Abstract
We report a case of missed diagnosis of maturity‐onset diabetes of the young type 5 uncovered during pregnancy with a previous diagnosis of type 1 diabetes. Maturity‐onset diabetes of the young type 5 cannot be excluded in early‐onset diabetes with positive islet‐related autoantibodies and type 1 diabetes‐prone human leukocyte antigen subtypes. Abdominal ultrasound should be used in all patients with pre‐gestational diabetes, and maturity‐onset diabetes of the young type 5 should be considered when renal abnormality is presented.
Keywords: Abdominal ultrasound, Glutamic acid decarboxylase antibody, Maturity‐onset diabetes of the young type 5 during pregnancy
Introduction
Maturity‐onset diabetes of the young (MODY) is an autosomal dominant form of early‐onset diabetes resulting from heterozygous mutations in various transcription factors and enzymes. It is crucial to make a correct molecular diagnosis, as this allows optimal treatment and genetic counseling. However, many MODY patients are misdiagnosed for a long time. We report a case of missed diagnosis of MODY5 uncovered during pregnancy with a previous diagnosis of type 1 diabetes.
Case Report
A 28‐year‐old female patient was diagnosed with type 1 diabetes at age 16 years with symptoms including polyuria, polydipsia and weight loss. Her body mass index (BMI) was 17.6 kg/m2, and her fasting glucose level was 19 mmol/L. No results on C‐peptides or islet‐related autoantibodies were available. Her glucose was well managed with insulin. She came to the Department of Endocrinology, Peking Union Medical College Hospital, Beijing, China, for preconception counseling. Her mother was diagnosed with type 2 diabetes when aged in her 50s, which was complicated by overweight (BMI 27 kg/m2) and hypertension. Her father and two older sisters were non‐diabetic. A physical examination showed no obvious abnormalities. Laboratory tests showed slightly elevated creatinine (Cr) and uric acid (UA) of 91 μmol/L (normal range 45–84 μmol/L) and 488 μmol/L (normal range 150–357 μmol/L), respectively. Urine protein was absent. Glycated hemoglobin was 5.7%, fasting C‐peptide was 0.19 ng/mL (normal range 0.8–4.2 ng/mL) and 2‐h postprandial C‐peptide was 0.21 ng/mL, with fasting glucose 9.5 mmol/L and 2‐h postprandial glucose 6.3 mmol/L. Although islet cell antibody and insulinoma‐associated antigen‐2 antibody (Ab) were both negative, glutamic acid decarboxylase (GAD) Ab was 16 (normal range <10). The woman successfully became pregnant when aged 30 years. Her glucose was well managed during the pregnancy, with an average insulin dose of 1 U/kg·day in the third trimester. However, during the 21st week of gestation, a fetal ultrasound indicated renal hyperechogenicity (Figure 1). Her Cr and UA levels were also elevated over her pre‐pregnancy levels at 107 and 577 μmol/L, respectively. MODY5 was then suspected. Later, renal ultrasound of the patient showed echo enhancement and multiple renal cysts (Figure 2). A boy was vaginally delivered at 39 + 5 weeks of gestation, with a birthweight of 2,900 g. The physical examination was normal and the neonate experienced neither hypoglycemia nor hyperglycemia. Six weeks after delivery, the patient's Cr and UA returned to pre‐pregnancy levels. Genetic tests were carried out with the patient's and her family members’ written informed consent. The MODY panel sequencing analysis revealed the heterozygous missense mutation c.541C>T (p.Arg181Ter) in exon 2 of the HNF1B gene (NM_000458.3). Deoxyribonucleic acid samples from the patient's child, her two sisters and her parents were all subjected to Sanger sequencing, revealing that her baby carried the same missense mutation. The human leukocyte antigen (HLA) genotype of the proband was shown to be heterozygous DRB1*0901‐DQB1*0303. Ethical approval for this study was given by the ethics committee of Peking Union Medical College Hospital (approval No. S‐K542), and the study conforms to the provisions of the Declaration of Helsinki.
