Abstract
Background
Although the clinical efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR‐TKIs) in EGFR‐mutant non‐small cell lung cancer (NSCLC) patients has been demonstrated, their efficacy in EGFR‐mutant NSCLCs with central nervous system (CNS) metastases and the role of radiotherapy remain unclear. This study aimed to determine if it is preferable to add upfront cranial radiotherapy to EGFR‐TKIs in patients with EGFR‐mutant NSCLC with newly diagnosed brain metastases.
Methods
We retrospectively analyzed the data of EGFR‐mutant NSCLC patients with CNS metastases who received EGFR‐TKIs as a first‐line therapy.
Results
A total of 104 patients were enrolled and 39 patients received upfront brain radiotherapy, while 65 patients received first and second generation EGFR‐TKIs first. The median time to treatment failure (TTF) was 7.8 months (95% confidence interval [CI]: 6.3–9.4). The median survival time (MST) was 24.0 months (95% CI: 20.1–30.1). The overall response rate of the CNS was 37%. The median CNS progression‐free survival (PFS) was 13.2 months (95% CI: 10.0–16.2). Brain radiotherapy prior to EGFR‐TKI prolonged TTF (11.2 vs. 6.8 months, P = 0.038) and tended to prolong CNS‐PFS (15.6 vs. 11.1 months, P = 0.096) but was not significantly associated with overall survival (MST 26.1 vs. 24.0 months, P = 0.525). Univariate and multivariate analyses indicated that poor performance status and the presence of extracranial metastases were poor prognostic factors related to overall survival.
Conclusion
EGFR‐TKI showed a favorable effect for EGFR‐mutant NSCLC patients with CNS metastases. Prolonged TTF and CNS‐PFS were observed with upfront brain radiotherapy.
Keywords: Brain metastases, EGFR mutation, EGFR tyrosine kinase inhibitor, non‐small cell lung cancer, radiotherapy
Key points
Significant findings of the study
In this retrospective study of EGFR‐mutant NSCLC patients with brain metastases who received EGFR‐TKIs for a first‐line drug therapy, upfront brain radiotherapy and TKI showed prolonged time to treatment failure compared to TKI alone.
What this study adds
This finding might support clinician's choice to give upfront brain RT for EGFR‐mutated NSCLC patients with brain metastases.
Introduction
The central nervous system (CNS) is a common site of metastasis of non‐small cell lung cancer (NSCLC). Brain metastases (BMs) are observed in approximately 10% of NSCLC patients at diagnosis and occur in 25%–30% of these patients throughout their clinical courses.1, 2 The complication of CNS metastasis is a poor prognostic factor in NSCLC patients. The prognosis for advanced NSCLC patients with CNS metastases is approximately 4–11 weeks if untreated.1, 2, 3
A retrospective analysis showed that patients with epidermal growth factor receptor (EGFR) ‐mutant NSCLC were more likely to develop BMs than those with EGFR‐wild‐type NSCLC (31.4% vs. 19.7%, P < 0.001); moreover, BMs from EGFR‐mutant NSCLC often disseminate (30.8% vs. 12.7%).4 Ultimately, CNS metastases can become lethal in EGFR‐mutant NSCLC patients. However, according to a previous study, survival after BMs was significantly longer for EGFR‐mutant cases than for EGFR‐wild‐type cases (hazard ratio: 2.23; 95% CI: 1.62–3.10, P < 0.001). This finding has been attributed to the benefit of EGFR‐tyrosine kinase inhibitors (TKIs) for EGFR‐mutant patients. EGFR‐TKIs such as gefitinib, erlotinib, and afatinib have been established as standard first‐line drug therapies in advanced NSCLC patients harboring EGFR mutations on the basis of a number of phase III trials.5, 6, 7, 8, 9, 10, 11
There is no consensus regarding the management of EGFR‐mutant NSCLC patients with BMs. The concentration of first‐generation EGFR‐TKIs in cerebrospinal fluid was much lower than that in plasma.12 Although several prospective trials examined the effect of EGFR‐TKIs on BMs without brain radiotherapy (RT), the results from these studies were inconclusive.13, 14, 15 Traditionally, patients with multiple BMs have been treated with whole‐brain radiotherapy (WBRT). Previous reports examining the efficacy of EGFR‐TKIs plus WBRT have provided conflicting results.16, 17 Therefore, whether WBRT in addition to EGFR‐TKI is especially needed for patients with asymptomatic BMs is unclear. Recently, evidence regarding the efficacy of stereotactic radiosurgery (SRS) for a few (four or less) small (< 3 cm) brain metastases has been gradually accumulated.18, 19 A retrospective study of EGFR‐mutant NSCLC patients with BMs suggested increased survival with a combination of upfront SRS and EGFR‐TKI compared to that with TKI monotherapy.20
The aim of this retrospective study was to determine the impact of adding upfront cranial radiotherapy to EGFR‐TKI in patients with EGFR‐mutant NSCLC with newly diagnosed brain metastases on time to treatment failure, intracranial progression‐free survival, and overall survival. We also evaluated the prognostic factors that could affect the treatment outcome.
Methods
Study design and patients
We retrospectively evaluated patient clinical outcomes and background for seven years between January 2008 and December 2014 in Niigata Lung Cancer Treatment Group facilities. The eligibility criteria were as follows: (i) pathologically confirmed NSCLC at stage IV, postoperative recurrence, or recurrence after treatment for locally advanced cancer; (ii) newly radiographically diagnosed brain metastasis and/or leptomeningeal metastases; (iii) confirmed EGFR mutation at any point during the clinical course; and (iv) initiated EGFR‐TKI as a first‐line drug therapy during the above period.
