Abstract
Recent clinical trials have demonstrated that anti‐PD‐1 blocking antibodies showed remarkable clinical efficacy in a subset of non‐small cell lung cancer (NSCLC) patients. Clinical trials usually exclude patients with renal dysfunction who are receiving hemodialysis (HD). Therefore, it is unclear whether these patients can be safely and effectively treated with pembrolizumab. Here, we present a non‐small cell lung cancer patient on HD who achieved complete remission after one dose of pembrolizumab without severe adverse events. We assessed pembrolizumab binding to peripheral blood T cells in this patient using a method that we recently developed. This is the first report to visualize pembrolizumab binding to T cells in a patient on HD during and after pembrolizumab treatment. The pharmacokinetics of pembrolizumab in this case were similar to those in patients with normal renal function, suggesting that severe renal dysfunction has little influence on the metabolism of pembrolizumab, and is not a contraindication for anti‐PD‐1 treatment. Immune checkpoint inhibitors, including pembrolizumab, may be a vital therapeutic option for lung cancer patients on HD.
Keywords: Hemodialysis, monitoring, PD‐1 blocking antibody, pembrolizumab
Introduction
Recent clinical trials demonstrated that PD‐1 blockade showed high clinical efficacy in patients with non‐small cell lung cancer (NSCLC) who had a high PD‐L1 tumor proportion score.1, 2, 3, 4 Clinical trials generally exclude patients with renal dysfunction who are receiving hemodialysis (HD), and it is therefore unclear whether these patients can be safely and effectively treated with anti‐PD‐1 therapy.
In this article we describe a NSCLC patient on HD who was treated with pembrolizumab. An obvious clinical response was obtained after only one treatment. The therapeutic effect was maintained and no recurrence had occurred at 50 weeks after discontinuation of treatment.
We have previously developed and reported a method to monitor binding of the anti‐PD‐1 antibody nivolumab to T cells in NSCLC patients.5 The same method could be used to detect pembrolizumab since both anti‐PD‐1 antibodies comprise fully humanized IgG4 and interfere with recognition by the PD‐1‐detecting antibody EH12.1.6, 7 In this study, we monitored pembrolizumab binding to peripheral blood T cells in a patient on HD, and found that the immunokinetics of pembrolizumab were similar to those in control patients with normal renal function.
Case report
A 72‐year‐old male patient was diagnosed with stage IIIB lung adenocarcinoma harboring neither EGFR mutation nor ALK rearrangement. Positron emission tomography (PET) showed a primary lesion in the right lower lobe and metastases to multiple lymph nodes, including the right supraclavicular lymph node (Fig 1). Although this patient was ineligible for cytotoxic chemotherapy due to anemia and HD,8 and could not undergo radiotherapy due to the large irradiated area in the lung, he was eligible to receive anti‐tumor treatment. PD‐L1 evaluation was performed by immunohistochemistry using the 22C3 antibody, and a biopsy sample showed a PD‐L1 tumor proportion score of 80%. Based on this clinical background, intravenous pembrolizumab 200 mg was administered as first‐line therapy. Three weeks after the first injection, he developed mild ileus and aspiration pneumonia which resolved with conservative treatment. The treatment was discontinued because immune‐related adverse events9 could not completely be ruled out as a cause of his condition. Despite the fact that the patient received only a single dose of pembrolizumab, his clinical response was maintained and follow‐up positron emission tomography/computed tomography revealed complete metabolic remission10 at 50 weeks after the dose (Fig 1). During his clinical course, peripheral blood was analyzed at three time points: at pretreatment, eight and 24 weeks after the injection. We previously developed a method to monitor nivolumab binding to T cells after discontinuation of treatment.5 This method was available for monitoring pembrolizumab binding in this patient. Briefly, we prepared two types of antibodies for the analysis: the first, EH12.1, binds to PD‐1 expressed on T cells, and the second, HP6025, is an anti‐IgG4 antibody identifying the PD‐1‐blocking antibodies consisted of humanized IgG4, nivolumab and pembrolizumab. EH12.1 recognizes a similar epitope as nivolumab and pembrolizumab. After treatment, EH12.1 does not detect PD‐1 expressed on T cells if PD‐1 is completely blocked by therapeutic antibodies, whereas HP6025 detects nivolumab and pembrolizumab is bound to T cells. This method simply identified the status of pembrolizumab binding to T cells in this patient. The binding status was classified as complete binding (CB), partial binding (PB), or no binding (NB).5 In this patient, T cells at eight and 24 weeks after injection showed CB and NB, respectively (Fig 2). We compared the immunokinetics of pembrolizumab binding in the current patient with that in a control group consisting of five lung adenocarcinoma patients with normal renal function who were treated with one to four doses of pembrolizumab (Fig 3a). Follow‐up in controls was performed between nine and 25 weeks after pembrolizumab discontinuation. One representative control patient showed decreased CB (red) and an absolute loss of CB at 25 weeks after the final dose (Fig 3b). The other four patients showed a similar trend in decreased CB, with an absolute CB loss at around 20–25 weeks (Fig 3c).
