Abstract
Aims/Introduction
Painful diabetic peripheral neuropathy (PDPN) has a significant impact on the patient's quality of life. The prevalence of PDPN in the Middle East and North Africa region has been reported to be almost double that of populations in the UK. We sought to determine the prevalence of PDPN and its associated factors in type 2 diabetes mellitus patients attending secondary care in Qatar.
Materials and Methods
This was a cross‐sectional study of 1,095 participants with type 2 diabetes mellitus attending Qatar's two national diabetes centers. PDPN and impaired vibration perception on the pulp of the large toes were assessed using the Douleur Neuropathique en 4 questionnaire with a cut‐off ≥4 and the neurothesiometer with a cut‐off ≥15 V, respectively.
Results
The prevalence of PDPN was 34.5% (95% confidence interval [CI] 31.7–37.3), but 80% of these patients had not previously been diagnosed or treated for this condition. Arabs had a higher prevalence of PDPN compared with South Asians (P < 0.05). PDPN was associated with impaired vibration perception adjusted odds ratio (AOR) 4.42 (95% CI 2.92–6.70), smoking AOR 2.43 (95% CI 1.43–4.15), obesity AOR 1.74 (95% CI 1.13–2.66), being female AOR 1.65 (95% CI: 1.03–2.64) and duration of diabetes AOR 1.08 (95% CI 1.05–1.11). Age, poor glycemic control, hypertension, physical activity and proteinuria showed no association with PDPN.
Conclusions
PDPN occurs in one‐third of type 2 diabetes mellitus patients attending secondary care in Qatar, but the majority have not been diagnosed. Arabs are at higher risk for PDPN. Impaired vibration perception, obesity and smoking are associated with PDPN in Qatar.
Keywords: Obesity, Painful diabetic peripheral neuropathy, Type 2 diabetes
Introduction
Painful diabetic peripheral neuropathy (PDPN) has a significant impact on the patient's quality of life1, 2, 3, as it is accompanied by depression, anxiety and sleep disturbance2. Estimates of the prevalence of PDPN in patients with type 2 diabetes mellitus vary, and range from 17.9 to 65.3%1, 4, 5, 6. In a large population‐based study (n = 15,692) from the UK4, we previously showed that PDPN occurred in 21.5% of patients with type 2 diabetes mellitus, and was more common in South Asians. In the Middle East and North Africa region, Jambart et al.5 reported a much higher prevalence of PDPN of 61.3% in Egypt, 57.5% in Jordan, 53.9% in Lebanon and 37.1% in the United Arab Emirates.
Despite having a serious impact on the patient's quality of life, PDPN is underdiagnosed and undertreated7, 8. Patients with painful symptoms are often unaware that the pain is related to diabetes, and do not report it to their clinician8, 9. Screening patients at high risk for PDPN should allow timely identification and treatment. Previous studies have shown that older age, a longer duration of diabetes, being female and the presence of diabetic peripheral neuropathy (DPN) increase the risk for PDPN1, 4, 5, 6, 10, 11. Additionally, obesity1, 5, 7, 12, low physical activity13, 14, smoking4, 12, poor glycemic control15, 16, low high‐density lipoprotein (HDL) cholesterol1, raised low‐density lipoprotein (LDL) cholesterol, triglycerides and creatinine13 are also independent risk factors of PDPN.
The aim of the present study was to establish the prevalence of PDPN in patients with type 2 diabetes mellitus in secondary care in Qatar, and explore the association with ethnicity and risk factors for this condition. We undertook a large cross‐sectional cohort study using the Douleur Neuropathique en 4 questionnaire (DN4), a validated, and highly sensitive and specific questionnaire for the diagnosis of PDPN17.
Methods
This was a cross‐sectional cohort study. Patients with diabetes aged ≥18 years were recruited from the two National Diabetes and Endocrine Centers in Doha, Qatar – Hamad General Hospital and Al‐Wakra Hospital. Participating clinicians reported on all patients satisfying the inclusion criteria examined between March 2017 and March 2018. No refusals were recorded, as the procedure was quick, simple and potentially valuable to the patient's health. Participants with other causes of neuropathy including vitamin B12 deficiency, hypothyroidism, HIV infection, leprosy, hepatitis C and chemotherapy were excluded from the study. We enrolled 1,163 individuals, and after excluding 66 patients with type 1 diabetes mellitus and two patients who did not complete the assessments, we were left with a sample size of 1,095.
