Abstract
Aims/Introduction
The severity of insulin resistance is higher in Japanese‐American people with American lifestyles than in native Japanese people with Japanese lifestyles. Recently, the role of gut microbiota in the control of host metabolic homeostasis and organ physiology has been recognized. In addition, gut microbiota alterations have been suggested to contribute to pathogenesis of insulin resistance. The principle aim of the present study was to evaluate the impact of a Westernized lifestyle on the gut microbiota of Japanese‐Americans versus native Japanese, and its correlation with insulin resistance.
Materials and Methods
A total of 14 native Japanese men living in Hiroshima, Japan, and 14 Japanese‐American men living in Los Angeles, USA, were included. A 75‐g oral glucose tolerance test was carried out for all participants to assess their glucose tolerance, and normal glucose tolerance was observed. We compared the insulin response with oral glucose load, the Matsuda Index, and the composition of the gut microbiota between the native Japanese and Japanese‐American men.
Results
Japanese‐American men showed higher area under the curve values for serum insulin concentrations during the oral glucose tolerance test and lower Matsuda Index than native Japanese men. Gut microbiota composition of the Japanese‐American men was different; in particular, they showed a relatively lower abundance of Odoribacter than native Japanese men. The ratio between relative abundance of Odoribacter and Matsuda Index was positively correlated between the two groups.
Conclusions
Our findings suggest that Westernized lifestyles alter gut microbiota, and its alteration might induce insulin resistance in non‐diabetic Japanese men.
Keywords: Gut microbiota, Insulin resistance, Lifestyle Westernization
Introduction
Japanese‐American people, while being of the same race as native Japanese people, lead a different lifestyle. Thus, they form an adequate population that can be used for investigating the effects of a Westernized lifestyle on the development of lifestyle‐related metabolic diseases among Japanese people. We undertook a medical survey targeting Japanese people, and their descendants, who emigrated to the USA (the Hawaii‐Los Angeles‐Hiroshima Study) since 19701. It was inferred that Japanese‐Americans with an American lifestyle in Hawaii or Los Angeles had a higher degree of insulin resistance2, 3, as well as a higher prevalence of diabetes mellitus and metabolic syndrome4, 5, 6 than native Japanese with a Japanese lifestyle in Hiroshima.
Recently, gut microbiota has been regarded as an essential organ, playing critical roles in controlling host metabolic homeostasis and organ physiology. In addition, gut microbiota alterations have been suggested to contribute to the pathogenesis of insulin resistance7, 8. Gut microbiota vary with race, diet and lifestyle9, 10, 11. Lifestyles impact the gut microbiota of people of different races living in different countries12, 13, 14, 15. However, there have been no reports regarding the alteration of gut microbiota by the westernized lifestyle in the same race and the correlation with insulin resistance.
We hypothesized that the alteration of gut microbiota by the westernized lifestyle might correlate with insulin resistance. In the present study, we compared the gut microbiota of native Japanese and Japanese‐American men to investigate the impact of a Westernized lifestyle on gut microbiota, and its correlation with insulin resistance.
Methods
Participants
The study participants comprised of 14 native Japanese men living in Hiroshima, Japan, who were enrolled in medical surveys carried out from April 2016 to March 2017, and 14 Japanese‐American men living in Los Angeles, USA, who were enrolled in medical surveys carried out in August 2015. The inclusion criteria were as follows: (i) aged ≥45 years to ≤75 years; and (ii) normal glucose tolerance, which was defined as a fasting serum glucose level <110 mg/dL (6.1 mmol/L) and 2‐h post‐load serum glucose level <140 mg/dL (7.8 mmol/L) after a 75‐g oral glucose tolerance test (OGTT). The exclusion criteria were as follows: (i) history of inflammatory bowel disease; and (ii) treatment with antibiotics in the 3 months before fecal sampling. After receiving an explanation of the study procedures, each participant provided written informed consent. This study was approved by the ethics committee of Hiroshima University (No. E‐139), and carried out in accordance with the Declaration of Helsinki.
