Abstract
Adipose tissue (AT) is composed not only of adipocytes, but also of macrophages, endothelial cells and preadipocytes. Macrophages are an important component of AT, and are involved in tissue homeostasis, tissue repair and fibrosis. AT‐resident macrophages are categorized into two subtypes, the M1‐like and M2‐like macrophages. M2‐like macrophages are reported to play anti‐inflammatory roles, and to be involved in clearing and removal of dying/dead adipocytes, and recruiting adipocyte progenitors (APs). M2‐like macrophages are also reported to be involved in the promotion of fibrosis in a transforming growth factor‐β‐dependent manner. However, the precise roles of M2‐like macrophages in the AT have not yet been clearly delineated. Recently, we generated genetically engineered transgenic mice in which CD206+ M2‐like macrophages can be conditionally depleted. Unexpectedly, we found that the depletion of CD206+ M2‐like macrophages resulted in the enhanced generation of smaller adipocytes, improved insulin sensitivity and proliferation of APs. We further clarified that the CD206+ M2‐like macrophages directly interact with the APs to regulate their growth/differentiation and adipogenesis, thereby controlling adiposity and systemic insulin sensitivity. In the present review, we discuss how CD206+ M2‐like macrophages provide a niche for APs and maintain glucose homeostasis.
Keywords: Adipocyte progenitors, Adipose tissue niche, M2‐like macrophages
Introduction
Recently, the prevalence of obesity has been increasing, posing a burden on the health of the population worldwide1. The increase in the prevalence of obesity has been attributed to an increasingly sedentary lifestyle and Westernization of the diet. Obesity or adipose tissue growth in response to a positive energy balance can occur through two distinct mechanisms: increase in the size of existing adipocytes (hypertrophy) or increase in the number of adipocytes (hyperplasia)2, 3, 4. The present authors, as well as others, have previously shown that the size of adipocytes is inversely related to the insulin sensitivity; namely, insulin resistance is associated with larger adipocytes, and insulin sensitivity with smaller adipocytes5, 6. When the pool size of adipocyte progenitors (APs) is sufficiently large, the adipose tissue undergoes hyperplasia through enhanced recruitment of APs that differentiate into smaller adipocytes in response to energy excess, thereby maintaining insulin sensitivity. When the pool size of APs is small, excess nutrients are taken up by the existing adipocytes, eventually resulting in an increase in the size of the cells, generation of reactive oxygen species, recruitment of macrophages and induction of inflammation, thereby leading to insulin resistance. It would appear that whether adipose tissue (AT) undergoes hyperplasia or hypertrophy depends on the pool size of the APs. However, the cellular and molecular pathways that control the pool size of the APs, thus regulating the adipocyte size and number in vivo, are still largely unknown.
AT contains several cell types, including APs, endothelial cells, fibroblasts, immune cells and so on, that interact with each other to maintain tissue homeostasis4, 7, 8. AT niches might regulate the APs pool by expressing some adhesion molecules or other factors to maintain AT homeostasis and tissue repair8, 9, 10. Although AT‐resident macrophages are considered to be metabolically beneficial macrophages to preserve adipocyte health in a lean state, they are also involved in AT remodeling during progression of obesity11. AT‐resident macrophages are categorized into two subtypes, namely, (M1‐like) pro‐inflammatory macrophages, which cause AT hypertrophy and worsen insulin resistance, and (M2‐like) anti‐inflammatory macrophages, which improve insulin sensitivity12, 13, 14. In addition, they play a role in adapting to excess energy states, because M2‐like macrophage dysfunction caused by genetic disruption of the M2 gene results in metabolic disorders under high‐fat diet conditions that are probably attributable to their anti‐inflammatory functions15. It has also been reported that M2‐like macrophages are involved in the removal of dying adipocytes4, 16, 17, recruitment of new progenitors17, 18, and promotion of white and beige adipocyte differentiation under certain conditions3, 17, 19, thus maintaining adipose tissue homeostasis and systemic insulin sensitivity.