This study was approved by the institutional review boards of Weill Cornell Medicine‐Qatar and Hamad Medical Corporation, and all participants gave informed consent to take part in the study. The research adhered to the tenets of the declaration of Helsinki.
Demographic and metabolic measures
Age, sex, duration of diabetes, height, weight and body mass index were recorded. Ethnicity was categorized as Qatari Arabs, other Arabs, South Asians and other ethnic groups. The average of two readings of systolic blood pressure and diastolic blood pressure blood pressure taken from the participant's left arm while seated with his/her arm at heart level, using a standard zero mercury sphygmomanometer after 10–15 min of rest. A non‐fasting blood sample of 10 mL was collected through venepuncture from each participant into vacutainer tubes containing ethylenediaminetetraacetic acid. The samples were kept at room temperature and transported within 2 h to a central certified laboratory at Hamad General Hospital, Hamad Medical Corporation, Doha, Qatar. Glycated hemoglobin (HbA1c), total cholesterol, HDL, LDL and triglycerides were measured by an autoanalyzer (Hitachi 747 autoanalyzer; Tokyo, Japan). Urinary albumin and creatinine levels were assessed on a random spot urine sample to evaluate the albumin‐to‐creatinine ratio. Patients with HbA1c ≥9% were considered to be poorly controlled. Hypertension was defined according to either an average systolic blood pressure ≥140 mmHg and/or the use of antihypertensive medication, as described in the World Health Organization/International Society of Hypertension Guidelines18. Current cigarette smoking was defined as having smoked at least one cigarette every day for 30 days preceding the study visit. Physical activity was defined as doing physical activity including walking for ≥30 min in a day at least three times a week. Obesity was classified according to the World Health Organization criteria19, with a body mass index ≥30 kg/m2. Proteinuria was defined as an albumin‐to‐creatinine ratio >30 mg/g.
Painful diabetic peripheral neuropathy assessment
The DN4 questionnaire has been validated for PDPN20, and can distinguish between nociceptive and neuropathic pain21. It consists of 10 questions: seven questions relating to the pain description (burning, painful cold, electric shocks) and associated abnormal sensations (tingling, pins and needles, numbness, itching), and the other three questions relate to a neurological examination in the painful area (hypoesthesia to touch and prick using disposable examination pins, and allodynia to brushing). The scoring is based on a “yes” (1 point) or “no” (0 point) answer, and each question is equally weighted. A score ≥4 has a high sensitivity (80%) and specificity (92%) for PDPN20. The questionnaire was administered by the investigator spoken in either English or Arabic. Previously diagnosed PDPN was self‐reported. Medications for painful neuropathy were recorded.
Impaired vibration perception assessment
Vibration perception threshold (VPT) was measured bilaterally on the pulp of the large toe using a neurothesiometer (Horwell; Scientific Laboratory Supplies, Wilford, UK). The strength of the vibration stimulus was gradually increased from null intensity to a value in voltage at which vibration was first detected by the participant. The test was repeated three times, and the average value was recorded. The range for VPT readings is 1–50 V. Impaired vibration perception was defined as a mean VPT ≥15 V22, 23.
Statistical analysis
Patients’ demographic and clinical characteristics were summarized using means and standard deviations for numeric variables, and frequency distribution for categorical variables. Variables were compared between patients with and without PDPN using an unpaired t‐test or Mann–Whitney test when the distribution was highly skewed for numeric variables, and the χ2‐test or Fisher's exact test when expected cell counts fell <5 for categorical variables.
Binary and multiple logistic regression analysis was carried out with age, duration of diabetes, diabetic neuropathy, sex, poor glycemic control, hypertension, obesity, physical activity, smoking, proteinuria and ethnic groups as independent variables, and PDPN as the dependent variable. The multiple logistic regression model included all variables with P‐value of ≤0.10 at the bivariate level. Adjusted odds ratios and their corresponding 95% confidence intervals are presented.
The demographic and clinical characteristics of the patients were compared between the different ethnic groups using the χ2‐test for categorical variables, such as hypertension and one‐way anova, for numeric variables, such as age. Multiple comparisons when required were carried out using the Bonferroni method.