Anthropometric data and biochemical analysis
Each participant was interviewed, and underwent physical measurements and venous blood sampling after an overnight fast. The collected blood samples were centrifuged, immediately frozen and stored until analysis. Serum glucose levels were measured by the hexokinase method. Enzyme immunoassay was used to measure serum immunoreactive insulin (IRI). Serum total cholesterol and triglyceride levels were assessed by an enzymatic method. High‐density lipoprotein cholesterol levels were measured by a homogenous assay. The Matsuda Index was determined as previously described3 and used as an index of insulin resistance. We chose the Matsuda Index, which reflects whole‐body insulin sensitivity, instead of the homeostasis model assessment of insulin resistance, which reflects hepatic insulin sensitivity16, as an index of insulin resistance, because gut microbiota is considered to be linked to whole‐body insulin sensitivity17, 18.
Nutritional evaluation
The dietary intake and dietary habits of all the participants were assessed by a nutritional assessment questionnaire during the examination period. Experienced dietitians analyzed nutrient composition using the nutritional management software, Chatty (Total Software Corp., Kagoshima, Japan). The dietary intake of macronutrients, saturated fatty acid, polyunsaturated fatty acid (PUFA; all expressed as the percentage of total energy intake) and fiber (g/day) were calculated.
Gut microbiota analysis
Fecal samples placed in clean, dry, screw‐top collection vials and returned within 24 h after collection were stored immediately at −80°C for gut microbiota analyses. Samples were placed in an insulated container with dry ice for transfer to the laboratory. Total deoxyribonucleic acid extraction from frozen fecal samples was carried out using a QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA, USA) as previously described19. Primer pairs, 341F and 785R, were used to amplify the V3‐V4 region of the 16S ribosomal ribonucleic acid gene as previously described20. Then, amplicons were purified, indexed and sequenced on an Illumina MiSeq platform (Illumina Incorporated, San Diego, CA, USA) following the 16S Metagenomic Sequencing Library Preparation protocol. After sequencing, the reads were quality‐trimmed using the CLC Genomics Workbench 7.5.3 (CLC Bio, Aarhus, Denmark). Quantitative Insights Into Microbial Ecology (v1.8.0)21 was used to analyze the 16S ribosomal ribonucleic acid sequence. Sequences were quality filtered using split_libraries_fastq.py Quantitative Insights Into Microbial Ecology script. USEARCH version 6.1 was used to carry out chimera detection22. Operational taxonomic unit data were generated using the UCLUST method with a 97% confidence threshold and classified taxonomically using the GreenGenes version 13.823. Subsequent analyses of diversity were carried out at a depth of 10,000 sequences per sample. Alpha diversity was computed using the Shannon Index. Beta diversity was estimated using weighted UniFrac analysis followed by a principle coordinate analysis. The relative abundance of bacterial groups between each group was calculated using Quantitative Insights Into Microbial Ecology.
Analyses of short‐chain fatty acids in feces
The concentration of short‐chain fatty acids in feces was measured by TechnoSuruga Laboratory Co., Ltd. (Shizuoka, Japan)24.
Statistical analysis
Data are described as the mean ± standard deviation, for normally distributed data, and median (25th–75th percentile) for data with skewed distribution. Because of the skewed distribution of the data for triglycerides, the area under the curve for serum IRI during the OGTT, the Matsuda Index and the relative abundance of some gut microbiota, these parameters were analyzed after logarithmic transformation. We carried out an unpaired Student's t‐test to analyze differences in the parameters between the two groups, and Pearson's correlation analysis to assess the correlation between age, body mass index (BMI), Matsuda Index, ratio of food intake and gut microbiota at genus level with relative abundance >0.1%. Heatmaps were generated by using the heatmap.2 function of the gplots package in R (version 3.1.3; R Foundation for statistical Computing, Vienna, Austria). Furthermore, we carried out single and multiple regression analyses to assess the associations between the Matsuda Index and age, BMI, and the relative abundance of gut microbiota. A P‐value <0.05 was considered to show statistical significance. IBM SPSS Statistics for Mac, version 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analyses.
Results
Table 1 shows the clinical characteristics of the study participants. The Japanese‐American men were older and had a lower ratio of PUFA intake than the native Japanese men. No significant differences were observed in BMI, glucose levels during the OGTT and fecal short‐chain fatty acids concentrations between the two groups.
Table 1.