Figure 1.
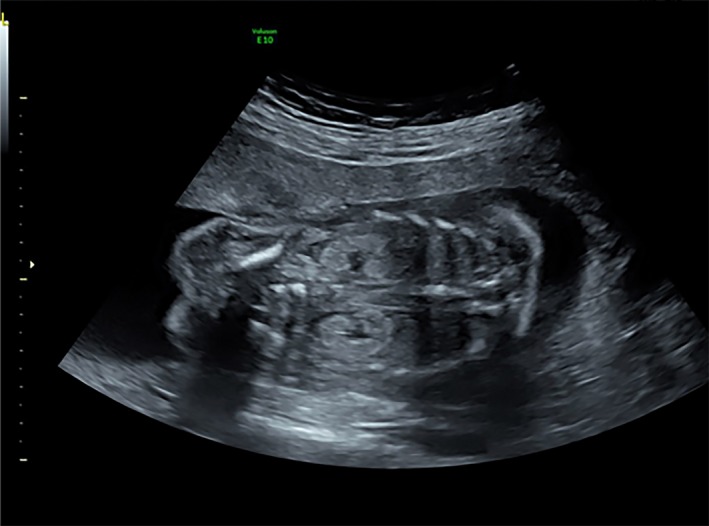
Renal ultrasound showing a hyperechogenic cortex.
Figure 2.
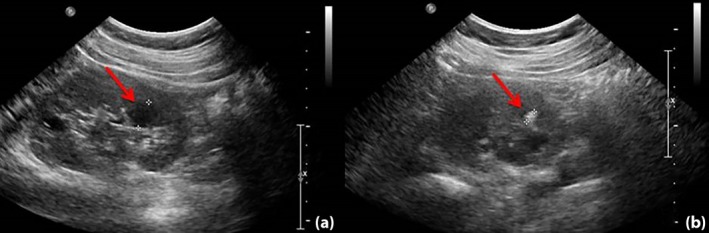
Renal ultrasound showing (a) a hyperechogenic cortex with multiple cysts and (b) suspected nephrocalcinosis or renal calculus.
Discussion
MODY5, also known as renal cysts and diabetes syndrome, has a 50% chance of being passed on to offspring. HNF1B‐related renal dysfunction could present in renal cysts and diabetes syndrome, which would lead to a poor prognosis. As for pregnant MODY5 patients, adverse outcomes during pregnancy could affect both the mothers and their offspring. In the present patient, although her glucose was well controlled, she experienced a higher level of Cr and UA than she did pre‐pregnancy. Preterm, premature rupture of membranes, low birthweight, transient neonatal renal dysfunction and transient neonatal hyperglycemia were recorded in other reports of MODY51, 2, 3, 4. Therefore, a correct diagnosis of MODY5 is of vital importance.
Different from MODY1–3, initiation of insulin is often early in MODY55, which makes it more difficult to be distinguished from type 1 diabetes. In contrast to type 1 diabetes, MODY patients are thought to be negative for islet‐related autoantibodies. Presenting with both positive anti‐GAD Ab and the early need for insulin treatment, the present patient was diagnosed as type 1 diabetes, but not MODY5, even though her Cr had slightly elevated before pregnancy. However, the positivity of autoantibodies might not be as rare as we thought before. Urbanova et al. reported that 25% of MODY1–3 patients examined were positive for anti‐GAD Ab or insulinoma‐associated antigen‐2 Ab6. Marconi reported a case of MODY5 with positive anti‐GAD Ab and insulinoma‐associated antigen‐2 Ab7, indicating that MODY5 could co‐occur with positive islet‐related autoantibodies. The HLA genotype of the present proband was determined to be DRB1*0901‐DQB1*0303, which has been reported as a major susceptible haplotype of type 1 diabetes in Asia8. Nevertheless, similar to the present case, MODY with risk of the HLA genotype has also been reported6, indicating that MODY should not be excluded even in patients with the susceptibility haplotype for type 1 diabetes. In conclusion, MODY5 could co‐exist with positive islet‐related autoantibodies and risk of the HLA subtype, which makes it difficult to be correctly diagnosed.