All analyses were carried out using IBM SPSS (version 23; SPSS Inc., Armonk, NY, USA). A two‐tailed P‐value of ≤0.05 was considered significant.
Results
Prevalence of PDPN
The cohort (n = 1,095) was aged 20–86 years (mean [SD], 54.3 [11.4]), 60.6% were men. The clinical and demographic characteristics of type 2 diabetes mellitus patients with and without PDPN are compared in Table 1. The prevalence of PDPN was 34.5% (95% confidence interval [CI] 31.7–37.3). Of the participants with PDPN, 80.2% had not been previously diagnosed with this condition and 86.0% had not been treated.
Table 1.
Demographic characteristics of adults with type 2 diabetes mellitus stratified by painful diabetic peripheral neuropathy status
| Painful diabetic neuropathy | P‐value | ||
|---|---|---|---|
| No | Yes | ||
| n (%) | 717 (65.5) | 378 (34.5) | NA |
| Age, years (mean ± SD) | 52.6 ± 11.4 | 57.5 ± 10.7 | <0.0001† |
| Sex, n (%) | |||
| Male | 453 (68.7) | 206 (31.3) | <0.01 |
| Female | 261 (60.7) | 169 (39.3) | |
| Diabetes duration, years (mean ± SD) | 8.2 ± 7.0 | 13.6 ± 7.9 | <0.0001† |
| HbA1c (mean ± SD) | |||
| % | 8.0 ± 2.0 | 8.4 ± 2.0 | 0.02 |
| mmol/mol | 64.9 ± 22.3 | 67.9 ± 21.8 | 0.02 |
| Poor glycemic control | |||
| Yes | 174 (60.4) | 114 (39.6) | <0.05 |
| No | 474 (67.6) | 227 (32.4) | |
| Cholesterol, mmol/L (mean ± SD) | 4.5 ± 1.2 | 4.4 ± 1.1 | NS |
| Triglyceride, mmol/L (mean ± SD) | 1.9 ± 1.3 | 1.7 ± 1.0 | NS† |
| HDL, mmol/L (mean ± SD) | 1.3 ± 0.2 | 1.1 ± 0.0 | NS |
| LDL, mmol/L (mean ± SD) | 2.6 ± 0.0 | 2.5 ± 0.0 | NS |
| Systolic blood pressure, mmHg (mean ± SD) | 131.1 ± 17.7 | 135.4 ± 18.3 | <0.001 |
| Diastolic blood pressure, mmHg (mean ± SD) | 78.5 ± 10.5 | 77.6 ± 9.5 | NS |
| Hypertension, n (%) | |||
| Yes | 371 (61.0) | 237 (39.0) | 0.001 |
| No | 294 (71.5) | 117 (28.5) | |
| Weight, kg (mean ± SD) | 83.4 ± 21.4 | 87.6 ± 18.6 | <0.0001† |
| BMI, kg/m2 (mean ± SD) | 30.7 ± 6.8 | 32.7 ± 7.0 | <0.0001 |
| Obesity, n (%) | |||
| Yes | 314 (60.2) | 208 (39.8) | <0.0001 |
| No | 318 (73.3) | 116 (26.7) | |
| Physical activity, n (%) | |||
| Yes | 240 (74.5) | 82 (25.5) | 0.001 |
| No | 330 (63.2) | 192 (36.8) | |
| Smoking, n (%) | |||
| Yes | 107 (69.0) | 48 (31.0) | NS |
| No | 501 (67.2) | 244 (32.8) | |
| Proteinuria, n (%) | |||
| Yes | 33 (51.6) | 31 (48.4) | <0.01 |
| No | 300 (67.1) | 147 (32.9) | |
| Vibration perception threshold, Volts (mean ± SD) | 9.8 ± 7.5 | 17.4 ± 10.6 | <0.0001 |
| Impaired vibration perception, n (%) | |||
| Yes | 126 (39.1) | 196 (60.9) | <0.0001 |
| No | 586 (76.8) | 177 (23.2) | |
| Previously diagnosed with PDPN, n (%) | 28 (4.0) | 73 (19.8) | <0.0001 |
| Treated for PDPN, n (%) | 22 (3.1) | 53 (14.0) | <0.0001 |
| Ethnic groups, n (%) | |||
| Qataris | 181 (54.7) | 150 (45.3) | <0.0001 |
| Other Arabs | 196 (64.3) | 109 (35.7) | |
| South Asians | 299 (74.2) | 104 (25.8) | |
| Others | 41 (73.2) | 15 (26.8) | |
Patients’ demographic and clinical characteristics summarized using means and standard deviations (SD) for numeric variables, and frequency distribution for categorical variables. Continuous parametric and non‐parametric variables were compared using the unpaired t‐test and †Mann–Whitney test, respectively. Categorical variables were compared using the χ2‐test. BMI, body mass index; HbA1c, glycated hemoglobin; NA, not applicable; NS, not significant; PDPN, painful diabetic peripheral neuropathy.