Baseline characteristics of participants
| Native Japanese | Japanese‐American | P‐value | |
|---|---|---|---|
| n | 14 | 14 | |
| Age (years) | 52.9 ± 4.4 | 62.2 ± 6.6 | <0.001 |
| BMI (kg/m2) | 22.3 ± 2.3 | 24.4 ± 3.9 | 0.102 |
| SBP (mmHg) | 131.8 ± 15.7 | 135.2 ± 20.8 | 0.627 |
| DBP (mmHg) | 81.4 ± 14.6 | 83.5 ± 9.2 | 0.657 |
| Total cholesterol (mg/dL) | 184.7 ± 29.4 | 204.2 ± 23.8 | 0.065 |
| HDL cholesterol (mg/dL) | 58.5 ± 13.4 | 59.4 ± 17.5 | 0.876 |
| Triglyceride (mg/dL) | 97.5 (57.0–125.5) | 97.0 (48.3–134.0) | 0.945 |
| Fasting glucose (mg/dL) | 92.0 ± 9.8 | 92.6 ± 6.9 | 0.860 |
| 1‐h glucose (mg/dL) | 136.1 ± 38.8 | 156.3 ± 28.2 | 0.129 |
| 2‐h glucose (mg/dL) | 112.5 ± 19.5 | 102.4 ± 28.2 | 0.281 |
| Total energy intake (kcal/day) | 2319 ± 682 | 2557 ± 817 | 0.409 |
| Ratio of carbohydrate intake (%TE) | 53.8 ± 2.8 | 48.1 ± 12.6 | 0.117 |
| Ratio of fat intake (%TE) | 28.4 ± 2.8 | 34.9 ± 13.7 | 0.104 |
| Ratio of SFA intake (%TE) | 8.2 ± 0.6 | 10.4 ± 4.2 | 0.072 |
| Ratio of PUFA intake (%TE) | 6.3 ± 1.1 | 5.4 ± 1.0 | 0.041 |
| Ratio of protein intake (%TE) | 12.8 ± 1.4 | 12.3 ± 2.9 | 0.566 |
| Dietary fiber intake (g/day) | 13.2 ± 5.6 | 16.4 ± 6.5 | 0.169 |
| Fecal SCFAs (μmol/g) and pH | |||
| Acetic acid | 52.1 ± 25.7 | 71.3 ± 40.1 | 0.144 |
| Propionic acid | 16.5 ± 9.7 | 20.1 ± 13.8 | 0.427 |
| Butyric acid | 11.2 ± 8.2 | 21.3 ± 20.1 | 0.101 |
| pH | 6.8 ± 0.7 | 6.5 ± 0.6 | 0.236 |
Data are presented as number, mean ± standard deviation or median (25th–75th percentile levels). %TE, percentage of total energy; BMI, body mass index; DBP, diastolic blood pressure; HDL, high‐density lipoprotein; PUFA, polyunsaturated fatty acid; SBP, systolic blood pressure; SCFA, short chain fatty acid; SFA, saturated fatty acid.
Insulin responses of native Japanese and Japanese‐American men to oral glucose load were compared (Figure 1). The 60‐min IRI after OGTT and the area under the curve for serum IRI during the OGTT values were higher in Japanese‐American men than in native Japanese men (P = 0.004; Figure 1a,b). Furthermore, the Matsuda Index was lower in Japanese‐American men than in native Japanese men (Figure 1c).
Figure 1.
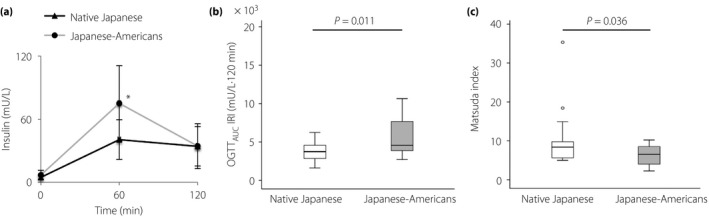
Insulin response after oral glucose load and insulin resistance in native Japanese and Japanese‐American men. (a) Serum immunoreactive insulin (IRI) levels during the oral glucose tolerance test (OGTT) in the native Japanese and Japanese‐American men. (b) Summary of the differences in the area under the curve for serum IRI during the OGTT (OGTT AUC IRI) values between the native Japanese and Japanese‐American men. (c) Matsuda Index of the native Japanese and Japanese‐American men. Data are presented as the mean ± standard deviation. The line in the middle of the box indicates the median value; the box extends from the 25th–75th percentiles. *Statistical significance P < 0.05.