Previous studies reported that M1‐like pro‐inflammatory macrophages regulate the expression of angiogenic genes in preadipocytes20, 21, suggesting the existence of direct interactions between macrophages and APs. It is still unknown how proliferation and differentiation of APs are regulated by M2‐like macrophages within the white adipose tissue (WAT) to control insulin sensitivity. We recently showed that conditional depletion of CD206+ M2‐like macrophages resulted in proliferation and differentiation of APs, increased the number of smaller adipocytes with upregulated expression of metabolically favorable genes, and the insulin‐sensitive phenotype in vivo 5. We further clarified that CD206+ M2‐like macrophages in adipose tissue constitute a microenvironment that inhibits proliferation of APs by secreting transforming growth factor (TGF)‐β.
Generation of CD206DTR Transgenic Mice
To investigate the role of M2‐like macrophages in the maintenance of adipose tissue health, we attempted to generate transgenic mice in which M2‐like macrophages could be transiently depleted at specific time‐points. To achieve this goal, we first attempted to identify a specific marker of adipose tissue M2‐like macrophages. We showed that almost all CD206+ cells are positive for F4/80+, showing that CD206 is a specific marker for M2‐like AT‐resident macrophages, as shown by flow cytometric analyses of the epididymal WAT (eWAT) of lean mice. Next, we generated genetically engineered CD206DTR mice based on transgenic expression of the diphtheria toxin receptor (DTR) under the control of the Mrc1 promoter, to enable specific ablation of the CD206+ M2‐like macrophages by administering diphtheria toxin (DT) (Figure 1a). The site‐specific chromosomal integration of the DTR‐expressing unit was confirmed by Southern blotting, and expression of the DTR protein in the eWAT was determined by immunofluorescence confocal imaging and western blotting. Administration of DT successfully ablated CD206+ cells in the adipose tissue of the CD206DTR mice without affecting the overall health of the mice (Figure 1b). Thus, CD206 represents an ideal marker to target M2‐like macrophages in the adipose tissue.
Figure 1.
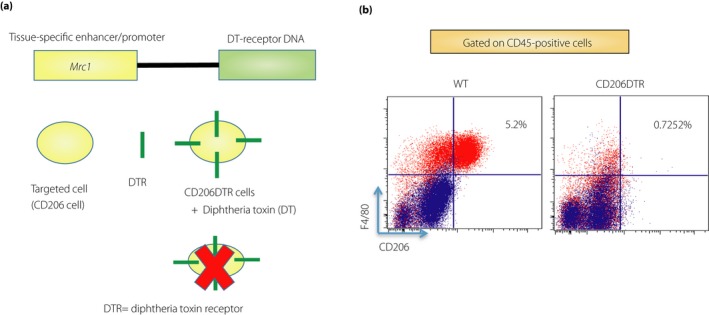
Generation of CD206DTR transgenic mice. (a) We generated genetically engineered CD206DTR based on the transgenic expression of diphtheria toxin receptor (DTR) under the control of the Mrc1 promoter to specifically ablate CD206+ M2‐like macrophages. Administration of diphtheria toxin (DT) successfully ablated CD206‐positive cells in adipose tissue of CD206DTR mice without affecting the overall health of the mice. (b) Representative flow cytometry analysis of F4/80 and CD206 expression (M2‐like) in the CD45+ stromal vascular fraction of epididymal white adipose tissue from wild‐type (WT) and CD206DTR mice. M2‐like macrophages were significantly reduced in CD206DTR mice.
Depletion of CD206+ M2‐like Macrophages Improved the Insulin Sensitivity
Obesity is associated with phenotypic transformation of anti‐inflammatory M2‐like macrophages to pro‐inflammatory M1‐like macrophages, thereby causing insulin resistance13. As M2‐like macrophages are considered to be metabolically beneficial macrophages, as compared with the pro‐inflammatory M1‐like macrophages12, 22, it was expected that depletion of CD206+ M2‐like macrophages might impair glucose metabolism. Surprisingly, however, the glucose tolerance and insulin sensitivity actually improved in the CD206+ M2‐like macrophage‐depleted mice. This unexpected finding was indeed found to be a specific outcome of depletion of the CD206+ M2‐like macrophages, as a glucose tolerance test carried out in the absence of DT administration showed no difference between the WT and CD206DTR mice. We observed enhanced phosphorylation of Akt in the eWAT, liver and skeletal muscle, consistent with the improved insulin sensitivity in the CD206+ M2‐macrophage‐depleted mice. Then, we evaluated the physiology of the adipose tissue by examining the size and number of adipocytes. In the CD206+ M2‐like macrophage‐depleted mice, the adipocytes in the eWAT were significantly reduced in size, whereas their number was significantly increased. We previously showed that smaller adipocytes were associated with enhanced systemic insulin sensitivity6. The improved insulin sensitivity was associated with increased glucose uptake in eWAT, and reduced gluconeogenesis in the liver and increased glucose disposal in muscle. Depletion of the CD206+ M2‐like macrophages in obese CD206DTR mice also enhanced generation of smaller adipocytes, reduced crown‐like structures and improved systemic insulin sensitivity5. Interestingly, depletion of CD206+ M2‐like macrophages in mice exposed to cold was also associated with further improvement of the insulin sensitivity23. Collectively, we found that depletion of CD206+ M2‐like macrophages was associated with improved glucose metabolism under both the lean and obese states.