The study protocol was approved by the institutional review board of each participating institution. The requirement for patient consent was waived due to the retrospective nature of the study and lack of potential harm to patients. The following variables were collected for analysis: age, sex, Eastern Cooperative Oncology Group‐Performance Status (ECOG‐PS), Karnofsky Performance Status (KPS), smoking history, pathological diagnosis, stage, EGFR mutation status, initial CNS symptom, number of BMs, size of the largest BM, presence of leptomeningeal metastases, extracranial metastases, kind of EGFR‐TKI, type of brain RT, site of disease progression, cause for discontinuation of EGFR‐TKI, 2nd line therapy, and cause of death. The date of initial EGFT‐TKI treatment, RT, discontinuation of systemic disease progression, CNS disease progression, most recent follow‐up and death were also recorded.
Treatment and response evaluation
Patients in this study were treated with EGFR‐TKI including gefitinib 250 mg, erlotinib 150 mg, or afatinib 40 mg once a day. WBRT was usually performed at a dose of 30 Gy in 10 fractions. For stereotactic irradiation (STI) of the brain, SRS or stereotactic radiotherapy (SRT) was performed. With SRS, the full radiation dose was delivered in one session, while with SRT, the dose of radiation was delivered over a course of several treatment sessions, instead of all at once. The median marginal dose of SRS was 20 Gy. SRT was usually performed at a dose of 30 Gy in four fractions.
At the baseline of disease diagnosis, computed tomography (CT) and/or positron emission tomography‐CT of the chest, abdomen, and pelvis, bone scintigraphy and brain magnetic resonance imaging (MRI) or brain CT were performed. CT for extracranial disease and MRI or CT for intracranial disease were usually repeated every 2–3 months. The tumor response was assessed as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD) according to response evaluation criteria in solid tumors (RECIST) 1.1.
Statistical analysis
The primary end point was time to treatment failure (TTF), and the secondary end points were PFS, CNS‐PFS, and overall survival (OS). TTF was defined from the initiation of treatment (EGFR‐TKI or local therapy for BM, whichever came first) to the end of treatment (disease progression, discontinuation of EGFR‐TKI from any cause, or death from any cause, whichever came first). PFS was measured from the initiation of treatment (EGFR‐TKI or local therapy for BM, whichever came first) to disease progression or death from any cause. CNS‐PFS was measured from the initiation of treatment (EGFR‐TKI or local therapy for BM, whichever came first) to CNS progression or death from any cause. OS was measured from the initiation of treatment (EGFR‐TKI or local therapy for BM, whichever came first) to death from any cause or last survival follow‐up.
We used Fisher's exact test, chi‐square test, or t‐test as appropriate to compare the characteristics of patients with and without upfront brain RT. Kaplan‐Meier survival curves were constructed for TTF, PFS, CNS‐PFS and OS; the differences between groups were identified using the log‐rank test. Univariate and multivariate Cox proportional hazards models were used to assess prognostic factors for OS. Each analysis was two‐sided, with a 5% significance level and 95% confidence interval (CI). All analyses were performed using JMP for Windows software version 13.0 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 104 NSCLC patients harboring EGFR mutations with CNS metastases who underwent EGFR‐TKI for the first‐line drug therapy from 10 institutions were identified. The median follow‐up was 21.5 months (range: 0.2–83.0). The baseline and initial treatment characteristics of these patients are summarized in Table 1. All patients were from Japan. Most were female (64%), never smokers (59%), and had adenocarcinoma (98%). The two major types of mutations, exon 19 deletion and exon 21 L858R point mutation, accounted for 95% of patients. More symptomatic BMs were observed in patients who received upfront brain RT (1st RT group) than in those who did not receive upfront brain RT (1st TKI group) (51% vs. 15%, P = 0.0001). Additionally, patients in the 1st RT group were more likely to have larger BMs (22 mm vs. 9 mm, P < 0.001). There was no significant difference in ECOG‐PS, number of BMs, extracranial metastases, leptomeningeal metastases, diagnosis‐specific Graded Prognostic Assessment (DS‐GPA), or EGFR mutations between the groups.