Next, the gut microbiota compositions of the two groups were compared (Figure 2). The alpha diversity of the gut microbiota estimated by the Shannon Index was not significantly different between the two groups (Figure 2a). Principle coordinate analysis after weighted UniFrac analysis showed separate clustering of groups (Figure 2b), and an analysis of multi‐response permutation procedures showed significant differences between the groups (A = 0.02, significance of delta = 0.04). Taxonomic analyses showed that the percentage of the Bacteroidetes was lower in the Japanese‐American men than in native Japanese men (P = 0.044), whereas the percentage of Firmicutes was higher in Japanese‐American men than in native Japanese men (Figure 2c); however, this difference was not statistically significant (P = 0.076). Subsequently, the genus of bacteria that had different distributions in the native Japanese and Japanese‐American men was identified. As a result, the percentages of the 16S ribosomal ribonucleic acid gene sequences representing Collinsella within the phylum Actinobacteria, and Parabacteroides and Odoribacter within the phylum Bacteroidetes were lower in the Japanese‐American men than native Japanese men, whereas Faecalibacterium within the phylum Firmicutes were higher (Figure 2d).
Figure 2.
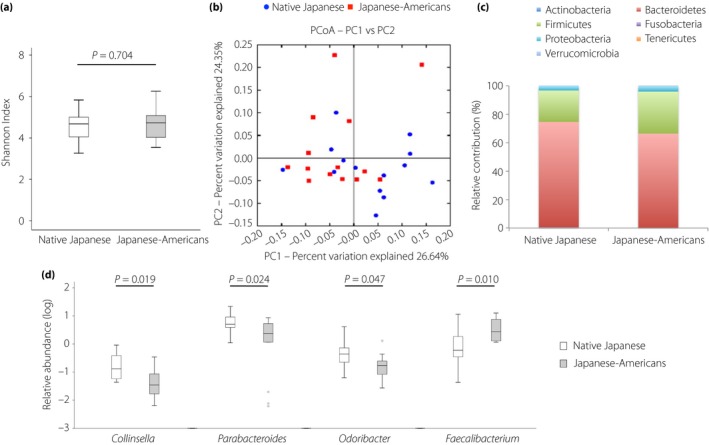
Difference in the gut microbiota compositions between the native Japanese and Japanese‐American men. (a) Alpha diversity as assessed by the Shannon Index for the fecal microbiota between the native Japanese and Japanese‐American men. (b) Principal coordinates analysis (PCoA) based on the weighted UniFrac distances for the fecal microbiota between the native Japanese and Japanese‐American men. (c) Relative abundance of phyla in fecal samples between the native Japanese and Japanese‐American men. (d) Relative abundance of genus in fecal samples between the native Japanese and Japanese‐American men. Data are presented as the mean ± standard deviation. The line in the middle of the box indicates the median value; the box extends from the 25th–75th percentiles.
Finally, the relationship between insulin resistance and the gut microbiota was investigated. Heat map showed that the Matsuda Index was positively correlated with the relative abundance of Odoribacter in both groups (Figure 3a,b). There was no correlation between age, BMI and the relative abundance of Odoribacter in two groups. Then, we investigated the associations between the gut microbiota and nutrition. The relative abundance of Odoribacter was correlated negatively with the total energy intake, and positively with the ratio of PUFA intake in Japanese‐American men, but not in native Japanese men (Figure S1a,b). There was no correlation between the ratio of PUFA intake and Matsuda Index in two groups (data not shown).
Figure 3.