Factors associated with PDPN
Participants with PDPN had a higher mean age (P < 0.0001), duration of diabetes (P < 0.0001), HbA1c (P = 0.02), systolic blood pressure (P < 0.001), weight (P < 0.0001) and body mass index (P < 0.0001), compared with participants without PDPN. VPT was significantly higher (17.4 vs 9.8 V, P < 0.0001). Total cholesterol, triglycerides, HDL, LDL and diastolic blood pressure were comparable between the two groups. A higher percentage of participants with PDPN had impaired vibration perception (60.9 vs 23.2%, P < 0.0001), and a greater proportion were women (39.3 vs 31.3%, P < 0.01), had poorer glycemic control (39.6 vs 32.4%, P < 0.05), hypertension (39.0 vs 28.5%, P = 0.001), proteinuria (48.4 vs 32.9%, P < 0.01) and obesity (39.8 vs 26.7%, P < 0.0001), and a lower percentage of those undertook physical activity (25.5 vs 36.8%, P = 0.001).
Logistic regression analysis showed that five factors were independently and significantly associated with PDPN (Table 2): impaired vibration perception (adjusted odds ratio [AOR] 4.42, 95% CI 2.92–6.70), smoking (AOR 2.43, 95% CI 1.43–4.15), obesity (AOR 1.74, 95% CI 1.13–2.66), being female (AOR 1.65, 95% CI 1.03–2.64) and duration of diabetes (AOR 1.08, 95% CI 1.05–1.11). Age, poor glycemic control, hypertension, physical activity, proteinuria and ethnicity showed no association with PDPN.
Table 2.
Logistic regression analysis between painful diabetic peripheral neuropathy and risk factors
| OR | 95% CI | P‐value | |
|---|---|---|---|
| Age | 1.01 | 0.99–1.03 | NS |
| Duration of diabetes | 1.08 | 1.05–1.11 | <0.0001 |
| Impaired vibration perception | 4.42 | 2.92–6.70 | <0.0001 |
| Female | 1.65 | 1.03–2.64 | <0.05 |
| Poor glycemic control | 1.40 | 0.93–2.11 | NS |
| Hypertension | 1.16 | 0.77–1.76 | NS |
| Obesity | 1.74 | 1.13–2.66 | <0.01 |
| Physical activity | 0.83 | 0.55–1.26 | NS |
| Smoking | 2.43 | 1.43–4.15 | 0.001 |
| Proteinuria | 1.04 | 0.51–2.16 | NS |
| Ethnic groups | |||
| Qataris | 1 | NS | |
| Other Arabs | 1.05 | 0.64–1.73 | NS |
| South Asians | 0.95 | 0.57–1.59 | NS |
| Others | 0.81 | 0.31–2.07 | NS |
Outcome variable: painful diabetic peripheral neuropathy. Independent variables: age, duration of diabetes, impaired vibration perception, female, poor glycemic control, hypertension, obesity, physical activity, smoking, proteinuria and ethnic groups were considered in the fitted model with a P‐value ≤0.05. CI, confidence interval; NS, not significant; OR, odds ratio.
