinoma (MIA). Note that the tumor consists of lepidic growth with a small central area of invasion <0.5 cm (150x); (d) IA: Lepidic growth of the tumor with central desmoid reaction is visible. The invasive area >0.5 cm in diameter (150x).
Figure 3.
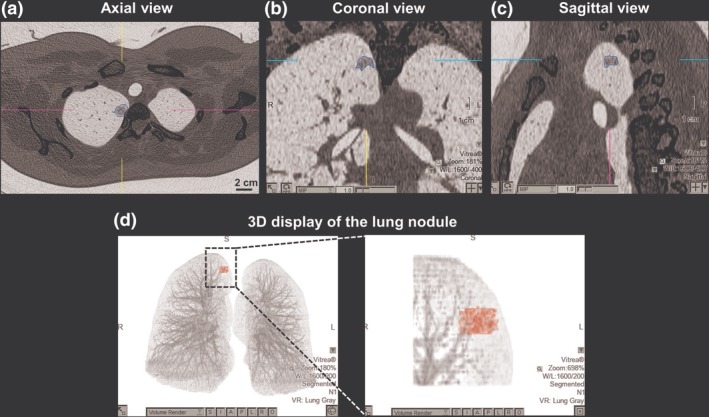
The tumor volume and value of the ground‐glass opacity (GGO) nodule were analyzed. The tumor was marginated by drawing freehand a circular line with the computer mouse and the scale was calculated with Vitrea Enterprise Suite software. (a) Axial view of the CT scan. A yellowish circular line was drawn by the radiological technologist. (b) & (c) Coronal and sagittal views of the CT scan. The radiological technologist adjusted the circular line precisely to the margin of the tumor. (d) Three‐dimensional display of the lung nodule. According to the graphic range marginated in axial, coronal, and sagittal view, the nodule was presented automatically by the software. The tumor volume and value were calculated and displayed.
Table 2.
Comparison of different histopathologic subtypes of early lung adenocarcinomas
| Parameters | AAH (n = 32) | AIS (n = 50) | MIA (n = 9) | IA (n = 1) | P‐value |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 9 (28.1) | 22 (44.0) | 6 (66.7) | 1 (100) | 0.100 |
| Female | 23 (71.9) | 28 (56.0) | 3 (33.3) | 0 (0) | |
| Age (years), median (range) | 49 (31–70) | 50 (28–72) | 62 (35–88) | 59 | 0.220 |
| Tumor size (cm), mean (range) | 0.74 (0.3–1.5) | 0.88 (0.5–1.8) | 1.20 (0.5–1.6) | 1.9 (1.9) | <0.001 |
| Location, n (%) | |||||
| Right upper lobe | 15 (46.9) | 25 (50.0) | 1 (11.1) | 1 (100) | 0.489 |
| Right middle lobe | 3 (9.4) | 3 (6.0) | 0 (0) | 0 (0) | |
| Right lower lobe | 3 (9.4) | 7 (14.0) | 4 (44.4) | 0 (0) | |
| Left upper lobe | 7 (21.9) | 11 (22.0) | 3 (33.3) | 0 (0) | |
| Left lower lobe | 4 (12.5) | 4 (8.0) | 1 (11.1) | 0 (0) | |
| CT findings | |||||
| Pure GGO lesion, n (%) | 25 (78.1) | 32 (64.0) | 2 (22.2) | 0 (0) | 0.010 |
| Part‐solid GGO lesion, n (%) | 7 (21.9) | 18 (36.0) | 7 (77.8) | 1 (100) | |
| Tumor volume (mm3), mean ± SD | 79 ± 92 | 199 ± 278 | 442 ± 351 | 321.9 | 0.001 |
| Mean tumor attenuation (HU), mean ± SD | −531 ± 164 | −549 ± 133 | −485 ± 132 | −356 | 0.390 |
| Maximum tumor attenuation (HU), mean ± SD | −162 ± 219 | −121 ± 238 | 69 ± 158 | 6 | 0.058 |
| Minimum tumor attenuation (HU), mean ± SD | −864 ± 149 | −887 ± 118 | −840 ± 92 | −650 | 0.236 |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IA, invasive adenocarcinoma; Preinvasive, AAH + AIS; Invasive, MIA + IA; GGO, ground‐glass opacity.