Figure 1.
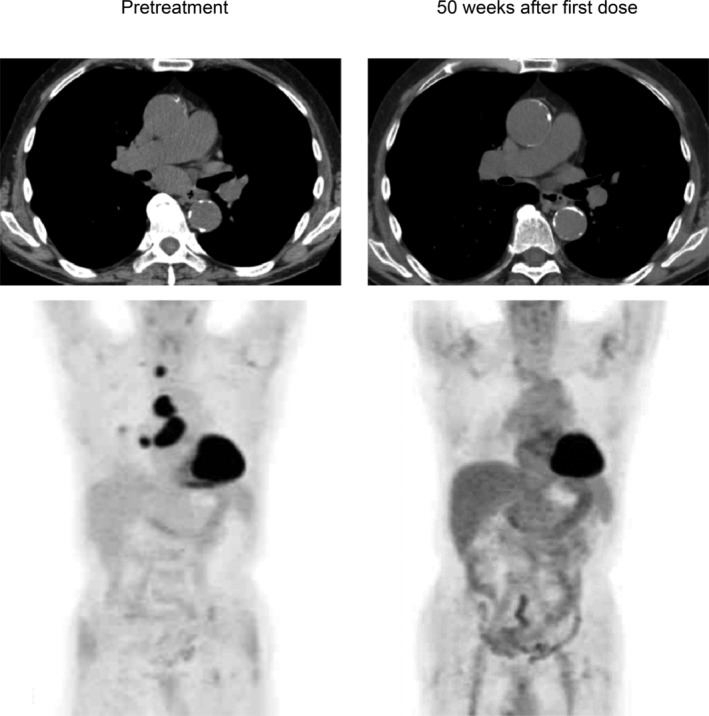
Axial computed tomography (CT) (upper lane) and positron emission tomography/CT images (lower lane) at indicated time points.
Figure 2.
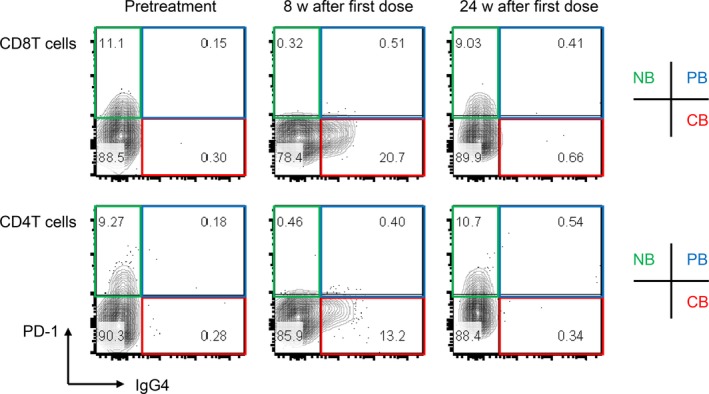
Staining of PD‐1 and IgG4 in blood CD8 and CD4 T cells from the patient on hemodialysis. Flow cytometry analysis was performed at pretreatment (pre) and at eight weeks and 24 weeks after discontinuation of pembrolizumab. CB, complete binding (red); NB, no binding (green); PB, partial binding (blue).
Figure 3.
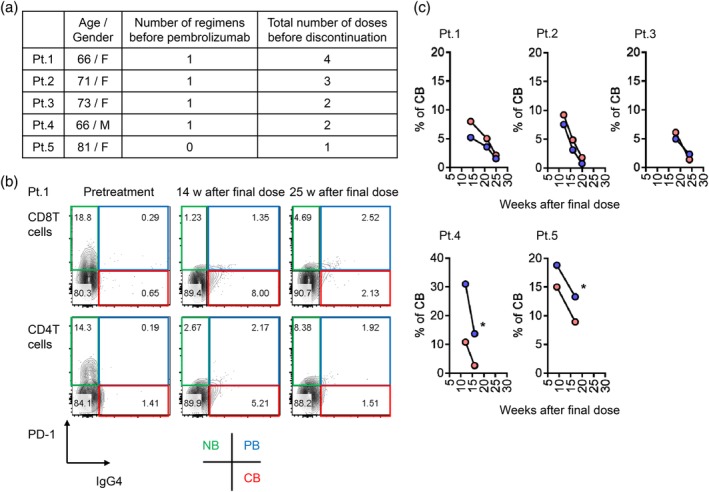
(a) Characteristics of control lung adenocarcinoma patients with normal renal function. (b) Representative staining results examining time‐dependent changes in pembrolizumab binding to T cells after drug discontinuation. Flow cytometry analysis was performed to evaluate PD‐1 and IgG4 staining in blood CD8 and CD4 T cells from patient 1 (Pt. 1). (c) The percent of complete binding of pembrolizumab in CD8 and CD4 T cells was followed up in five NSCLC patients (*follow‐up discontinued due to hospital change or death). ( ) CD8 T cells and (
) CD8 T cells and ( ) CD4 T cells.