Table 1.
Patient characteristics
| Total | 1st TKI | 1st RT | |||||
|---|---|---|---|---|---|---|---|
| Characteristics | N = 104 | % | N = 65 | % | N = 39 | % | P‐value |
| Sex | 1.000 | ||||||
| Male | 37 | 36% | 24 | 37% | 14 | 36% | |
| Female | 67 | 64% | 41 | 63% | 25 | 64% | |
| Age, median (range, years) | 67 | (36–91) | 67 | (36–86) | 71 | (41–91) | 0.3866 |
| Body height, median (range, cm) Bodyweight median (range, kg) |
157 51 |
(130–180) (33–87) |
156 49 |
(130–180) (34–87) |
158 52 |
(130–170) (33–69) |
0.7262 0.9162 |
| Smoking history | 0.8374 | ||||||
| Never smoker | 61 | 59% | 39 | 60% | 22 | 56% | |
| ECOG performance status | 0.8468 | ||||||
| 0–1 | 66 | 63% | 43 | 66% | 23 | 59% | |
| 2 | 20 | 19% | 11 | 17% | 9 | 23% | |
| 3 | 16 | 15% | 10 | 15% | 6 | 15% | |
| 4 | 2 | 2% | 1 | 2% | 1 | 3% | |
| Stage | 0.0003 | ||||||
| Stage IV | 82 | 79% | 59 | 91% | 23 | 59% | |
| Recurrence after operation or (chemo) radiotherapy | 22 | 21% | 6 | 9% | 16 | 41% | |
| Pathology | 0.1383 | ||||||
| Adeno | 102 | 98% | 65 | 100% | 37 | 95% | |
| Adenosquamous | 2 | 2% | 0 | 0% | 2 | 5% | |
| Number of BMs | 0.8683 | ||||||
| 1 | 32 | 31% | 21 | 32% | 12 | 31% | |
| 2 | 17 | 16% | 9 | 14% | 8 | 21% | |
| 3 | 6 | 6% | 4 | 6% | 2 | 5% | |
| 4 | 8 | 8% | 6 | 9% | 2 | 5% | |
| 5≤ | 41 | 39% | 25 | 38% | 15 | 38% | |
| Size of the largest BM, median (range, mm) | 12 | (3–57) | 9 | (3–55) | 22 | (6–57) | <0.001 |
| Initial brain symptom | 0.0001 | ||||||
| Symptomatic | 30 | 29% | 10 | 15% | 20 | 51% | |
| Asymptomatic | 74 | 71% | 55 | 85% | 19 | 49% | |
| Primary leptomeningeal carcinomatosis | 1.0000 | ||||||
| Yes | 8 | 8% | 5 | 8% | 3 | 8% | |
| No | 96 | 92% | 60 | 92% | 36 | 92% | |
| Extracranial metastasis | 0.2481 | ||||||
| Yes | 77 | 74% | 51 | 78% | 26 | 67% | |
| No | 27 | 26% | 14 | 22% | 13 | 33% | |
| DS‐GPA | 0.5859 | ||||||
| 0 | 11 | 11% | 6 | 9% | 5 | 13% | |
| 0.5 | 17 | 16% | 12 | 18% | 5 | 13% | |
| 1 | 21 | 20% | 15 | 23% | 6 | 15% | |
| 1.5 | 18 | 17% | 12 | 18% | 6 | 15% | |
| 2 | 17 | 16% | 7 | 11% | 10 | 26% | |
| 2.5 | 8 | 8% | 6 | 9% | 2 | 5% | |
| 3 | 10 | 10% | 6 | 9% | 4 | 10% | |
| 3.5 | 2 | 2% | 1 | 2% | 1 | 3% | |
| EGFR mutation | 0.1383 | ||||||
| Ex 19 deletion | 52 | 50% | 37 | 57% | 15 | 38% | |
| Ex 21 L858R | 47 | 45% | 24 | 37% | 23 | 59% | |
| Ex 18 L861Q | 3 | 3% | 2 | 3% | 1 | 3% | |
| Ex 18 G719X | 2 | 2% | 2 | 3% | 0 | 0% | |
| EGFR TKI | 0.1774 | ||||||
| Gefitinib | 77 | 74% | 53 | 82% | 26 | 77% | |
| Erlotinib | 24 | 23% | 10 | 15% | 12 | 31% | |
| Afatinib | 3 | 3% | 2 | 3% | 1 | 3% | |
| Upfront brain radiotherapy | — | ||||||
| No | 65 | 63% | 65 | 100% | 19 | 49% | |
| STI only | 19 | 18% | 16 | 41% | |||
| WBRT only | 16 | 15% | 4 | 10% | |||
| STI + WBRT | 4 | 4% | |||||
BM, brain metastasis; DS‐GPA, diagnosis‐specific graded prognostic assessment; ECOG, Eastern Cooperative Oncology Group; RT, radiotherapy; STI, stereotactic irradiation; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiotherapy.
A total of 39 of 104 patients received upfront brain RT, while 65 patients received EGFR‐TKI therapy first. Of these 39 patients, 19 received STI (SRS and SRT), 16 received WBRT, and four received both STI and WBRT. Three of these 39 patients received EGFR TKI concurrently with WBRT, while the others received TKI therapy sequentially. Median interval between initiation of radiotherapy and initiation of EGFR TKI was 19 days (range: 0–277).
Treatment outcome
The Kaplan‐Meier curve for the TTF, CNS‐PFS, and OS of the entire cohort is shown in Fig 1. The median TTF, CNS‐PFS, and OS of the entire cohort were 7.8 months (95% CI: 6.3–9.4), 13.2 months (95% CI: 10.0–16.2), and 24.0 months (95% CI: 20.1–30.1), respectively (Fig 1a–c). As shown in Fig 1d, TTF was significantly longer for patients who received brain RT prior to EGFR‐TKI (1st RT) than for those who did not receive upfront brain RT (1st TKI) (11.2 vs. 6.8 months, P = 0.038) (Fig 1d). For patients age <70, female, smoker, ECOG‐PS < 2, KPS > 70, max size of BMs ≥15 mm, and L858R, TTF was longer in the 1st RT group than in the 1st TKI group (Fig S1). Although nonsignificant, patients in the 1st RT group tended to have longer CNS‐PFS (15.6 vs. 11.1 months, P = 0.096) (Fig 1e). There was no significant difference in OS (MST 26.1 vs. 24.0 months, P = 0.525) (Fig 1f). Moreover, there was no significant difference in TTF, CNS‐PFS, and OS between patients who received STI and those who received WBRT as brain RT prior to EGFR‐TKI (Fig S2).
Figure 1.