With regard to the results of CT findings, we found a trend that the percentage of part‐solid GGO lesions and mean tumor volume significantly increased with the progression of the invasiveness (the percentage of part‐solid GGO lesions: 21.9% in AAH, 36.0% in AIS, 77.8% in MIA, and 100% in IA, P = 0.01; mean tumor volume: 79 mm3 in AAH, 199 mm3 in AIS, 442 mm3 in MIA, and 321.9 mm3 in IA, P = 0.001).
Characteristics of invasiveness
To investigate invasiveness of the GGO lesions, we grouped AAH and AIS into the preinvasive group and grouped MIA and IA into the invasive group, as shown in Table 3. There were no significant differences in gender, age, and location. Tumor sizes in the invasive group were significantly larger than those in preinvasive group (1.27 cm vs. 0.83 cm, P < 0.001). The CT findings were evaluated and preinvasive nodules were more likely to present in a pure GGO pattern, whereas the pure GGO pattern was only present in 20% of invasive nodules (P = 0.004). The tumor volume was also significantly different between the preinvasive and invasive groups (P = 0.001). Furthermore, maximal tumor attenuation revealed a significant difference between the preinvasive and invasive group (P = 0.002). After the cutoff value of tumor size was set at 1 cm, the results showed that in the pure GGO group there was no significant difference between tumor size and invasiveness; on the contrary, a nodule larger than 1 cm was a significant factor to predict invasiveness in the part‐solid group (P = 0.012, OR = 14.88) (Table 4).
Table 3.
Relationships between the clinical characteristics and histopathologic invasiveness
| Parameters | Preinvasive (n = 82) | Invasive (n = 10) | P‐value |
|---|---|---|---|
| Gender, n (%) | |||
| Male | 31 (37.8) | 7 (70) | 0.086 |
| Female | 51 (62.2) | 3 (30) | |
| Age (years), median (range) | 50 (28–72) | 61 (35–88) | 0.169 |
| Tumor size (cm), mean (range) | 0.83 (0.3–1.8) | 1.27 (0.5–1.9) | <0.001 |
| Location, n (%) | |||
| Right upper lobe | 40 (48.8) | 2 (20.0) | 0.129 |
| Right middle lobe | 6 (7.3) | 0 (0) | |
| Right lower lobe | 10 (12.2) | 4 (40.0) | |
| Left upper lobe | 18 (22.0) | 3 (30.0) | |
| Left lower lobe | 8 (9.8) | 1 (10.0) | |
| CT findings | |||
| pure GGO lesion, n (%) | 57 (69.5) | 2 (20.0) | 0.004† |
| part‐solid GGO lesion, n (%) | 25 (30.5) | 8 (80.0) | |
| Tumor volume (mm3), mean ± SD | 152 ± 231 | 430 ± 333 | 0.001 |
| Mean tumor attenuation (HU), mean ± SD | −541 ± 145 | −472 ± 131 | 0.147 |
| Maximum tumor attenuation (HU), mean ± SD | −137 ± 230 | 63 ± 151 | 0.002 |
| Minimum tumor attenuation (HU), mean ± SD | −876 ± 131 | −821 ± 106 | 0.193 |
Preinvasive, AAH + AIS; Invasive, MIA + IA.
Odds ratio = 9.12, 95% Confidence interval = 1.81–46.05.
Table 4.
Relationship between invasiveness and tumor size in different GGO groups
| Preinvasive | Invasive | OR (95% C.I.) | P‐value | |
|---|---|---|---|---|
| Pure GGO, n (%) | ||||
| ≤ 1 cm | 51 (89.5) | 2 (100) | 1 | |
| > 1 cm | 6 (10.5) | 0 (0) | 0.96 (0.91–1.02) | 1 |
| Part‐solid GGO, n (%) | ||||
| ≤ 1 cm | 17 (68.0) | 1 (12.5) | 1 | |
| > 1 cm | 8 (32.0) | 7 (87.5) | 14.88 (1.56–142.20) | 0.012 |
Preinvasive, AAH + AIS; Invasive, MIA + IA; OR, odds ratio; C.I., confidence interval; GGO, ground‐glass opacity.
Correlation between GGO nodule morphology and histopathologic invasiveness
Except for the CT attenuation value, we analyzed the GGO nodule morphology for invasiveness in the pure GGO group and part‐solid GGO group. The nodular margin, bubble lucency, and air bronchogram (Fig 4) were analyzed in the different groups, respectively. All these three features revealed no significant difference between preinvasive and invasive nodules in both pure GGO, or part‐solid GGO groups. According to this result, morphology of the GGO nodule could not be considered as the predictor for the invasiveness evaluation (Table 5).