CD206+ M2‐like Macrophages Regulate the Proliferation of Adipocyte Progenitors
AT is composed of distinct cell types, besides adipocytes and preadipocytes. The cells that are committed to the adipocyte lineage are termed as preadipocytes, whereas those that are less committed to this lineage and show proliferative capacity are termed as APs. Most APs remain quiescent under the basal condition, and this quiescence might be controlled by various signals from adipocytes and surrounding stromal cells, including endothelial cells, and macrophages. In addition to the increased number of smaller adipocytes, we also found an increase in the stromal vascular fraction population in the eWAT. Next, we examined which type of stromal vascular fraction cells in the eWAT proliferated after depletion of CD206+ M2‐like macrophages. As macrophages are reportedly involved in the regulation of progenitor/stem cell niche activity4, 21, 24, 25, 26, we investigated the possible involvement of CD206+ M2‐like macrophages in the regulation of APs proliferation. Non‐endothelial and non‐hematopoietic (CD31−/CD45−) Sca‐1+/platelet‐derived growth factor receptor α+ (PDGFRα+) populations showed larger increments in the expression levels of cell cycling indicator genes in the CD206+ M2‐like macrophage‐depleted mice, as shown by flow cytometry analysis, indicating that the proliferation of APs was indeed activated in the CD206+ M2‐like macrophage‐depleted mice27, 28. For further confirmation, the fate of the proliferating cells was examined by injecting the mice with 5‐ethynyl‐2′‐deoxyuridine (EdU). Two hours after the EdU injection, EdU+PDGFRα+ cells were detected at higher frequencies in the CD206+ M2‐like macrophage‐depleted mice than in the WT mice. We further found that 96 h post‐EdU injection, EdU+ nuclei were detected in the cells that were positive for perilipin. Thus, depletion of CD206+ M2‐like macrophages promoted the proliferation of APs, which finally differentiated into mature adipocytes.
Involvement of TGF‐β Signaling in CD206+ M2‐like Macrophages‐based Proliferation of APs
Macrophages have been reported to express large amounts of TGF‐β129, 30, 31 and to be involved in inhibiting proliferation/differentiation of various cell types, including adipocytes32, 33, 34, 35. We examined the possible involvement of TGF‐β signaling in the upregulated proliferation of APs in the CD206+ M2‐like macrophage‐depleted mice. Among the genes involved in TGF‐β signaling, TGF‐β1 was specifically upregulated in the CD206+ M2‐like macrophages to the same degree as that of the Mrc1 messenger ribonucleic acid (mRNA). Confocal imaging studies showed co‐localization of the CD206+ M2‐like macrophages with TGF‐β1 in the eWAT. We also showed that CD206+ M2‐like macrophages were even located in close vicinity of the PDGFRα+ APs, consistent with a previous report4. Co‐localization of APs with p27Kip1, a factor downstream of TGF‐β signaling in cell growth inhibition, was also confirmed in the PDGFRα‐CreERT2‐eGFP (PRα) mice. Expression of other factors downstream of TGF‐β signaling was downregulated in the eWAT of the CD206+ M2‐like macrophage‐depleted mice. To validate this hypothesis, we generated another genetically engineered mouse model with specific knockout of TGF‐β1 expression in CD206+ M2‐like macrophages (CD206‐CreERT2/TGF‐β1flox/flox mice). The expressions of the cell cycle‐associated genes were upregulated in the APs of TGF‐β1 knockout mice. We also observed the number of APs was increased in the eWAT of TGF‐β1 knockout mice, supporting our hypothesis that TGF‐β1 expressed in CD206+ M2‐like macrophages is involved in the inhibition of APs proliferation.