Ethnicity and PDPN
The prevalence of PDPN differed between ethnic groups (Figure 1; Table 3). Qataris (45.3%) and other Arabs (35.7%) had a higher prevalence of PDPN compared with South Asians (25.8%). However, the prevalence of impaired vibration perception was comparable between ethnic groups. The prevalence of obesity was comparable between Qataris (66.8%) and other Arabs (70.9%), but significantly higher than in South Asians (34.2%). The percentage of Qataris (20.8%) and other Arabs (35.5%) who undertook physical activity was significantly lower than in South Asians (54.3%). The percentage of Qataris with proteinuria was significantly higher than in South Asians (9.4 vs 3.0%), and comparable with other Arabs and other ethnicities. Qataris were significantly older than other Arabs, South Asians and other ethnicities (58.2 vs 53.8 vs 51.8 and 52.5 years, respectively), and had a significantly longer duration of diabetes (13.4 vs 9.1 vs 8.1 and 9.9 years, respectively). The percentage of Qataris with hypertension was significantly higher than other Arabs (65.3 vs 53.9%). There were significantly fewer smokers amongst Qataris compared with other Arabs (10.4 vs 23.9%).
Figure 1.
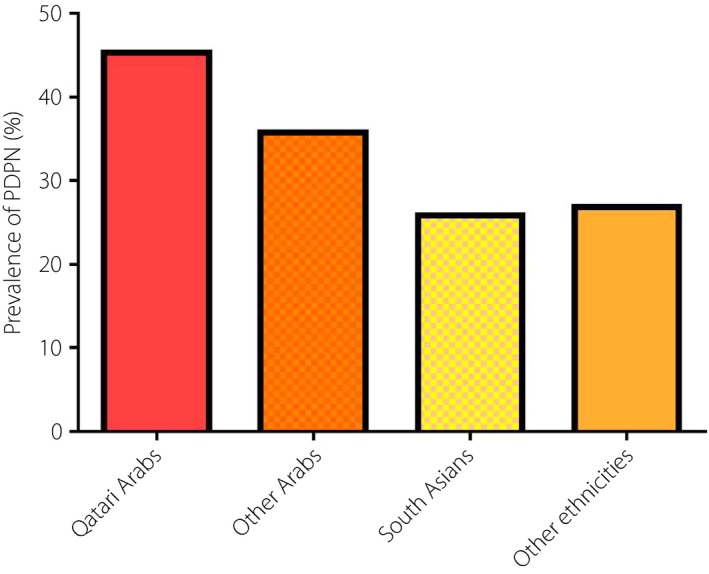
Prevalence of painful diabetic peripheral neuropathy (PDPN) between ethnic groups.
Table 3.
Differences in the prevalence of painful diabetic peripheral neuropathy and other risk factors between different ethnic groups
| Qataris | Other Arabs | South Asians | Others | |
|---|---|---|---|---|
| n | 331 | 305 | 403 | 56 |
| PDPN, n (%) | 150 (45.3)a | 109 (35.7)a | 104 (25.8)b | 15 (26.8)ab |
| Mean age, years (SD) | 58.2 (12.0)a | 53.8 (11.7)b | 51.8 (9.7)b | 52.5 (10.5)b |
| Mean duration of diabetes, years (SD) | 13.4 (7.8)a | 9.1 (7.2)b | 8.1 (7.0)b | 9.9 (8.4)b |
| Impaired vibration perception, n (%) | 108 (33.0)a | 91 (30.0)a | 102 (25.6)a | 21 (37.5)a |
| Female, n (%) | 211 (64.1)a | 109 (35.9)b | 89 (22.3)c | 21 (39.5)bc |
| Poor glycemic control, n (%) | 100 (33.8)a | 86 (31.5)a | 152 (41.4)a | 21 (39.6)a |
| Hypertension, n (%) | 196 (65.3)a | 153 (53.9)b | 229 (59.9)ab | 30 (56.6)ab |
| Obesity, n (%) | 185 (66.8)ab | 188 (70.9)b | 125 (34.2)c | 24 (49.0)ac |
| Physical activity, n (%) | 52 (20.8)a | 87 (35.5)b | 170 (54.3)c | 13 (36.1)abc |
| Smoking, n (%) | 27 (10.4)a | 62 (23.9)b | 57 (16.9)ab | 9 (20.5)ab |
| Proteinuria, n (%) | 31 (9.4)a | 15 (4.9)a,b | 12 (3.0)b | 6 (10.7)a |
a,b,c,dWithin each row, columns with similar letters are not statistically significant and those with different letters are significantly different. PDPN, painful diabetic peripheral neuropathy; SD, standard deviation.