Figure 4.
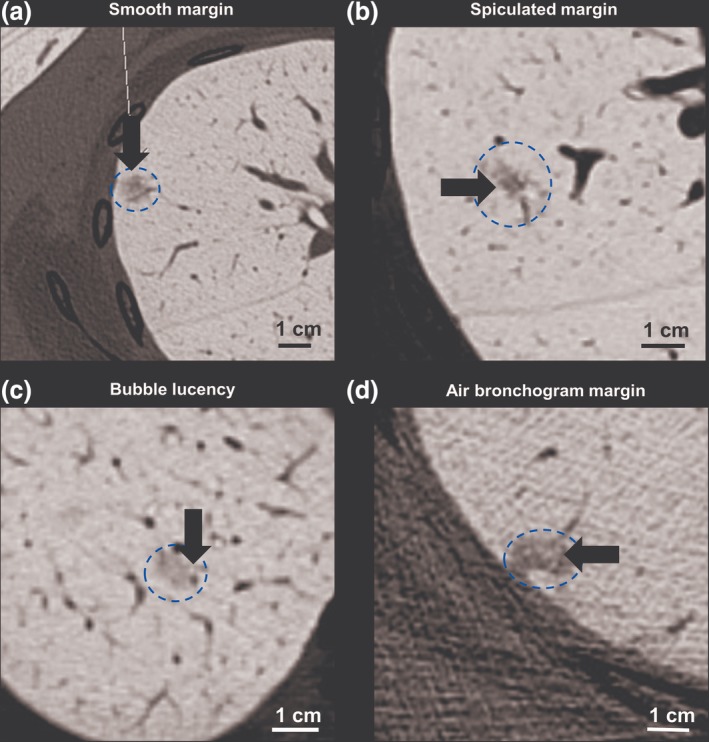
Morphology of the ground‐glass GGO nodules visible on the CT scan. (a) A smooth margin (arrow) of one pure GGO nodule was noted in a 50‐year‐old female with a final pathologic report of adenocarcinoma in situ (AIS). (b) Spiculated margin (arrow) of part‐solid GGO nodule. The final histopathology was minimally invasive adenocarcinoma (MIA). (c) Bubble lucency (arrow) in a pure GGO nodule. This image was from a 51‐year‐old female with AIS. (d) Air bronchogram (arrow). There is one V‐shape air‐filled bronchus visible in a GGO nodule which post‐operatively was diagnosed as MIA.
Table 5.
Comparison of the relationship between the invasiveness and GGO morphology in pure GGO and part‐solid GGO groups
| Pure GGO (n = 59) | Part‐solid GGO (n = 33) | |||||
|---|---|---|---|---|---|---|
| Parameters | Preinvasive (n = 57) | Invasive (n = 2) | P‐value | Preinvasive (n = 25) | Invasive (n = 8) | P‐value |
| Margin, n (%) | ||||||
| Smooth | 43 (75.4) | 1 (50.0) | 0.447 | 9 (36.0) | 1 (12.5) | 0.382 |
| Spiculated | 14 (24.6) | 1 (50.0) | 16 (64.0) | 7 (87.5) | ||
| Bubble lucency sign, n (%) | ||||||
| Yes | 29 (50.9) | 0 (0.0) | 0.492 | 10 (40.0) | 2 (25.0) | 0.678 |
| No | 28 (49.1) | 2 (100.0) | 15 (60.0) | 6 (75.0) | ||
| Air bronchogram, n (%) | ||||||
| Yes | 48 (84.2) | 1 (50.0) | 0.313 | 15 (60.0) | 4 (50.0) | 0.695 |
| No | 9 (15.8) | 1 (50.0) | 10 (40.0) | 4 (50.0) | ||
Preinvasive, AAH + AIS; Invasive, MIA + IA; GGO, ground‐glass opacity.