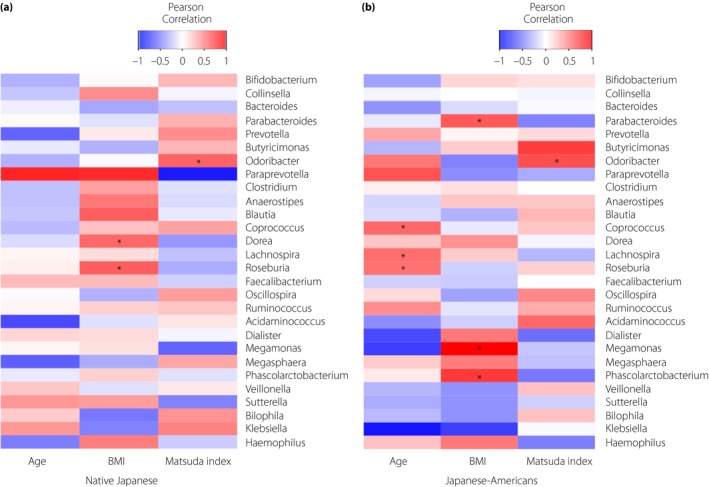
Heat map showing Pearson's correlations between age, body mass index (BMI), the Matsuda Index and gut microbiota at the genus level with relative abundance >0.1% in the (a) native Japanese men and (b) Japanese‐American men. *Statistical significance P < 0.05.
Multiple regression analyses showed that the relative abundance of Odoribacter was a factor that positively explained the Matsuda Index, after adjusting for age and BMI in native Japanese men (β = 0.556, P = 0.006), but this association disappeared after adjusting for age and BMI in Japanese‐American men (β = 0.621, P = 0.108; Table S1).
Discussion
In the present study, we compared the gut microbiota of the two cohorts of Japanese participants who were of the same race but lived in different countries, and investigated alterations in their gut microbiota and its resulting correlation with insulin resistance. We found that the gut microbiota composition in the Japanese‐American men differed from that in the native Japanese men; in particular, there was lower relative abundance of Odoribacter. The degree of insulin resistance was higher in the Japanese‐American men than in native Japanese men. The ratio between relative abundance of Odoribacter and the Matsuda index was positively correlated between the two groups. The results of the present study show that leading a Westernized lifestyle might induce insulin resistance through alterations in the gut microbiota in Japanese people.
The composition of the gut microbiota in the Japanese‐American men was different from that observed in the native Japanese men. Previous studies have shown that a Westernized lifestyle decreased alpha diversity12, 13, 14, 15. A higher intake of dietary fiber in non‐Westernized societies is considered to contribute to higher alpha diversity25. In the present study, no significant difference in the alpha diversity was observed between the two groups, and decreasing dietary fiber intake in the Japanese population26 might have contributed to the lack of significant difference seen for alpha diversity in this study.
The abundance profiles of bacterial taxa differed between the two groups. At the phylum level, previous reports have shown that people with a Westernized lifestyle had increased proportions of Firmicutes and decreased proportions of Bacteroidetes12, 14, which is in accordance with the results of the present study. In this study, Bacteroidetes, not Firmicutes, predominated, which differed from previous observations in Japanese participants27, 28. This difference between studies might be due to the difference in factors including BMI and physical activity of the participants, and methods such as bacterial deoxyribonucleic acid extraction from feces and next‐generation sequencing. At the genus level, those with a Westernized lifestyle had consistently increased proportions of Faecalibacterium, Ruminococcus, Bifidobacterium, Bacteroides and Blautia, and decreased proportions of Prevotella 12, 13, 14, 15. In accordance with previous reports, we found an increased proportion of Faecalibacterium in the Japanese‐American men compared with the native Japanese men, but no other differences in genera were observed in the present study. Previous reports have compared the gut microbiota of individuals in non‐industrialized societies with those with a westernized lifestyle, such as comparing gut microbiota of Europeans and with to that of children in Burkina Faso12, children and adults in Malawi and Amazonian Americans13, adult Hadza hunter‐gatherers in Tanzania14, and adult Papua New Guineans15. Differences in the alteration of gut microbiota in the present study compared with the previous reports might be influenced by ethnicity and the levels of urbanization29, 30.