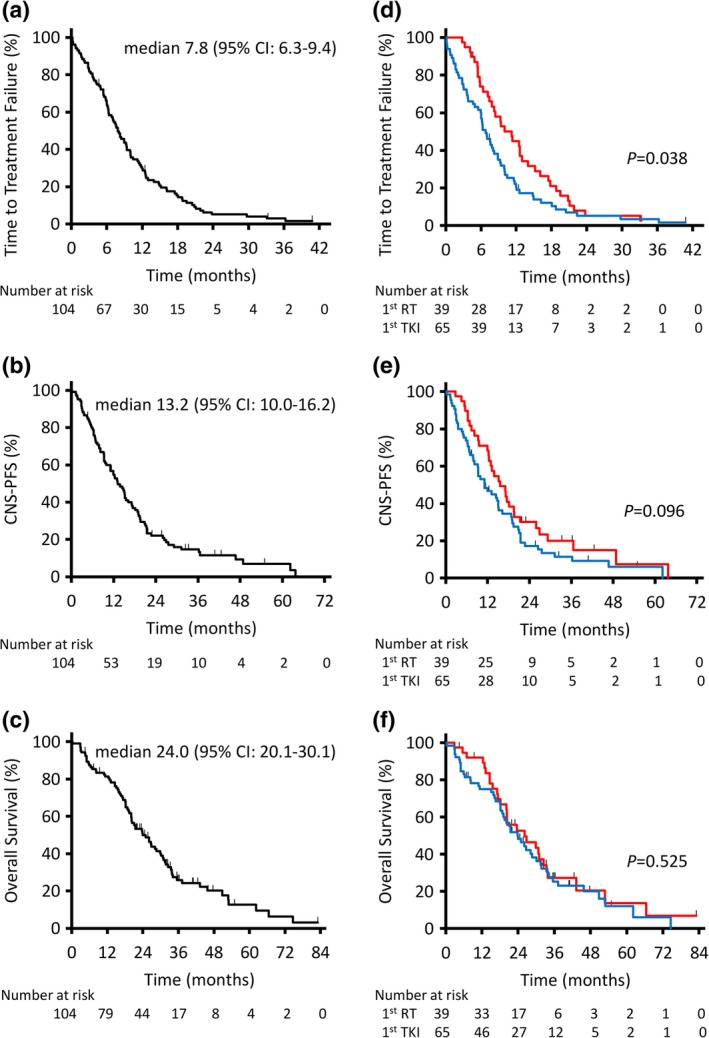
Kaplan‐Meier analysis of time to treatment failure, CNS‐PFS, and overall survival in the entire population (a, b, and c). Kaplan‐Meier analysis of time to treatment failure, CNS‐PFS, and overall survival compared between patients treated with upfront brain radiotherapy (RT) and those treated without upfront RT (d, e, and f). CI, confidence interval; CNS, central nervous system; PFS, progression‐free survival.  1st RT and
1st RT and  1st TKI.
1st TKI.
The overall response rate (ORR) and CNS‐RR of the entire cohort were 62% and 37%, respectively. There was no significant difference in ORR and CNS‐RR between the 1st RT group and the 1st TKI group (ORR; 64% vs. 60%, P = 0.8353, CNS‐RR; 36% vs. 37%, P = 1.0000) (Table 2).
Table 2.
Treatment response
| Total | 1st TKI | 1st RT | ||
|---|---|---|---|---|
| Response | N = 104 | N = 65 | N = 39 | P‐value |
| Best overall response | ||||
| CR | 1 | 0 | 1 | — |
| PR | 63 | 39 | 24 | — |
| SD (include non‐CR/non‐PD) | 22 | 13 | 9 | — |
| PD | 10 | 8 | 2 | — |
| Not evaluated | 8 | 5 | 3 | — |
| Response rate | 64 (62%) | 39 (60%) | 25 (64%) | 0.8353 |
| Disease control rate | 86 (83%) | 52 (80%) | 34 (87%) | 0.4289 |
| Best CNS response | ||||
| CR | 15 | 10 | 5 | — |
| PR | 23 | 14 | 9 | — |
| SD (include non‐CR/non‐PD) | 46 | 27 | 19 | — |
| PD | 7 | 6 | 1 | — |
| Not evaluated | 13 | 8 | 5 | — |
| Response rate | 38 (37%) | 24 (37%) | 14 (36%) | 1.0000 |
| Disease control rate | 84 (81%) | 51 (78%) | 33 (85%) | 0.6082 |
CR, complete response; PD, progression of disease; PR, partial response; RT, radiotherapy; SD, stable disease; TKI, tyrosine kinase inhibitor; WBRT, whole brain radiotherapy.
Patterns of treatment failure
Of the 104 patients, 88 developed disease progression during their follow‐up period. Compared to the 1st RT population, the 1st TKI population was more likely to present with an intracranial first site of progression, although the difference was nonsignificant (46% vs. 28%, P = 0.1151) (Table 3). Among the patients who developed disease progression only in intracranial lesions, EGFR‐TKI treatment were continued beyond PD in four out of 20 in 1st TKI patients and one out of eight 1st RT patients, respectively.
Table 3.
Patterns of disease progression
| Total | 1st TKI | 1st RT | |||||
|---|---|---|---|---|---|---|---|
| Pattern | N = 88 | % | N = 56 | % | N = 32 | % | P‐value |
| Intracranial lesions only | 28 | 32% | 20 | 36% | 8 | 25% | 0.3482 |
| Extracranial lesions only | 52 | 59% | 30 | 54% | 22 | 69% | 0.3696 |
| Both intra‐ and extracranial lesions | 7 | 8% | 6 | 11% | 1 | 3% | 0.4146 |
| Not available | 1 | 1% | 0 | 0% | 1 | 3% | — |
| Intracranial lesion only + both lesions | 35 | 40% | 26 | 46% | 9 | 28% | 0.1151 |
TKI, tyrosine kinase inhibitor; RT, radiotherapy.
Cause of death
Of the 104 patients, 79 died during their follow‐up period. Thirty‐one patients (39%) died of disease progression, including intracranial lesions. The cause of death was not significantly different between the 1st TKI group and the 1st RT group (Table 4).
Table 4.
Direct cause of death
| Total | 1st TKI | 1st RT | |||||
|---|---|---|---|---|---|---|---|
| Cause of death | N = 79 | % | N = 49 | % | N = 30 | % | P‐value |
| Intracranial lesions | 18 | 23% | 10 | 20% | 8 | 27% | 0.5855 |
| Extracranial lesions | 28 | 35% | 18 | 37% | 10 | 33% | 0.8123 |
| Both intra‐ and extracranial lesions | 13 | 16% | 9 | 18% | 4 | 13% | 0.7565 |
| Treatment related death | 2 | 3% | 1 | 2% | 1 | 3% | 1.0000 |
| Other disease | 6 | 8% | 4 | 8% | 2 | 7% | 1.0000 |
| Not available | 12 | 15% | 7 | 14% | 5 | 17% | — |
RT, radiotherapy; TKI, tyrosine kinase inhibitor.