Discussion
Surgical intervention remains the cornerstone in the treatment of lung cancer and is the only way to offer long‐term survival.11 It has been reported that five‐year‐survival in patients with AIS and MIA presenting with GGO nodules on chest CT could be 100% following surgical resection.5 However, in another study, the five‐year survival rates in stage IA1, IA2, and IA3 which presented in GGO nodules were 97.8%, 89.3%, and 88.5%, respectively.12, 13 Hence, the surgical timing for the GGO nodules should be considered at the MIA stage. Previous reports have revealed that pure GGO nodules could be diagnosed as IA ultimately after surgical resection14, 15 and IA could also be misdiagnosed as a clinically noninvasive tumor on a preoperative CT scan just according to the CT features.16 In this study, we found that the part‐solid GGO nodules were significantly different in the invasiveness compared to the pure GGO nodules. A solid component is a risk factor for invasiveness and could be considered as the surgical indication when physicians encounter a new diagnosed of GGO nodule. Previous studies have also mentioned that a solid component in GGO nodules was related to invasiveness.17, 18, 19 Furthermore, the correlation of the solid component diameter and invasiveness was significantly positive,7 and this concept was also announced in the report by Zhang et al. which revealed the most powerful factor to predict IA was the diameter of the solid component.20 Moreover, the patients with solid‐predominant nodules were at a greater risk of recurrence compared to those with GGO‐predominant nodules.21 Therefore, part‐solid GGO nodules should be regarded as having a higher risk of invasiveness and surgical intervention should be considered.
Tumor size of the GGO nodule was another critical risk factor of potential malignancy.22, 23 We also found that tumor size was a significant factor for the invasiveness. A cutoff diameter of 10 mm for preinvasive GGO nodules and 14 mm for invasive GGO nodules have been previously reported.6 Eguchi et al. also reported a cutoff diameter of 11 mm for evaluating the invasiveness in pure GGO nodules.24 Recently, Li et al. reported that the GGO nodules showed a tendency to be invasive adenocarcinoma if the tumor diameter was larger than 13.5 mm.25 Until now, there has been no definitive surgical policy for pure GGO nodules and generally, a pure GGO nodule larger than 1 cm should be considered indicative for surgery. Previous studies have also revealed that all the IA presented in pure GGO nodules were larger than 1 cm.16 Our study revealed that a nodule diameter larger than 1 cm was a significant factor to predict invasiveness in the part‐solid group.
Quantitative analysis of attenuation of the GGO nodule by histogram distribution has offered physicians a way to distinguish preinvasiveness from invasiveness and the report by Li et al. showed that the tumor volume and mean attenuation could be a predictive factor for the invasiveness.26 In the study by Zhao et al. mean CT value was chosen for distinguishing the preinvasiveness from the invasiveness.27 We also found a similar result in our study that there was a correlation between the tumor volume and invasiveness. The mean CT value did not reveal the significant difference in the present study, despite the values being similar in comparison with the data in the study by Zhao et al. However, maximal tumor attenuation was another significant factor for the invasiveness reported in our study and was compatible with the study by Li et al.28 Moreover, the mean attenuation of the solid component has been evaluated in a previous study and revealed a significant difference between preinvasiveness and invasiveness.29 Thus, tumor attenuation could be considered as a useful tool to evaluate invasiveness.
The morphology between preinvasive nodules and invasive nodules has also been previously reported and the smooth margin noted, mainly in preinvasive lesions.6 An irregular margin of the GGO nodules was positively associated with invasive lesions.27 In our study, we did not find a correlation between the nodular margin and invasiveness, and we infer that the small size of the invasive group may be the major cause. Bubble lucency of the GGO nodule was chosen as the CT feature in a previous study to distinguish the IA from AIS/MIA, although the result revealed no significant difference.20 In our study, we used bubble lucency to evaluate the invasiveness and the result indicated that this feature could not be considered as the predictive tool. An air bronchogram had been used to evaluate invasiveness although the efficacy in invasiveness prediction is still controversial. However, an air bronchogram was found to be a good prognostic factor in the study by Yoshino et al.30 but, in contrast, another study showed that the presence of an air bronchogram was more frequently noted in IA than in AIS.31 An air bronchogram was not a significant factor to predict invasiveness in our study. Hence, according to our results and those of other studies, morphology of the GGO nodules should not be regarded as a good predictor for invasiveness.
There were some limitations in our study. First, it was a single‐institution retrospective study and we only selected the patients with small GGO nodules (less than 2 cm) with a C/T ratio less than 0.25. Therefore, there may have been a bias in the population which could not be applied to other types of GGO nodules where the tumor size was greater than 2 cm or C/T ratio larger than 0.25. Second, the total sample size was small, especially in the invasive group. Further studies are required to re‐evaluate the invasiveness with an increase in the sample sizes of the invasive group. Third, the nodule margin was drawn freehand and thus there may have been slight personal error in tumor volume and CT value evaluation. In future, to reduce the possibility of personal error, further software upgrades are required in order to be able to evaluate the nodular margin more precisely, instead of relying upon manual margination. Nonetheless, this study still offers an important reference for evaluating the invasiveness of the small and low C/T ratio of GGO nodules to assist physicians in formulating a treatment strategy.