Subsequently, a notable finding of the present study was the decreased proportion of Odoribacter observed in the Japanese‐American men compared with native Japanese men, and that the relative abundance of this gut microbiota was positively correlated to the Matsuda Index in both groups. To date, no study has focused on the relationship between gut microbiota and insulin resistance in Japanese populations. The abundance of Prevotella copri was found to be positively correlated to insulin resistance in non‐diabetic Danish individuals31, and the Bacteroidetes‐to‐Firmicutes ratio was inversely correlated to peripheral insulin sensitivity in non‐diabetic European men in a study from the Netherlands32. To the best of our knowledge, the correlation between Odoribacter and insulin resistance has not been reported on so far. Taking into consideration that fecal metagenomic markers for type 2 diabetes mellitus were different between European and Chinese individuals33, the gut microbiota that correlate to insulin resistance might vary by race, lifestyle and geographical location.
On estimating the role of Odoribacter in insulin sensitivity, it was found that Odoribacter produced butyrate34. Oral butyrate treatment was reported to improve insulin sensitivity in healthy lean men35. In the present study, there was no difference in the fecal butyrate concentration between the two groups, and thus butyrate might not have been the main contributor to the differences in the insulin resistance. Odoribacter is also reported to produce sulfobacin B36; sulfobacin B treatment is reported to suppress lipopolysaccharide‐induced inflammation in mice37. Considering that the levels of C‐reactive protein, an inflammatory marker, are reportedly lower in native Japanese than in Japanese‐Americans3, Odoribacter‐produced sulfobacin B might exert an anti‐inflammatory effect and partially contribute to improved insulin sensitivity.
Finally, the ratio of PUFA intake is shown to be less in Japanese‐American men than in native Japanese men (P = 0.041; Table 1). Considering our previous report that the ratio of serum eicosapentaenoic acid to arachidonic acid was lower in Japanese‐American than native Japanese38, Japanese‐Americans possibly have a lower intake of PUFA, especially omega‐3 fatty acids, than native Japanese. The Western diet is characterized by high intake of simple carbohydrates, and saturated and omega‐6 fatty acids, and low intake of omega‐3 fatty acids and fiber39, 40, 41. In the present study, Japanese‐American men showed a higher trend in the ratio of SFA intake and less ratio of PUFA intake than native Japanese men, which could be indicators of a Westernized lifestyle. In this study, the relative abundance of Odoribacter was correlated positively with the ratio of PUFA in Japanese‐American men, but not in native Japanese men (Figure S1a,b). There was no report about the relationship between PUFA intake and gut microbiota in Japanese. In Western countries, there was also no report about the relationship between PUFA intake and Odoribacter 42. These data suggest that gut microbiota response to PUFA intake could also vary by race and lifestyle.
The present study had several limitations. First, the participants in this study were limited to native Japanese men and Japanese‐American men participating in one medical survey with a sample size that was relatively small. To ensure reproducible results, a larger study trial is required. In addition, considering that men and women have different gut microbiota, and the fact that the impact on insulin sensitivity associated with it also varies32, we restricted the study to only men. Further studies that include women are required to understand the differences of the impact of a Westernized lifestyle on the gut microbiota of both sexes and its correlation with insulin resistance. Second, the present study comprised only Japanese people. While elucidating the alteration of gut microbiota according to a Westernized lifestyle in the same race and identifying a correlation with insulin resistance is a strong point of this study, the results cannot be generalized to other ethnic groups. Finally, this was a cross‐sectional study, thus, causal relationships could not be observed. To investigate the mechanism by which Westernized lifestyles alter the gut microbiota and subsequently induce insulin resistance, future experiments, such as serum or fecal metabolomics, are necessary.
In conclusion, the present study showed that Japanese men with Westernized lifestyles had altered gut microbiota, and this correlated with insulin resistance in those without diabetes. Thus, these gut microbiota alterations might be involved in the pathogenesis of insulin resistance. Lifestyle‐related diseases, such as type 2 diabetes mellitus, are increasing worldwide, especially in Asia. The present study could provide additional insights into the pathogenesis of Westernized lifestyle‐related metabolic disorders, and might offer novel biomarkers and therapeutic targets in patients with those disorders.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1 | Heat map showing Pearson's correlations between the ratio of food intake and gut microbiota at the genus level with relative abundance >0.1% in the (a) native Japanese men and (b) Japanese‐American men.
Table S1 | Relationship of the Matsuda Index with each parameter: age, body mass index, and Odoribacter by single and multiple regression analyses.