) CD4 T cells.
Discussion
Few case reports have reported the successful administration of anti‐PD‐1 antibodies in cancer patients receiving HD.11, 12, 13 Here, we present a patient on HD who achieved complete remission after one dose of the anti‐PD‐1 antibody pembrolizumab, without severe adverse events. Renal impairment reportedly has little effect on the pharmacokinetics of pembrolizumab.14 However, no studies have visualized anti‐PD‐1 antibody binding to T cells after anti‐PD‐1 antibody discontinuation in patients on HD. Monitoring of antibody binding to T cells in the patient in this case revealed that CB was maintained at eight weeks and binding was completely lost at 24 weeks after a single pembrolizumab injection. Similar immunokinetics were confirmed in control patients treated with a small number of pembrolizumab doses, suggesting that HD had little effect on pembrolizumab metabolism, though the time points of sample collection could not be standardized among individuals. Importantly, clinical efficacy in this case was maintained independently of pembrolizumab binding at ≥24 weeks after one dose,15 suggesting that either T cell immune surveillance began to function efficiently after treatment or viable cancer cells were cleared before pembrolizumab binding was lost.
Pembrolizumab may be a vital treatment option for lung cancer patients on HD who have no contraindications. Moreover, our monitoring strategy for anti‐PD‐1 antibody binding in patients on HD could be useful for understanding the metabolism of anti‐PD‐1 antibodies and the maintenance of anti‐tumor T cell immunity after complete loss of antibody binding.
Disclosure
The authors do not report any conflict of interest.
References
- 1. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366: 2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 3. Reck M, Rodriguez‐Abreu D, Robinson AG et al Updated analysis of KEYNOTE‐024: Pembrolizumab versus platinum‐based chemotherapy for advanced non‐small‐cell lung cancer with PD‐L1 tumor proportion score of 50% or greater. J Clin Oncol 2019; 37: 537–46. [DOI] [PubMed] [Google Scholar]
- 4. Mok TSK, Wu YL, Kudaba I et al Pembrolizumab versus chemotherapy for previously untreated, PD‐L1‐expressing, locally advanced or metastatic non‐small‐cell lung cancer (KEYNOTE‐042): A randomised, open‐label, controlled, phase 3 trial. Lancet 2019; 393: 1819–30. [DOI] [PubMed] [Google Scholar]
- 5. Osa A, Uenami T, Koyama S et al Clinical implications of monitoring nivolumab immunokinetics in non‐small celllung cancer patients. JCI Insight 2018; 3: 59125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kamphorst AO, Pillai RN, Yang S e a. Proliferation of PD‐1+ CD8 T cells in peripheral blood after PD‐1‐targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017; 114: 4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelba H, Bochem J, Pawelec G, Garbe C, Wistuba‐Hamprecht K, Weide B. Accurate quantification of T‐cells expressing PD‐1 in patients on anti‐PD‐1 immunotherapy. Cancer Immunol Immunother 2018; 67: 1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Funakoshi T, Horimatsu T, Nakamura M et al Chemotherapy in cancer patients undergoing haemodialysis: A nationwide study in Japan. ESMO Open 2018; 3: e000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and tolerability of PD‐1/PD‐L1 inhibitors compared with chemotherapy in patients with advanced cancer: A meta‐analysis. Oncologist 2017; 22: 470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hicks RJ. The role of PET in monitoring therapy. Cancer Imaging 2005; 5: 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ansari J, Ali M, Farrag A, Ali AM, Alhamad A. Efficacy of nivolumab in a patient with metastatic renal cell carcinoma and end‐stage renal disease on dialysis: Case report and literature review. Case Reports Immunol 2018; 2018: 1623957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlo MI, Feldman DR. Response to nivolumab in a patient with metastatic clear cell renal cell carcinoma and end‐stage renal disease on dialysis. Eur Urol 2016; 70: 1082–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang R, Shirai K. Safety and efficacy of pembrolizumab in a patient with advanced melanoma on haemodialysis. BMJ Case Rep 2016; bcr2016216426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahamadi M, Freshwater T, Prohn M e a. Model‐based characterization of the pharmacokinetics of pembrolizumab: A humanized anti‐PD‐1 monoclonal antibody in advanced solid tumors. CPT Pharmacometrics Syst Pharmacol 2017; 6: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garon EB, Hellmann MD, Rizvi NA et al Five‐year overall survival for patients with advanced nonsmall‐cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE‐001 study. J Clin Oncol 2019; Jco1900934. [DOI] [PMC free article] [PubMed] [Google Scholar]