In conclusion, when GGO nodules are detected on a CT scan, the physician has to decide if the nodules are potentially invasive and whether surgical intervention should be performed at the MIA stage. The clinical features including tumor size, solid‐component, tumor volume and maximal tumor attenuation are significant factors in the prediction of invasiveness. In particular, unlike the NCCN guideline, we suggest that part‐solid GGO nodules with a diameter greater than 1 cm should be given surgical consideration due to their high possibility of invasiveness.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This study was supported by a grant from the Ministry of Science and Technology (MOST 106‐2320‐B‐009‐001‐MY3) in Taiwan. This work was also financially supported by the Center For Intelligent Drug Systems and Smart Bio‐devices (IDS2B) of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68 (1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Sobue T, Moriyama N, Kaneko M et al Screening for lung cancer with low‐dose helical computed tomography: Anti‐lung cancer association project. J Clin Oncol 2002; 20 (4): 911–20. [DOI] [PubMed] [Google Scholar]
- 3. Lee HY, Lee KS. Ground‐glass opacity nodules: Histopathology, imaging evaluation, and clinical implications. J Thorac Imaging 2011; 26 (2): 106–18. [DOI] [PubMed] [Google Scholar]
- 4. Behera M, Owonikoko TK, Gal AA et al Lung adenocarcinoma staging using the 2011 IASLC/ATS/ERS classification: A pooled analysis of adenocarcinoma in situ and minimally invasive adenocarcinoma. Clin Lung Cancer 2016; 17 (5): e57–64. [DOI] [PubMed] [Google Scholar]
- 5. Murakami S, Ito H, Tsubokawa N et al Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015; 90 (2): 199–204. [DOI] [PubMed] [Google Scholar]
- 6. Lee SM, Park CM, Goo JM, Lee HJ, Wi JY, Kang CH. Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground‐glass nodules: Differentiation by using CT features. Radiology 2013; 268 (1): 265–73. [DOI] [PubMed] [Google Scholar]
- 7. Wilshire CL, Louie BE, Manning KA et al Radiologic evaluation of small lepidic adenocarcinomas to guide decision making in surgical resection. Ann Thorac Surg 2015; 100 (3): 979–88. [DOI] [PubMed] [Google Scholar]
- 8. Moon Y, Sung SW, Lee KY, Sim SB, Park JK. Pure ground‐glass opacity on chest computed tomography: Predictive factors for invasive adenocarcinoma. J Thorac Dis 2016; 8 (7): 1561–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naidich DP, Bankier AA, MacMahon H et al Recommendations for the management of subsolid pulmonary nodules detected at CT: A statement from the Fleischner society. Radiology 2013; 266 (1): 304–17. [DOI] [PubMed] [Google Scholar]
- 10. MacMahon H, Naidich DP, Goo JM et al Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner society 2017. Radiology 2017; 284 (1): 228–43. [DOI] [PubMed] [Google Scholar]
- 11. Lackey A, Donington JS. Surgical management of lung cancer. Semin Intervent Radiol 2013; 30 (2): 133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goldstraw P, Chansky K, Crowley J et al The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11 (1): 39–51. [DOI] [PubMed] [Google Scholar]
- 13. Hattori A, Hirayama S, Matsunaga T et al Distinct clinicopathologic characteristics and prognosis based on the presence of ground glass opacity component in clinical stage IA lung adenocarcinoma. J Thorac Oncol 2019; 14 (2): 265–75. [DOI] [PubMed] [Google Scholar]
- 14. Jin X, Zhao SH, Gao J et al CT characteristics and pathological implications of early stage (T1N0M0) lung adenocarcinoma with pure ground‐glass opacity. Eur Radiol 2015; 25 (9): 2532–40. [DOI] [PubMed] [Google Scholar]
- 15. Son JY, Lee HY, Kim JH et al Quantitative CT analysis of pulmonary ground‐glass opacity nodules for distinguishing invasive adenocarcinoma from non‐invasive or minimally invasive adenocarcinoma: The added value of using iodine mapping. Eur Radiol 2016; 26 (1): 43–54. [DOI] [PubMed] [Google Scholar]