Subgroup analysis of OS
In univariate analysis for OS of the entire cohort, ECOG‐PS < 2 (P < 0.0001), KPS ≥70 (P = 0.017), postoperative recurrence and recurrence after treatment for locally advanced cancer (P = 0.005), absence of extracranial metastases (P = 0.0001), and GPA ≥2 (P = 0.0021) were associated with prolonged OS (Table 5). In multivariate analysis for OS, ECOG‐PS < 2 (P = 0.0192) and absence of extracranial metastases (P = 0.0044) were independent factors associated with prolonged OS.
Table 5.
Univariable and multivariable analyses of overall survival
| Univariable | Multivariable | ||||
|---|---|---|---|---|---|
| Variable | Risk group | HR (95%CI) | P‐value | HR (95%CI) | P‐value |
| Age | < 60 years old | 0.79 (0.45–1.32) | 0.379 | ||
| < 70 years old | 0.82 (0.52–1.30) | 0.390 | |||
| Sex | Female | 0.67 (0.42–1.08) | 0.101 | ||
| Smoking status | Never | 0.81 (0.51–1.31) | 0.387 | ||
| ECOG performance status | < 2 | 0.35 (0.22–0.57) | <0.0001 | 0.39 (0.19–0.85) | 0.0192 |
| KPS | ≥ 70 | 0.43 (0.26–0.72) | 0.0017 | 0.78 (0.37–1.74) | 0.526 |
| Stage | Recurrence vs. IV | 0.43 (0.21–0.79) | 0.0050 | 0.58 (0.28–1.07) | 0.0845 |
| Number of BMs | ≤ 4 | 0.69 (0.44–1.12) | 0.132 | ||
| Max size of BMs | < 15 mm | 0.75 (0.47–1.19) | 0.213 | ||
| Initial brain symptom | No | 0.72 (0.44–1.19) | 0.196 | ||
| Extracranial metastases | No | 0.38 (0.23–0.63) | 0.0001 | 0.48 (0.28–0.80) | 0.0044 |
| DS‐GPA | ≥ 2 | 0.47 (0.28–0.77) | 0.0021 | 0.82 (0.46–1.43) | 0.480 |
| EGFR mutation | Ex 19 del v L858R | 0.87 (0.54–1.39) | 0.552 | ||
| Primary Leptomeningeal metastases | No vs. Yes | 0.60 (0.29–1.44) | 0.232 | ||
| EGFR‐TKI | GEF vs. ERL | 0.75 (0.44–1.32) | 0.302 | ||
| Brain radiotherapy prior to TKI | 1st vs. others | 0.86 (0.53–1.36) | 0.523 | ||
BM, brain metastasis; CI, confidence interval; DS‐GPA, diagnosis‐specific Graded Prognostic Assessment; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio; KPS, Karnofsky performance status; LM, leptomeningeal metastases; TKI, tyrosine kinase inhibitor.
Discussion
We retrospectively evaluated the clinical outcomes and background of 104 EGFR‐mutant NSCLC patients with CNS metastases who were administered EGFR‐TKIs as a first‐line drug therapy. Patients who received upfront brain RT showed more favorable TTF and CNS‐PFS than did those who received EGFR‐TKI first without upfront brain RT. Notably, in our study, the 1st RT group showed significantly longer TTF, even though the 1st RT population had larger and more symptomatic BMs than did the 1st TKI population. These findings suggested that this population benefited from brain RT prior to EGFR‐TKI.
In previous reports, the median PFS ranged from 6.6 to 11.4 months, the median CNS‐PFS ranged from 14.5 to 19.7 months, the median OS ranged from 13.6 to 31.7 months, the ORR ranged from 68.2 to 87.8%, and the CNS‐RR ranged from 36.5 to 82.6% for EGFR‐mutant patients with BMs who were treated with EGFR‐TKI.14, 15, 21, 22, 23 In our study, TTF, CNS‐PFS, and OS were similar to those data. In contrast, the ORR of 62% and CNS‐RR of 37% were relatively low. This result can be explained by data unavailability; that is, the treatment response of eight patients in ORR and 13 patients in CNS‐RR were not evaluated, although they were included in the study. In addition, regarding CNS‐RR, we considered irradiated brain lesions as unmeasurable unless there had been demonstrated progression in the lesions according to RECIST, resulting in an evaluation of false SD, even though those lesions were shrinking. In fact, the disease control rate of 81% in this study as well as the rates of TTF, CNS‐PFS, and OS were not inferior to the results from previous reports.
In the current study, patients were treated with first and second generation EGFR‐TKI as a first‐line chemotherapy. Recently, evidence regarding the efficacy of a third generation TKI, osimertinib, which selectively inhibits both EGFR‐TKI‐sensitizing and T790M resistance mutations, has accumulated. In a phase III trial, osimertinib showed significantly longer PFS and less toxicity than did first generation EGFR‐TKIs as a first‐line therapy for EGFR‐mutant NSCLC patients.24 In addition, compared to first generation EGFR‐TKIs, osimertinib demonstrated significantly longer PFS in patients with BMs.24 These favorable antitumor effects in patients with BMs might be due to the high concentration of osimertinib in cerebrospinal fluid.25 Nonetheless, further prospective trials are required to evaluate the efficacy of brain RT in EGFR‐mutant patients with CNS metastases before the initiation of osimertinib.