Acknowledgments
We are grateful to the members of the Hiroshima Kenjin‐kai Association of Southern California for their participation. We thank Dr Chikako Ito from Grand Tower Medical Court and Dr Ken Okusaki from Mihara Medical Association Hospital for carrying out the sample collection from native Japanese participants. We extend our gratitude to the Analysis Center of Life Science and the Natural Science Center for Basic Research and Development, Hiroshima University, for the use of their facilities. We also thank TechnoSuruga Laboratory Co., Ltd. for carrying out the short‐chain fatty acids measurements in feces. This work was financially supported by the Japan Society for the Promotion of Science KAKENHI Grant JP16K19538, and Grants for young researchers from the Japan Association for Diabetes Education and Care.
J Diabetes Investig 2019; 10: 1463–1470
References
- 1. Kawate R, Yamakido M, Nishimoto Y, et al Diabetes mellitus and its vascular complications in Japanese migrants on the Island of Hawaii. Diabetes Care 1979; 2: 161–170. [DOI] [PubMed] [Google Scholar]
- 2. Nakanishi S, Okubo M, Yoneda M, et al A comparison between Japanese‐Americans living in Hawaii and Los Angeles and native Japanese: the impact of lifestyle westernization on diabetes mellitus. Biomed Pharmacother 2004; 58: 571–577. [DOI] [PubMed] [Google Scholar]
- 3. Kubota M, Yoneda M, Maeda N, et al Westernization of lifestyle affects quantitative and qualitative changes in adiponectin. Cardiovasc Diabetol 2017; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hara H, Egusa G, Yamakido M, et al The high prevalence of diabetes mellitus and hyperinsulinemia among the Japanese‐Americans living in Hawaii and Los Angeles. Diabetes Res Clin Pract 1994; 24(Suppl): S37–S42. [DOI] [PubMed] [Google Scholar]
- 5. Yoneda M, Yamane K, Jitsuiki K, et al Prevalence of metabolic syndrome compared between native Japanese and Japanese‐Americans. Diabetes Res Clin Pract 2008; 79: 518–522. [DOI] [PubMed] [Google Scholar]
- 6. Shiwa M, Yoneda M, Nakanishi S, et al Japanese lifestyle during childhood prevents the future development of obesity among Japanese‐Americans. PLoS ONE 2015; 10: e0120804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bouter KE, van Raalte DH, Groen AK, et al Role of the gut microbiome in the pathogenesis of obesity and obesity‐related metabolic dysfunction. Gastroenterology 2017; 152: 1671–1678. [DOI] [PubMed] [Google Scholar]
- 8. Okubo H, Nakatsu Y, Kushiyama A, et al Gut microbiota as a therapeutic target for metabolic disorders. Curr Med Chem 2018; 25: 984–1001. [DOI] [PubMed] [Google Scholar]
- 9. Chen L, Zhang YH, Huang T, et al Gene expression profiling gut microbiota in different races of humans. Sci Rep 2016; 6: 23075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu GD, Chen J, Hoffmann C, et al Linking long‐term dietary patterns with gut microbial enterotypes. Science 2011; 334: 105–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kau AL, Ahern PP, Griffin NW, et al Human nutrition, the gut microbiome and the immune system. Nature 2011; 474: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Filippo C, Cavalieri D, Di Paola M, et al Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 2010; 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yatsunenko T, Rey FE, Manary MJ, et al Human gut microbiome viewed across age and geography. Nature 2012; 486: 222–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schnorr SL, Candela M, Rampelli S, et al Gut microbiome of the Hadza hunter‐gatherers. Nat Commun 2014; 5: 3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Martinez I, Stegen JC, Maldonado‐Gomez MX, et al The gut microbiota of rural papua new guineans: composition, diversity patterns, and ecological processes. Cell Rep 2015; 11: 527–538. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999; 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 17. Carvalho BM, Saad MJ. Influence of gut microbiota on subclinical inflammation and insulin resistance. Mediators Inflamm 2013; 2013: 986734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (Bethesda) 2016; 31: 283–293. [DOI] [PubMed] [Google Scholar]