- 16. Moon Y, Park JK, Lee KY, Ahn S, Shin J. Predictive factors for invasive adenocarcinoma in patients with clinical non‐invasive or minimally invasive lung cancer. J Thorac Dis 2018; 10 (11): 6010–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eguchi T, Kadota K, Park BJ, Travis WD, Jones DR, Adusumilli PS. The new IASLC‐ATS‐ERS lung adenocarcinoma classification: What the surgeon should know. Semin Thorac Cardiovasc Surg 2014; 26 (3): 210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takahashi M, Shigematsu Y, Ohta M, Tokumasu H, Matsukura T, Hirai T. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014; 147 (1): 54–9. [DOI] [PubMed] [Google Scholar]
- 19. Saji H, Matsubayashi J, Akata S et al Correlation between whole tumor size and solid component size on high‐resolution computed tomography in the prediction of the degree of pathologic malignancy and the prognostic outcome in primary lung adenocarcinoma. Acta Radiol 2015; 56 (10): 1187–95. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Shen Y, Qiang JW, Ye JD, Zhang J, Zhao RY. HRCT features distinguishing pre‐invasive from invasive pulmonary adenocarcinomas appearing as ground‐glass nodules. Eur Radiol 2016; 26 (9): 2921–8. [DOI] [PubMed] [Google Scholar]
- 21. Moon Y, Sung SW, Lee KY, Park JK. Clinicopathological characteristics and prognosis of non‐lepidic invasive adenocarcinoma presenting as ground glass opacity nodule. J Thorac Dis 2016; 8 (9): 2562–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oh JY, Kwon SY, Yoon HI et al Clinical significance of a solitary ground‐glass opacity (GGO) lesion of the lung detected by chest CT. Lung Cancer 2007; 55 (1): 67–73. [DOI] [PubMed] [Google Scholar]
- 23. Lee SM, Park CM, Goo JM et al Transient part‐solid nodules detected at screening thin‐section CT for lung cancer: Comparison with persistent part‐solid nodules. Radiology 2010; 255 (1): 242–51. [DOI] [PubMed] [Google Scholar]
- 24. Eguchi T, Yoshizawa A, Kawakami S et al Tumor size and computed tomography attenuation of pulmonary pure ground‐glass nodules are useful for predicting pathological invasiveness. PLOS One 2014; 9 (5): e97867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li M, Wang Y, Chen Y, Zhang Z. Identification of preoperative prediction factors of tumor subtypes for patients with solitary ground‐glass opacity pulmonary nodules. J Cardiothorac Surg 2018; 13 (1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Q, Fan L, Cao ET, Li QC, Gu YF, Liu SY. Quantitative CT analysis of pulmonary pure ground‐glass nodule predicts histological invasiveness. Eur J Radiol 2017; 89: 67–71. [DOI] [PubMed] [Google Scholar]
- 27. Zhao W, Xu Y, Yang Z et al Development and validation of a radiomics nomogram for identifying invasiveness of pulmonary adenocarcinomas appearing as subcentimeter ground‐glass opacity nodules. Eur J Radiol 2019; 112: 161–8. [DOI] [PubMed] [Google Scholar]
- 28. Li W, Wang X, Zhang Y, Li X, Li Q, Ye Z. Radiomic analysis of pulmonary ground‐glass opacity nodules for distinction of preinvasive lesions, invasive pulmonary adenocarcinoma and minimally invasive adenocarcinoma based on quantitative texture analysis of CT. Chin J Cancer Res 2018; 30 (4): 415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Qiang JW, Ye JD, Ye XD, Zhang J. High resolution CT in differentiating minimally invasive component in early lung adenocarcinoma. Lung Cancer 2014; 84 (3): 236–41. [DOI] [PubMed] [Google Scholar]
- 30. Yoshino I, Nakanishi R, Kodate M et al Pleural retraction and intra‐tumoral air‐bronchogram as prognostic factors for stage I pulmonary adenocarcinoma following complete resection. Int Surg 2000; 85 (2): 105–12. [PubMed] [Google Scholar]
- 31. Lim HJ, Ahn S, Lee KS et al Persistent pure ground‐glass opacity lung nodules >/= 10 mm in diameter at CT scan: Histopathologic comparisons and prognostic implications. Chest 2013; 144 (4): 1291–9. [DOI] [PubMed] [Google Scholar]