To further confirm the impact of TGF‐β signaling, we carried out inhibitor analyses in vitro. We cultured primary adipocytes with M2‐induced bone marrow‐derived macrophages in the presence and absence of LY2109761 (TGF‐βRI/II inhibitor). M2‐induced bone marrow‐derived macrophages inhibited adipogenesis of primary adipocytes, whereas this effect was reversed by LY2109761. We also found that TGF‐β1,2,3 neutralizing antibody (1D11) enhanced proliferation of APs, suggesting that CD206+ M2‐like macrophages suppressed the proliferation of APs and subsequent adipogenesis through a TGF‐β‐dependent manner. In this regard, it is expected that CD206+ M2‐like macrophage‐targeting drugs might be therapeutically effective by breaking the interactions between the M2‐like macrophages and APs.
Depletion of CD206+ M2‐like Macrophages Promotes Browning of White adipocytes
Whether M2‐like macrophages promote or inhibit the proliferation of beige progenitors is still controversial. There are two conflicting reports on the role of M2‐like macrophages in the browning of WAT. Previous reports showed that upon cold exposure, M2‐like macrophages produce catecholamine, hence enhancing browning of WAT3, 19, 36, 37, 38, 39, 40, 41. Whereas a recent study showed that type 2 cytokines/M2‐like macrophages upon cold exposure do not produce catecholamine and have no effect on browning of WAT42, suggesting that M2‐like macrophages do not promote browning of inguinal WAT. Exposure of the mice to a cold environment resulted in enhanced browning of inguinal WAT in CD206+ M2‐like macrophage‐depleted mice23, showing that CD206+ M2‐like macrophages regulate beige adipogenesis, independent of catecholamine synthesis.
Role of CD206+ M2‐like Macrophages in the Regulation of Beige Progenitor's Proliferation
The mechanisms underlying cold‐induced beige adipogenesis is under debate; while some argue that beige adipocytes that are converted into white‐like adipocytes at room temperature turn into beige adipocytes under cold exposure43, 44, others argue that a cold environment stimulates the proliferation of beige progenitors, to increase beige adipogenesis45, 46. Our study showed an increase in the number of Ki‐67+ cells in CD206+ M2‐like macrophage‐depleted mice upon cold exposure, as compared with their control littermates, indicating an increase in the number of proliferating cells in the inguinal WAT of the CD206+ M2‐like macrophage‐depleted mice. Consistent with this, we observed upregulated expression of cell cycle‐related genes in the inguinal WAT of the CD206+ M2‐like macrophage‐depleted mice. We also observed upregulated expression of Cd137 and Tmem26, beige progenitor markers19, 45, 47, in the inguinal WAT of the cold‐stimulated CD206+ M2‐like macrophage‐depleted mice, indicating that partial depletion of CD206+ M2‐like macrophages might regulate the proliferation of brown adipocytes or beige progenitors. Our flow‐cytometric analysis further confirmed a significant increase in the number of CD137+ beige progenitors in the cold‐stimulated CD206+ M2‐like macrophage‐depleted mice, as compared with their control littermates. Altogether, we showed that depletion of CD206+ M2‐like macrophages enhanced proliferation of beige progenitors.
Conclusion
In conclusion, we showed that adipose tissue M2‐like macrophages provide a niche for APs through the TGF‐β pathway, maintaining their pool, preventing their exhaustion or overproliferation. M2‐like macrophages also contribute to the maintenance of functions of APs by placing them in a state of hibernation, to avoid unnecessary cell division that promotes cell senescence (Figure 2). When macrophages are low in number or weak in function, it is possible that the pool of APs is smaller. In that case, animals might suffer from the lipodystrophic diabetes phenotype of insulin resistance under overnutrition. In fact, Trib1−/− mice, which lack adipose tissue macrophages, developed the lipodystrophic phenotype of insulin resistance15. We presume that the mice develop glucose intolerance essentially in the same way as mice having a genetic defect in the development of M2‐like macrophages, including defects in the interleukin‐4/13‐stat6 pathway, peroxisome proliferator‐activated receptor‐γ pathway, peroxisome proliferator‐activated receptor‐δ pathway and Kruppel‐like factor 4 pathway22, 48, 49, 50. This would also be applicable to ΔdblGATA mice, which lack eosinophils51, 52.