The deterioration of renal function during pregnancy and the patient's fetus's renal abnormality alerted the clinicians of her diagnosis of MODY5, and her renal ultrasound and genetic analysis confirmed HNF1B mutation (p.Arg181Ter)‐related disease. Renal abnormality is a very commonly observed clinical feature in HNF1B‐associated disease9, and can be detected on antenatal ultrasound as early as the 17th week of gestation in HNF1B affected fetuses10. Thus, abdominal ultrasound is of great value in the diagnosis of MODY5. Furthermore, abdominal ultrasound is cost‐effective and easy to be carried out. Thus, it might be reasonable that all patients with pre‐gestational diabetes should undergo an abdominal ultrasound to avoid missed diagnosis of MODY5.
In conclusion, the present case showed that islet‐related autoantibodies and the susceptibility haplotype for type 1 diabetes could coexist with MODY5, abdominal ultrasound should be carried out for all diabetes patients before pregnancy, and the HNF1B gene should be screened in the presence of renal abnormality.
Disclosure
The authors declare no conflict of interest.
J Diabetes Investig 2019; 10: 1590–1592
References
- 1. Bellanne‐Chantelot C, Chauveau D, Gautier JF, et al Clinical spectrum associated with hepatocyte nuclear factor‐1beta mutations. Ann Intern Med 2004; 140: 510–517. [DOI] [PubMed] [Google Scholar]
- 2. Thirumalai A, Holing E, Brown Z, et al A case of hepatocyte nuclear factor‐1beta (TCF2) maturity onset diabetes of the young misdiagnosed as type 1 diabetes and treated unnecessarily with insulin. J Diabetes 2013; 5: 462–464. [DOI] [PubMed] [Google Scholar]
- 3. Mikuscheva A, Mckenzie E, Mekhail A. 21‐year‐old pregnant woman with MODY‐5 diabetes. Case Rep Obstet Gynecol 2017; 2017: 6431531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Zhao Y, Zhang J, et al A case of a novel mutation in HNF1beta‐related maturity‐onset diabetes of the young type 5 with diabetic kidney disease complication in a Chinese family. J Diabetes Complications 2017; 31: 1243–1246. [DOI] [PubMed] [Google Scholar]
- 5. Dubois‐Laforgue D, Cornu E, Saint‐Martin C, et al Diabetes, associated clinical spectrum, long‐term prognosis, and genotype/phenotype correlations in 201 adult patients with hepatocyte nuclear factor 1B (HNF1B) molecular defects. Diabetes Care 2017; 40: 1436–1443. [DOI] [PubMed] [Google Scholar]
- 6. Urbanova J, Rypackova B, Prochazkova Z, et al Positivity for islet cell autoantibodies in patients with monogenic diabetes is associated with later diabetes onset and higher HbA1c level. Diabet Med 2014; 31: 466–471. [DOI] [PubMed] [Google Scholar]
- 7. Abreu M, Lingvay I. A Novel HNF1B mutation and its associated clinical presentation. Endocrine Rev 2014; 35 (Suppl 3: 0984). [Google Scholar]
- 8. Kawabata Y, Ikegami H, Kawaguchi Y, et al Asian‐specific HLA haplotypes reveal heterogeneity of the contribution of HLA‐DR and ‐DQ haplotypes to susceptibility to type 1 diabetes. Diabetes 2002; 51: 545–551. [DOI] [PubMed] [Google Scholar]
- 9. Horikawa Y. Maturity‐onset diabetes of the young as a model for elucidating the multifactorial origin of type 2 diabetes mellitus. J Diabetes Investig 2018; 9: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bingham C, Ellard S, Allen L, et al Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor‐1 beta. Kidney Int 2000; 57: 898–907. [DOI] [PubMed] [Google Scholar]