- 19. Mirsepasi H, Persson S, Struve C, et al Microbial diversity in fecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes 2014; 7: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kishida Y, Okubo H, Ohno H, et al Effect of miglitol on the suppression of nonalcoholic steatohepatitis development and improvement of the gut environment in a rodent model. J Gastroenterol 2017; 52: 1180–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caporaso JG, Kuczynski J, Stombaugh J, et al QIIME allows analysis of high‐throughput community sequencing data. Nat Methods 2010; 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010; 26: 2460–2461. [DOI] [PubMed] [Google Scholar]
- 23. McDonald D, Price MN, Goodrich J, et al An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J 2012; 6: 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamamoto B, Suzuki Y, Yonezu T, et al Cha‐Koji, comprising green tea leaves fermented with Aspergillus luchuensis var kawachii kitahara, increases regulatory T cell production in mice and humans. Biosci Biotechnol Biochem 2018; 2: 1–8. [DOI] [PubMed] [Google Scholar]
- 25. Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota‐accessible carbohydrates. Cell Metab 2014; 20: 779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nakaji S, Sugawara K, Saito D, et al Trends in dietary fiber intake in Japan over the last century. Eur J Nutr 2002; 41: 222–227. [DOI] [PubMed] [Google Scholar]
- 27. Nishijima S, Suda W, Oshima K, et al The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res 2016; 23: 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takagi T, Naito Y, Inoue R, et al Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J Gastroenterol 2019; 54: 53–63. [DOI] [PubMed] [Google Scholar]
- 29. Gupta VK, Paul S, Dutta C. Geography, ethnicity or subsistence‐specific variations in human microbiome composition and diversity. Front Microbiol 2017; 8: 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mancabelli L, Milani C, Lugli GA, et al Meta‐analysis of the human gut microbiome from urbanized and pre‐agricultural populations. Environ Microbiol 2017; 19: 1379–1390. [DOI] [PubMed] [Google Scholar]
- 31. Pedersen HK, Gudmundsdottir V, Nielsen HB, et al Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016; 535: 376–381. [DOI] [PubMed] [Google Scholar]
- 32. Most J, Goossens GH, Reijnders D, et al Gut microbiota composition strongly correlates to peripheral insulin sensitivity in obese men but not in women. Benef Microbes 2017; 8: 557–562. [DOI] [PubMed] [Google Scholar]
- 33. Karlsson FH, Tremaroli V, Nookaew I, et al Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013; 498: 99–103. [DOI] [PubMed] [Google Scholar]
- 34. Gomez‐Arango LF, Barrett HL, McIntyre HD, et al Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 2016; 68: 974–981. [DOI] [PubMed] [Google Scholar]
- 35. Bouter K, Bakker GJ, Levin E, et al Differential metabolic effects of oral butyrate treatment in lean versus metabolic syndrome subjects. Clin Transl Gastroenterol 2018; 9: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Walker A, Pfitzner B, Harir M, et al Sulfonolipids as novel metabolite markers of Alistipes and Odoribacter affected by high‐fat diets. Sci Rep 2017; 7: 11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jun Maeda MN, Takikawa H, Yoshida H, et al Inhibitory effects of sulfobacin B on DNA polymerase and inflammation. Int J Mol Med 2010; 26: 751–758. [DOI] [PubMed] [Google Scholar]
- 38. Yoneda M, Kubota M, Shiwa M, et al Impact of Lifestyle Changes on Japanese‐Americans: Results from the Hawaii‐Los Angeles– Hiroshima Study. J Endocrinol Diabetes Obes 2014; 2: 1019. [Google Scholar]
- 39. Zimmet P, Alberti KGMM, Shaw J. Global and societal implications of the diabetes epidemic. Nature 2001; 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 40. Myles IA. Fast food fever: reviewing the impacts of the Western diet on immunity. Nutr J 2014; 13: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Statovci D, Aguilera M, MacSharry J, et al The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol 2017; 8: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costantini L, Molinari R, Farinon B, et al Impact of omega‐3 fatty acids on the gut microbiota. Int J Mol Sci 2017; 18: E2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Heat map showing Pearson's correlations between the ratio of food intake and gut microbiota at the genus level with relative abundance >0.1% in the (a) native Japanese men and (b) Japanese‐American men.
Table S1 | Relationship of the Matsuda Index with each parameter: age, body mass index, and Odoribacter by single and multiple regression analyses.