Discussion
This is the first large observational study to establish the prevalence of PDPN and its associated factors in secondary care in Qatar. PDPN occurs in approximately one‐third of patients with type 2 diabetes mellitus; however, alarmingly, four‐fifths had not been previously diagnosed or treated. PDPN, a manifestation of small fiber damage24, 25, 26, occurred in more than one‐quarter of patients without impaired vibration perception, and in half of the patients with impaired vibration perception. Impaired vibration perception, obesity and smoking were associated with PDPN. Arabs also have a higher prevalence of PDPN compared with Asians. This might be attributed to the higher percentage of women and obesity, and a lower percentage undertaking physical activity in the Arab population.
The prevalence of PDPN in type 2 diabetes mellitus patients in Qatar was lower than previous studies from the Middle East and North Africa region, even though they also used the DN4 pain questionnaire, and showed that the prevalence of PDPN was 65.3% in Saudi Arabia6, 61.3% in Egypt5, 57.5% in Jordan, 53.9% in Lebanon, and 37.1% in United Arab Emirates and Kuwait. This difference could be attributed to different populations and control of various risk factors, although age, duration of diabetes and the percentage of those with obesity were comparable to this study. However, the percentage of those with poor glycemic control in Saudi Arabia was higher compared with the current study (59.5 vs 39.6%)27. Poor glycemic control is common in the Middle East27, 28, 29, 30, and has been reported to be a significant risk factor for both DPN and PDPN15, 16. In the UK, the prevalence of PDPN in type 2 diabetes mellitus patients is lower (21.5–26.4%) than in Qatar4, 10, and may be attributed to a lower HbA1c (7.26 vs 8.14%) and shorter duration of diabetes (4–8 vs 10.1 years). One of the earlier UK studies10 was carried out in patients with type 1 diabetes mellitus and type 2 diabetes mellitus in primary care, and the prevalence of PDPN is known to be lower in primary care12 and in type 1 diabetes mellitus patients1, 4, 7.
The physical quality of life of patients with PDPN decreases at a significantly faster rate over 3 years compared with type 2 diabetes mellitus patients without PDPN3. Patients with PDPN are also at high risk for depression, anxiety and sleep disturbance2. However, the underdiagnosis and treatment of PDPN continue to pose a considerable problem for patients. Other studies have also reported that a large proportion of patients with PDPN were not diagnosed, 61.5% in Germany7 and 12.5% in the UK8. Major hurdles limiting the diagnosis of PDPN are that patients with painful symptoms do not attribute them to diabetes and fail to report them to their physician8, 9, and of course screening is not currently advocated for PDPN, only for those at high risk of foot ulceration31. Given that we have identified age, duration of diabetes and the presence of impaired vibration perception as major determinants for PDPN1, 5, 6, 10, one could advocate screening for PDPN in at least diabetes patients who are older, have a longer duration of diabetes and impaired vibration perception. Furthermore, we have identified that obesity is associated with PDPN, which has also been reported in some1, 5, 7, 12, but not other studies6, 11. Low physical activity has been reported as a risk factor13, 14, but in the present study, we showed no association after adjusting for other risk factors. Smoking has also been associated with PDPN in some4, 12, but not other studies1, 4, 5, 6, 11. Improved glycemic control reduces the development and progression of DPN in type 1 diabetes mellitus32, but has shown limited benefit in type 2 diabetes mellitus33. Low HDL cholesterol, and raised LDL cholesterol and triglycerides have been independently associated with PDPN1. Creatinine is associated with PDPN, whereas albuminuria13 and proteinuria have no association. A previous study of individuals with prediabetes showed that lifestyle intervention reduced neuropathic symptoms, and improved small fiber function and structure14.
The prevalence of painful neuropathic symptoms4 and PDPN9 differs between ethnic groups. In our previous study in the UK4, we showed that South Asians were 50% more likely to have painful neuropathic symptoms compared with Europeans and Afro‐Caribbeans, after adjusting for age and duration of diabetes. However, in the present study, South Asians had a lower prevalence of PDPN compared with Qatari Arabs and other Arabs, which might be attributed to a lower proportion with obesity, fewer women and higher physical activity in this group. Indeed, this and other studies4, 5 have shown that women have a 50–65% increase in the odds for PDPN. The ethnic difference might also reflect genetic differences in the prevalence of abnormalities in voltage‐gated channels on nociceptors in different ethnic groups34, 35.