Several mechanisms have been proposed to explain the effect of combinational therapy with radiation and EGFR‐TKI. Previous studies have shown the high radiosensitivity of EGFR‐mutant NSCLC.26, 27 Moreover, WBRT or focal RT can cause early and delayed blood brain barrier disruption, which presumably leads to an increase in TKI permeability.28 In addition, EGFR‐TKIs could enhance the radiation response at several levels, including cell cycle arrest, apoptosis induction, accelerated cellular repopulation, and DNA damage repair.29 Based on these biological rationales, radiation and EGFR‐TKI are expected to provide good combinational therapy. In our study, the 1st RT population showed more favorable TTF and CNS‐PFS than did the 1st TKI population. However, OS showed no significant difference between these groups. In fact, of the 65 1st TKI patients, 43 (66%) received second line drug therapy, while 21 of the 39 1st RT patients (54%) received second line drug therapy (P = 0.2207). Of the 43 1st TKI patients who received second line drug therapy, 28 (65%) received platinum doublet therapy as the second line treatment, while 11 of the 21 1st RT patients (52%) received that (P = 0.2874). Of the 65 1st TKI patients, 34 (52%) received brain RT at any point during their clinical course after EGFR TKI therapy. Thus, the majority of the patients in both groups received subsequent drug therapy, and the majority of the 1st TKI group received some kind of brain RT eventually. The absence of significant difference in OS might be attributed to these successful subsequent therapies or salvage local therapies. In the meantime, a recent meta‐analysis showed that upfront RT followed by TKI had longer OS as well as CNS‐PFS, especially for patients with limited number of BMs.30
Recent real world data collected by EORTC Lung Cancer Group showed that NSCLC patients with driver mutations generally received more intensive local treatments.31 Further, a recent large‐scale retrospective study of EGFR‐mutant NSCLC patients with BMs reported that survival time was improved by the combination of upfront SRS and EGFR‐TKI compared to that by upfront WBRT and EGFR‐TKI or TKI monotherapy (46 months vs. 30 months vs. 25 months, P < 0.001).20 Interestingly, this tendency was observed in patients with a favorable prognosis, DS‐GPA 2–4, and in those with a less favorable prognosis, DS‐GPA 0–1.5, suggesting that the improved OS seen in the upfront SRS group was not secondary to selection bias or differences between patient cohorts. In our study, there was no significant difference in TTF, CNS‐PFS and OS between patients who received STI and those who received WBRT as brain RT prior to EGFR‐TKI (Fig S2). In any case, a prospective study comparing 1st STI followed by EGFR‐TKI with 1st TKI in EGFR‐mutant NSCLC patients with BMs is required. Moreover, whether WBRT should be added to STI as a treatment option in patients with BMs remains unclear.
Brain radiotherapy could deteriorate QOL and impair cognitive function in patients. In our retrospective study, we could not analyze toxicities of brain radiotherapy due to lack of data. According to a systematic review reported by Hendriks et al. most of nine trials showed no increased neurotoxicity in TKI plus brain radiotherapy compared to TKI alone.32 Whereas, another retrospective study reported increase of memory impairment in TKI plus WBRT compared to TKI alone.17 Two randomized phase 3 studies of solid tumors with one to three BMs showed that SRS plus WBRT impaired health‐related QOL and cognitive function, respectively, and did not improve OS compared to SRS alone.33, 34 In this regard, WBRT in addition to SRS may not be routinely recommended in patients with limited number of BMs.
Sperduto et al. developed DS‐GPA to evaluate the prognosis of patients with BMs35; DS‐GPA is now used as a prognostic factor in an American Society for Radiation Oncology guideline for radiotherapeutic and surgical management for newly diagnosed BMs.36 For NSCLC, DS‐GPA consists of four prognostic factors, including age, KPS, extracranial metastases, and number of BMs. In our study, univariate and multivariate analyses indicated that the existence of extracranial metastases and poor PS (ECOG‐PS ≥2) are independent poor prognostic factors for OS in EGFR‐mutant NSCLCs. These findings almost corresponded to DS‐GPA as they were related to the factors comprising DS‐GPA. Two recent phase II studies have shown a survival benefit from WBRT on SRS in patients with good DS‐PGA,37, 38 suggesting that potential long‐term survivors could receive the benefit of WBRT. Similar to these findings, our patients younger than 70 or with good PS in the 1st RT group had longer TTF than those in the 1st TKI group (Fig S1). However, OS in the 1st RT group was not significantly prolonged. Whether the addition of WBRT to STI is especially beneficial for potential long‐time survivors, for example, those with good DS‐GPA, should be determined. Recently, Sperduto et al. updated GPA for NSCLC by adding the factor of gene status to DS‐GPA39; this change apparently contributes to an improved reflection of actual clinical practice.
Our study has several limitations that should be acknowledged. First, owing to its retrospective nature, which lacks randomization, bias might yield a difference in clinical outcomes between the groups. Second, the choice of treatment was not random because it was determined by both physicians and patients in as many as 10 different facilities whose location or equipment varies. Third, the sample size of this study was small, which may have affected its statistical power. Fourth, adverse events were not analyzed due to the lack of clinical data. In particular, a complication of leukoencephalopathy was supposed to be monitored because it strongly triggers neurocognitive dysfunction and a decrease in QOL. Leukoencephalopathy can be an important factor to consider when determining treatment.
In conclusion, our retrospective analysis demonstrated a favorable response of CNS metastases treated with EGFR‐TKI in EGFR‐mutant NSCLC patients. Furthermore, prolonged TTF and CNS‐PFS were observed with upfront brain RT. Further research through prospective studies is needed to determine the optimal timing of brain RT for the most appropriate population in EGFR‐mutant NSCLC patients with CNS metastases.