Figure 2.
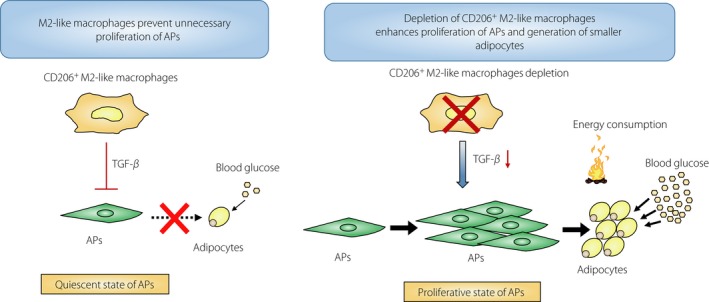
Schematic diagram. M2‐like macrophages prevent unnecessary proliferation of adipocyte progenitors (APs) through transforming growth factor (TGF)‐β signaling. Depletion of CD206+ M2‐like macrophages enhances proliferation of APs and generation of smaller adipocytes.
As shown in Figure 2, M2‐like macrophages prevent unnecessary proliferation of APs by serving as a niche for APs. The niche formed by M2‐like macrophages also responds to the nutritional state of the organism. When animals are fed HFD or overnourished, the M2‐like macrophages–APs interactions might be disrupted as a result of activation of peroxisome proliferator‐activated receptor‐γ in the APs through downregulation of some surface molecules involved in the interactions with the M2‐like macrophages. Then, inhibitory action of CD206+ M2‐like macrophages through TGF‐β is released, and APs start to proliferate and differentiate into adipocytes, thereby mitigating nutritional overload of the existing adipocytes, preventing their further enlargement, reducing oxidative stress and inflammation, a sign of pathological adipose tissue expansion.
It is also a matter of debate as to whether cold exposure induces proliferation and differentiation of beige progenitors into beige adipocytes or makes the whitened beige adipocytes back into beige adipocytes again. Our data are consistent with the former hypothesis, although our findings do not rule out the latter hypothesis. How do CD206+ M2‐like macrophages regulate browning of inguinal WAT? Various studies suggested that M2‐like macrophages promote browning of white adipocytes3, 36, 38, 39, 41. Our findings that depletion of M2‐like macrophages induces browning of white adipocytes seem contradictory. However, if we hypothesized that M2‐like macrophages also serve as a niche for beige progenitors, we are able to reconcile our findings with the previous reports. Thus, when the number and functions of M2‐like macrophages are well preserved, and the pool size of white and beige progenitors is sufficient, the animals might show healthy adipose tissue expansion, but not pathological expansion, under the HFD condition. Our “niche theory” explains the homeostatic functions of the M2‐like macrophages in WAT for metabolic adaptations to excessive nutrient intake, which has long remained a mystery (Figure 3).
Figure 3.
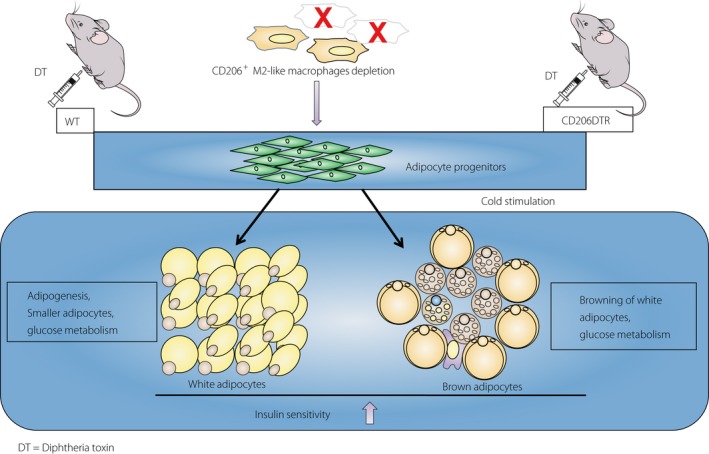
Summary. Depletion of CD206+ M2‐like macrophages induces white and beige progenitors proliferation and improves insulin sensitivity. DT, diphtheria toxin; WT, wild type.