We recognize that recruiting patients with diabetes from secondary healthcare centers and not primary care centers was a major limitation of the present study and limited the generalizability of the results to all people with diabetes in Qatar. However, those two hospitals are the only National Diabetes and Endocrine Centers in Qatar, and the recruited participants were of diverse backgrounds. The cross‐sectional design of the present study also limited the interpretation of cause and effect in relation to risk factors. The strengths of the present study were the large sample size and the inclusion of a wide range of risk factors to identify those associated independently with PDPN. Furthermore, PDPN was diagnosed using the DN4 questionnaire, which has been validated in Arabic21, and used in other studies in the Middle East and North Africa region to establish the prevalence of PDPN5, 6.
In conclusion, one‐third of patients with type 2 diabetes mellitus attending secondary care in Qatar have PDPN. It remains a neglected complication of diabetes, as ~80% of patients were not diagnosed or treated for this condition. Impaired vibration perception, obesity and smoking are associated with PDPN, suggesting that patients with these risk factors should be screened for PDPN, and treated for relief of symptoms and with lifestyle interventions to limit progression.
Disclosure
The authors declare no conflict of interest.
Acknowledgments
We thank all the participants for their efforts, will and commitment to be involved in the study. Funding source: Qatar National Research Fund, funding ID: Grant BMRP‐5726113101; and Pfizer Gulf FZ LLC, funding ID: W1230787.
J Diabetes Investig 2019; 10: 1558–1564
References
- 1. Van Acker K, Bouhassira D, De Bacquer D, et al Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab 2009; 35: 206–213. [DOI] [PubMed] [Google Scholar]
- 2. Bohlega S, Alsaadi T, Amir A, et al Guidelines for the pharmacological treatment of peripheral neuropathic pain: expert panel recommendations for the middle East region. J Int Med Res 2010; 38: 295–317. [DOI] [PubMed] [Google Scholar]
- 3. daCosta DiBonaventura M, Cappelleri JC, Joshi AV. A longitudinal assessment of painful diabetic peripheral neuropathy on health status, productivity, and health care utilization and cost. Pain Med 2011; 12: 118–126. [DOI] [PubMed] [Google Scholar]
- 4. Abbott CA, Malik RA, van Ross ER, et al Prevalence and characteristics of painful diabetic neuropathy in a large community‐based diabetic population in the U.K. Diabetes Care 2011; 34: 2220–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jambart S, Ammache Z, Haddad F, et al Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res 2011; 39: 366–377. [DOI] [PubMed] [Google Scholar]
- 6. Halawa MR, Karawagh A, Zeidan A, et al Prevalence of painful diabetic peripheral neuropathy among patients suffering from diabetes mellitus in Saudi Arabia. Curr Med Res Opin 2010; 26: 337–343. [DOI] [PubMed] [Google Scholar]
- 7. Ziegler D, Landgraf R, Lobmann R, et al Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract 2018; 139: 147–154. [DOI] [PubMed] [Google Scholar]
- 8. Daousi C, MacFarlane IA, Woodward A, et al Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med 2004; 21: 976–982. [DOI] [PubMed] [Google Scholar]
- 9. Eichholz M, Alexander AH, Cappelleri JC, et al Perspectives on the impact of painful diabetic peripheral neuropathy in a multicultural population. Clin Diabetes Endocrinol 2017; 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies M, Brophy S, Williams R, et al The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care 2006; 29: 1518–1522. [DOI] [PubMed] [Google Scholar]
- 11. Jacovides A, Bogoshi M, Distiller LA, et al An epidemiological study to assess the prevalence of diabetic peripheral neuropathic pain among adults with diabetes attending private and institutional outpatient clinics in South Africa. J Int Med Res 2014; 42: 1018–1028. [DOI] [PubMed] [Google Scholar]
- 12. Aslam A, Singh J, Rajbhandari S. Prevalence of painful diabetic neuropathy using the self‐completed leeds assessment of neuropathic symptoms and signs questionnaire in a population with diabetes. Can J Diabetes 2015; 39: 285–295. [DOI] [PubMed] [Google Scholar]