Disclosure
Satoshi Watanabe received honoraria from AstraZeneca, Chugai Pharma, Bristol‐Myers, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical; Tetsuya Abe received honoraria from AstraZeneca, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Eli Lilly Japan, Novartis, Taiho Pharmaceutical; Kosuke Ichikawa received honoraria from AstraZeneca, Chugai Pharma, Bristol‐Myers, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical; Kenichi Koyama received honoraria from AstraZeneca, MSD, Chugai Pharma, Ono Pharmaceutical, Eli Lilly, Iyaku Jounal; Satoru Miura received honoraria from Bristol‐Myer, Ono Pharmaceutical, Boehringer Ingelheim, Eli Lilly, MSD, Chugai Pharma, AstraZeneca, Taiho Pharmaceutical, Kyowa Hakko Kirin, Mochida Pharmaceutical; Hiroshi Tanaka received honoraria from AstraZeneca, Chugai Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Bristol‐Myers, Taiho Pharmaceutical, Eli Lilly, MSD, Astellas Pharmaceutical, Pfizer, Takeda pharmaceutical, Novartis, Merck; Masaaki Okajima received honoraria from AstraZeneca, Chugai Pharma, Bristol‐Myers, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical, MSD; Hiroki Tsukada received honoraria from AstraZeneca, Taisho Toyama Pharmaceutical, Astellas Pharma, Boehringer Ingelheim, Ono Pharmaceutical, Meiji Seika Pharma, Daiichi Sankyo, Shionogi, GlaxoSmithKline, Novartis, MSD, Sumitomo Dainippon Pharma, Takeda Pharmaceutical, Kyorin Pharmaceutical, Asahi Kasei Pharma, Fujifilm Pharma; Kazuhiro Sato received honoraria from AstraZeneca, Boehringer Ingelheim, Ono Pharmaceutical, MSD, Pfizer; Toshiaki Kikuchi received honoraria from Chugai Pharma, Boehringer Ingelheim, Eli Lilly Japan. The other authors indicated no financial relationships.
Supporting information
Figure S1 Forest plot of hazard ratios (HR) for time to treatment failure (TTF) by baseline characteristics in EGFR‐mutant NSCLC patients with brain metastases who received EGFR tyrosine kinase inhibitors with upfront radiotherapy (RT) and EGFR tyrosine kinase inhibitors without upfront RT as first‐line therapy. ECOG‐PS, Eastern Cooperative Oncology Group performance status; KPS, Karnofsky performance status; BM, brain metastasis; DS‐GPA, diagnosis‐specific Graded Prognostic Assessment; TKI, tyrosine kinase inhibitor; RT, radiotherapy; HR, hazard ratio; CI, confidence interval.
Figure S2 Kaplan‐Meier analysis of time to treatment failure, CNS‐PFS, and overall survival comparing in the patients treated with upfront brain STI, in those treated with upfront WBRT and in those treated without upfront radiotherapy (A, B, and C). CNS, central nervous system; PFS, progression‐free survival; STI, stereotactic irradiation; WBRT, whole brain radiotherapy; TKI, tyrosine kinase inhibitor.
Acknowledgments
The authors thank the members of the Niigata Lung Cancer Treatment Group and all of the participants who made this study possible for their dedication and cooperation throughout the course of this work. This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
References
- 1. Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol 2005; 23: 6207–19. [DOI] [PubMed] [Google Scholar]
- 2. Komaki R, Cox JD, Stark R. Frequency of brain metastasis in adenocarcinoma and large cell carcinoma of the lung: Correlation with survival. Int J Radiat Oncol Biol Phys 1983; 9: 1467–70. [DOI] [PubMed] [Google Scholar]
- 3. Sørensen JB, Hansen HH, Hansen M, Dombernowsky P. Brain metastases in adenocarcinoma of the lung: Frequency, risk groups, and prognosis. J Clin Oncol 1988; 6: 1474–80. [DOI] [PubMed] [Google Scholar]
- 4. Iuchi T, Shingyoji M, Itakura M et al Frequency of brain metastases in non‐small‐cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015; 20: 674–9. [DOI] [PubMed] [Google Scholar]
- 5. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 6. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 7. Zhou C, Wu YL, Chen G et al Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first‐line treatment of EGFR mutation‐positive advanced non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802). Ann Oncol 2015; 26: 1877–83. [DOI] [PubMed] [Google Scholar]
- 8. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Hu CP et al Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 10. Yang JC, Wu YL, Schuler M et al Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015; 16: 141–51. [DOI] [PubMed] [Google Scholar]
- 11. Oizumi S, Kobayashi K, Inoue A et al Quality of life with gefitinib in patients with EGFR‐mutated non‐small cell lung cancer: Quality of life analysis of North East Japan Study Group 002 Trial. Oncologist 2012; 17: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Togashi Y, Masago K, Masuda S et al Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non‐small cell lung cancer. Cancer Chemother Pharmacol 2012; 70: 399–405. [DOI] [PubMed] [Google Scholar]
- 13. Jamal‐Hanjani M, Spicer J. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of epidermal growth factor receptor‐mutant non‐small cell lung cancer metastatic to the brain. Clin Cancer Res 2012; 18: 938–44. [DOI] [PubMed] [Google Scholar]
- 14. Iuchi T, Shingyoji M, Sakaida T et al Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR‐mutant lung adenocarcinoma. Lung Cancer 2013; 82: 282–7. [DOI] [PubMed] [Google Scholar]
- 15. Park SJ, Kim HT, Lee DH et al Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non‐small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer 2012; 77: 556–60. [DOI] [PubMed] [Google Scholar]
- 16. Jiang T, Su C, Li X et al EGFR TKIs plus WBRT demonstrated no survival benefit other than that of TKIs alone in patients with NSCLC and EGFR mutation and brain metastases. J Thorac Oncol 2016; 11: 1718–28. [DOI] [PubMed] [Google Scholar]
- 17. Chen Y, Yang J, Li X et al First‐line epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor alone or with whole‐brain radiotherapy for brain metastases in patients with EGFR‐mutated lung adenocarcinoma. Cancer Sci 2016; 107: 1800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aoyama H, Shirato H, Tago M et al Stereotactic radiosurgery plus whole‐brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2016; 295: 2483–91. [DOI] [PubMed] [Google Scholar]