Disclosure
Kazuyuki Tobe received research grants from Japan Diabetes Foundation, The Uehara Memorial Foundation, The Naito Foundation, The Mitsubishi Foundation, (Translational Research program; Strategic PRomotion for practical application of INnovative medical Technology [TR‐SPRINT] from) Japan Agency for Medical Research and Development, AMED, AstraZeneca K.K., Merck & Co., Inc., Medical Review Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Novo Nordisk Pharma, Ltd., Kowa Pharmaceutical Co., Ltd., Astellas Pharma Inc., Fuji Chemical Industries Co., Ltd., Japan Eli Lilly Co., Ltd., Akurey Marketing Co., Ltd, Sanofi Co., Ltd., Daiichi Sankyo Co., Ltd., MSD Co., Ltd., Asahi Kasei Pharma Co., Ltd., Teijin Pharma Co., Ltd., Japan Boehringer Ingelheim Co., Ltd. and Ono Pharmaceutical Co., Ltd. AN was supported by Grants‐in‐Aid for the Japan Society for the Promotion of Science (JSPS) Fellow (18F18102 to TK and AN).
Acknowledgments
We thank all members of our laboratory of the First Department of Internal Medicine, Faculty of Medicine for their valuable suggestions and support provided for finalization of this review. AN and KT wrote the manuscript. The content of this article was finalized according to the opinions of both authors.
J Diabetes Investig 2019; 10: 1394–1400
Contributor Information
Allah Nawaz, Email: nawaz@med.u-toyama.ac.jp.
Kazuyuki Tobe, Email: tobe@med.u-toyama.ac.jp.
References
- 1. Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and management of obesity. N Engl J Med 2017; 376: 254–266. [DOI] [PubMed] [Google Scholar]
- 2. Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annu Rev Pathol 2011; 6: 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qiu Y, Nguyen KD, Odegaard JI, et al Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014; 157: 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee Y‐H, Petkova Anelia P, Granneman James G. Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 2013; 18: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nawaz A, Aminuddin A, Kado T, et al CD206 + M2‐like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun 2017; 8: 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okuno A, Tamemoto H, Tobe K, et al Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest 1998; 101: 1354–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol 2008; 9: 11–21. [DOI] [PubMed] [Google Scholar]
- 8. Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids 2005; 73: 9–15. [DOI] [PubMed] [Google Scholar]
- 9. Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132: 598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scadden DT. The stem‐cell niche as an entity of action. Nature 2006; 441: 1075–1079. [DOI] [PubMed] [Google Scholar]
- 11. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujisaka S, Usui I, Bukhari A, et al Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet‐induced obese mice. Diabetes 2009; 58: 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Science 2013; 339: 172–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh T, Kidoya H, Naito H, et al Critical role of Trib1 in differentiation of tissue‐resident M2‐like macrophages. Nature 2013; 495: 524–528. [DOI] [PubMed] [Google Scholar]
- 16. Fischer‐Posovszky P, Wang QA, Asterholm IW, et al Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology 2011; 152: 3074–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YH, Thacker RI, Hall BE, et al Exploring the activated adipogenic niche: interactions of macrophages and adipocyte progenitors. Cell Cycle 2014; 13: 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee YH, Petkova AP, Mottillo EP, et al In vivo identification of bipotential adipocyte progenitors recruited by beta3‐adrenoceptor activation and high‐fat feeding. Cell Metab 2012; 15: 480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee MW, Odegaard JI, Mukundan L, et al Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 2015; 160: 74–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest 2011; 121: 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takikawa A, Mahmood A, Nawaz A, et al HIF‐1alpha in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 2016; 65: 3649–3659. [DOI] [PubMed] [Google Scholar]
- 22. OdegaardJI RG, Goforth MH. Macrophage‐specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007; 447: 1116–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Igarashi Y, Nawaz A, Kado T, et al. Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci Rep 2018; 8: 14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Soucie EL, Weng Z, Geirsdóttir L, et al Lineage‐specific enhancers activate self‐renewal genes in macrophages and embryonic stem cells. Science 2016; 351: aad5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tang W, Zeve D, Suh JM, et al White fat progenitor cells reside in the adipose vasculature. Science 2008; 322: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cinti S, Mitchell G, Barbatelli G, et al Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005; 46: 2347–2355. [DOI] [PubMed] [Google Scholar]