- 13. Ziegler D, Rathmann W, Meisinger C, et al Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre‐diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain 2009; 13: 582–587. [DOI] [PubMed] [Google Scholar]
- 14. Smith AG, Russell J, Feldman EL, et al Lifestyle intervention for pre‐diabetic neuropathy. Diabetes Care 2006; 29: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 15. Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the U.S. population. Diabetes Care 1993; 16: 1446–1452. [DOI] [PubMed] [Google Scholar]
- 16. Smith AG, Singleton JR. Impaired glucose tolerance and neuropathy. Neurologist 2008; 14: 23–29. [DOI] [PubMed] [Google Scholar]
- 17. Unal‐Cevik I, Sarioglu‐Ay S, Evcik D. A comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain: validity and reliability of the Turkish version of DN4. J Pain 2010; 11: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 18. Moser M. World Health Organization‐international society of hypertension guidelines for the management of hypertension‐do these differ from the U.S. recommendations? Which guidelines should the practicing physician follow? J Clin Hypertens 1999; 1: 48–54. [PubMed] [Google Scholar]
- 19. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 2000; 894: i–xii, 1‐253. [PubMed] [Google Scholar]
- 20. Spallone V, Morganti R, D'Amato C, et al Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med 2012; 29: 578–585. [DOI] [PubMed] [Google Scholar]
- 21. Harifi G, Ouilki I, El Bouchti I, et al Validity and reliability of the Arabic adapted version of the DN4 questionnaire (Douleur Neuropathique 4 Questions) for differential diagnosis of pain syndromes with a neuropathic or somatic component. Pain Pract 2011; 11: 139–147. [DOI] [PubMed] [Google Scholar]
- 22. Wiles PG, Pearce SM, Rice PJ, et al Vibration perception threshold: influence of age, height, sex, and smoking, and calculation of accurate centile values. Diabet Med 1991; 8: 157–161. [DOI] [PubMed] [Google Scholar]
- 23. Garrow AP, Boulton AJ. Vibration perception threshold–a valuable assessment of neural dysfunction in people with diabetes. Diabetes Metab Res Rev 2006; 22: 411–419. [DOI] [PubMed] [Google Scholar]
- 24. Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care 2006; 29: 883–887. [DOI] [PubMed] [Google Scholar]
- 25. Vlckova‐Moravcova E, Bednarik J, Belobradkova J, et al Small‐fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med 2008; 25: 692–699. [DOI] [PubMed] [Google Scholar]
- 26. Quattrini C, Tavakoli M, Jeziorska M, et al Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes 2007; 56: 2148–2154. [DOI] [PubMed] [Google Scholar]
- 27. Akbar DH, Mira SA, Zawawi TH, et al Subclinical diabetic neuropathy. A common complication in Saudi diabetics. Neurosciences 2000; 5: 110–114. [PubMed] [Google Scholar]
- 28. Youssef AA, El Mahalli AA, Akl OA, et al Quality of diabetes care in primary care setting in egypt: an example of health sector reform in developing countries. J Egypt Public Health Assoc 2006; 81: 301–320. [PubMed] [Google Scholar]
- 29. Uddin I, Ahmad TJ, Kurkuman AR, et al Diabetes education: its effects on glycemic control. Ann Saudi Med 2001; 21: 120–122. [DOI] [PubMed] [Google Scholar]
- 30. Habib SS, Aslam M. Risk factors, knowledge and health status in diabetic patients. Saudi Med J 2003; 24: 1219–1224. [PubMed] [Google Scholar]
- 31. Pop‐Busui R, Boulton AJ, Feldman EL, et al Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med 1996; 124: 90–96. [DOI] [PubMed] [Google Scholar]
- 33. Callaghan BC, Little AA, Feldman EL, et al Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012: CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wadhawan S, Pant S, Golhar R, et al NaV channel variants in patients with painful and nonpainful peripheral neuropathy. Neurol Genet 2017; 3: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blesneac I, Themistocleous AC, Fratter C, et al Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Int J Endocrinol 2018; 159: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]