- 19. Chang EL, Wefel JS, Hess KR et al Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole‐brain irradiation: A randomised controlled trial. Lancet Oncol 2009; 10: 1037–44. [DOI] [PubMed] [Google Scholar]
- 20. Magnuson WJ, Lester‐Coll NH, Wu AJ et al Management of brain metastases in tyrosine kinase inhibitor‐naive epidermal growth factor receptor‐mutant nonsmall‐cell lung cancer: A retrospective multi‐institutional analysis. J Clin Oncol 2017; 35: 1070–7. [DOI] [PubMed] [Google Scholar]
- 21. Bai H, Xiong L, Han B. The effectiveness of EGFR‐TKIs against brain metastases in EGFR mutation‐positive non‐small‐cell lung cancer. Onco Targets Ther 2017; 10: 2335–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fan Y, Xu Y, Gong L et al Effects of icotinib with and without radiation therapy on patients with EGFR mutant non‐small cell lung cancer and brain metastases. Sci Rep 2017; 7: 45193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magnuson WJ, Yeung JT, Guillod PD, Gettinger SN, Yu JB, Chiang VL. Impact of deferring radiation therapy in patients with epidermal growth factor receptor‐mutant non‐small cell lung cancer who develop brain metastases. Int J Radiat Oncol Biol Phys 2016; 95: 673–9. [DOI] [PubMed] [Google Scholar]
- 24. Soria JC, Ohe Y, Vansteenkiste J et al Osimertinib in untreated EGFR‐mutated advanced non‐small‐cell lung cancer. N Engl J Med 2018; 378: 113–25. [DOI] [PubMed] [Google Scholar]
- 25. Ballard P, Yates JW, Yang Z et al Preclinical comparison of osimertinib with other EGFR‐TKIs in EGFR‐mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130–40. [DOI] [PubMed] [Google Scholar]
- 26. Das AK, Sato M, Story MD et al Non‐small‐cell lung cancers with kinase domain mutations in the epidermal growth factor receptor are sensitive to ionizing radiation. Cancer Res 2006; 66: 9601–8. [DOI] [PubMed] [Google Scholar]
- 27. Johung KL, Yao X, Li F et al A clinical model for identifying radiosensitive tumor genotypes in non‐small cell lung cancer. Clin Cancer Res 2013; 19: 5523–32. [DOI] [PubMed] [Google Scholar]
- 28. Khalifa J, Amini A, Popat S et al Brain metastases from NSCLC: Radiation therapy in the era of targeted therapies. J Thorac Oncol 2016; 11: 1627–43. [DOI] [PubMed] [Google Scholar]
- 29. Chinnaiyan P, Huang S, Vallabhaneni G et al Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res 2005; 65: 3328–35. [DOI] [PubMed] [Google Scholar]
- 30. Wang C, Lu X, Lyu Z, Bi N, Wang L. Comparison of up‐front radiotherapy and TKI with TKI alone for NSCLC with brain metastases and EGFR mutation: A meta‐analysis. Lung Cancer 2018; 122: 94–9. [DOI] [PubMed] [Google Scholar]
- 31. Levy A, Faivre‐Finn C, Hasan B et al Diversity of brain metastases screening and management in non‐small cell lung cancer in Europe: Results of the European Organisation for Research and Treatment of Cancer Lung Cancer Group survey. Eur J Cancer 2018; 93: 37–46. [DOI] [PubMed] [Google Scholar]
- 32. Hendriks J, Schoenmaekers JD, Zindler DB et al Safety of cranial radiotherapy concurrent with tyrosine kinase inhibitors in non‐small cell lung cancer patients: A systematic review. Cancer Treat Rev 2015; 41: 634–45. [DOI] [PubMed] [Google Scholar]
- 33. Soffietti R, Kocher M, Abacioglu UM et al A European organisation for research and treatment of cancer phase III trial of adjuvant whole‐brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality‐of‐life results. J Clin Oncol 2013; 31: 65–72. [DOI] [PubMed] [Google Scholar]
- 34. Brown PD, Jaeckle K, Ballman KV et al Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA 2016; 316: 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sperduto PW, Kased N, Roberge D et al Summary report on the graded prognostic assessment: An accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J Clin Oncol 2012; 30: 419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsao MN, Rades D, Wirth A et al Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence‐based guideline. Pract Radiat Oncol 2012; 2: 210–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sperduto PW, Shanley R, Luo X et al Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole‐brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1‐3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 2014; 90: 526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aoyama H, Tago M, Shirato H et al Japanese Radiation Oncology Study GroupI. stereotactic radiosurgery with or without wholebrain radiotherapy for brain metastases: Secondary analysis of the JROSG 99‐1 randomized clinical trial. JAMA Oncol 2015; 1: 457–64. [DOI] [PubMed] [Google Scholar]
- 39. Sperduto PW, Yang TJ, Beal K et al Estimating survival in patients with lung cancer and brain metastases: An update of the Graded Prognostic Assessment for lung cancer using molecular markers (Lung‐molGPA). JAMA Oncol 2017; 3: 827–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Forest plot of hazard ratios (HR) for time to treatment failure (TTF) by baseline characteristics in EGFR‐mutant NSCLC patients with brain metastases who received EGFR tyrosine kinase inhibitors with upfront radiotherapy (RT) and EGFR tyrosine kinase inhibitors without upfront RT as first‐line therapy. ECOG‐PS, Eastern Cooperative Oncology Group performance status; KPS, Karnofsky performance status; BM, brain metastasis; DS‐GPA, diagnosis‐specific Graded Prognostic Assessment; TKI, tyrosine kinase inhibitor; RT, radiotherapy; HR, hazard ratio; CI, confidence interval.
Figure S2 Kaplan‐Meier analysis of time to treatment failure, CNS‐PFS, and overall survival comparing in the patients treated with upfront brain STI, in those treated with upfront WBRT and in those treated without upfront radiotherapy (A, B, and C). CNS, central nervous system; PFS, progression‐free survival; STI, stereotactic irradiation; WBRT, whole brain radiotherapy; TKI, tyrosine kinase inhibitor.