- 27. Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo . Nat Cell Biol 2013; 15: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo . Cell 2008; 135: 240–249. [DOI] [PubMed] [Google Scholar]
- 29. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009; 27: 451–483. [DOI] [PubMed] [Google Scholar]
- 31. Lech M, Anders HJ. Macrophages and fibrosis: How resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta 2013; 1832: 989–997. [DOI] [PubMed] [Google Scholar]
- 32. Ignotz RA, Massague J. Type beta transforming growth factor controls the adipogenic differentiation of 3T3 fibroblasts. Proc Natl Acad Sci U S A 1985; 82: 8530–8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nishimura EK, Suzuki M, Igras V, et al Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell 2010; 6: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamazaki S, Ema H, Karlsson G, et al Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 2011; 147: 1146–1158. [DOI] [PubMed] [Google Scholar]
- 35. Margoni A, Fotis L, Papavassiliou AG. The transforming growth factor‐beta/bone morphogenetic protein signalling pathway in adipogenesis. Int J Biochem Cell Biol 2012; 44: 475–479. [DOI] [PubMed] [Google Scholar]
- 36. Lee YH, Kim SN, Kwon HJ, et al Adipogenic role of alternatively activated macrophages in beta‐adrenergic remodeling of white adipose tissue. Am J Physiol Regul Integr Comp Physiol 2016; 310: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chung KJ, Chatzigeorgiou A, Economopoulou M, et al A self‐sustained loop of inflammation‐driven inhibition of beige adipogenesis in obesity. Nat Immunol 2017; 18: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hui X, Gu P, Zhang J, et al Adiponectin enhances cold‐induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab 2015; 22: 279–290. [DOI] [PubMed] [Google Scholar]
- 39. Brestoff JR, Kim BS, Saenz SA, et al Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 2015; 519: 242–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Azua IR, Mancini G, Srivastava RK, et al Adipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophages. J Clin Invest 2017; 127: 4148–4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nguyen KD, Qiu Y, Cui X, et al Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011; 480: 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fischer K, Ruiz HH, Jhun K, et al Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat Med 2017; 23: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rosenwald M, Perdikari A, Rulicke T, et al Bi‐directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667. [DOI] [PubMed] [Google Scholar]
- 44. Barbatelli G, Murano I, Madsen L, et al The emergence of cold‐induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Endocrinol Metab 2010; 298: E1244–E1253. [DOI] [PubMed] [Google Scholar]
- 45. Wu J, Boström P, Sparks LM, et al Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang QA, Tao C, Gupta RK, et al Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013; 19: 1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang W, Kissig M, Rajakumari S, et al Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc Natl Acad Sci 2014; 111: 14466–14471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nelms K, Keegan AD, Zamorano J, et al The IL‐4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol 1999; 17: 701–738. [DOI] [PubMed] [Google Scholar]
- 49. Bouhlel MA, Derudas B, Rigamonti E, et al PPARgamma activation primes human monocytes into alternative M2 macrophages with anti‐inflammatory properties. Cell Metab 2007; 6: 137–143. [DOI] [PubMed] [Google Scholar]
- 50. Odegaard JI, Ricardo‐Gonzalez RR, Eagle AR, et al Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity‐induced insulin resistance. Cell Metab 2008; 7: 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yu C, Cantor AB, Yang H, et al Targeted deletion of a high‐affinity GATA‐binding site in the GATA‐1 promoter leads to selective loss of the eosinophil lineage in vivo . J Exp Med 2002; 195: 1387–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ikutani M, Tsuneyama K, Kawaguchi M, et al Prolonged activation of IL‐5‐producing ILC2 causes pulmonary arterial hypertrophy. JCI Insight 2017; 2: e90721. [DOI] [PMC free article] [PubMed] [Google Scholar]


