Abstract
Aims/Introduction
The risk of end‐stage kidney disease increases in proportion to the decline in the estimated glomerular filtration rate (eGFR). Although protective effects of sodium–glucose cotransporter 2 inhibitors (SGLT2i) on the eGFR decline were shown in several large‐scale clinical trials, there are no studies investigating patients with a high risk of end‐stage kidney disease. We investigated the efficacy and safety of SGLT2i in advanced renal dysfunction patients (stage G3 or G4 of chronic kidney disease) with a rapid decline in eGFR.
Materials and Methods
This retrospective, longitudinal study enrolled patients with type 2 diabetes who were treated with SGLT2i, and whose eGFR was <60 mL/min/1.73 m2 and had declined >20% over 2 years (%ΔeGFR−2y) before initiating SGLT2i. The primary end‐point was the change in eGFR 2 years after initiation (%ΔeGFR+2y) compared with %ΔeGFR−2y.
Results
A total of 17 patients among 553 patients treated with SGLT2i for ≥2 years were included in the study. The average age, glycated hemoglobin and eGFR at SGLT2i initiation were 68.5 years, 7.3% and 38.3 mL/min/1.73 m2, respectively. %ΔeGFR+2y in patients who were treated with SGLT2i was significantly increased compared with the patients not treated with SGLT2i (2.3 and −21.7%, respectively; P < 0.0001). A multiple regression analysis showed that only the proportion of the rate of eGFR decline was the independent factor associated with improvement of %ΔeGFR+2y. There was no increase in serious adverse events including acute kidney injury.
Conclusions
SGLT2i was safe, and prevented further eGFR decline in patients with type 2 diabetes and advanced renal dysfunction.
Keywords: Estimated glomerular filtration rate, Kidney disease, Sodium–glucose cotransporter 2 inhibitors inhibitor
Introduction
The incidence and prevalence of diabetes has increased significantly throughout the world, and the costs of caring for people with diabetic kidney disease are extraordinarily high1. The annual number of dialysis inductions in Japan resulting from diabetes was 16,103 in 20162, 3, and the Japanese Ministry of Health, Labor and Welfare started, and have been actively promoting, a program to prevent the aggravation of diabetic nephropathy. Identification and effective screening of high‐risk patients are important, along with administration of effective medications to reduce the progression from diabetic kidney disease to end‐stage kidney disease (ESKD)4.
Extensive studies of patients with type 1 diabetes at the Joslin Clinic over a period of 25 years showed that the predominant clinical feature of diabetic nephropathy was progressive renal decline (estimated glomerular filtration rate [eGFR] loss >3.5 mL/min/year), and that the prevalence of decliners (patients with renal decline) was 10, 32 and 50% in patients with normoalbuminuria, microalbuminuria and macroalbuminuria, respectively5. Predominantly based on North American and European studies, a 30–40% decline in eGFR over a few years is strongly associated with the risk of ESKD, and has been proposed as a surrogate end‐point for ESKD in clinical research6. A cohort study to investigate the association between the decline in eGFR and the subsequent risk of ESKD was carried out in Japanese patients with type 2 diabetes4, 7. In the study, a 20% decline in eGFR over 2 years, in addition to the 30–40% decline, was the candidate surrogate end‐point for ESKD in diabetic kidney disease (hazard ratio of subsequent ESKD was 3.9 [95% CI 2.2–7.0] at 2 years and 5.4 [95% CI 2.3–12.8] at 3 years in the study)7.
In a consensus report by the American Diabetes Association and the European Association for the Study of Diabetes on managing patients with hyperglycemia, sodium–glucose cotransporter 2 inhibitors (SGLT2i) are recommended for patients with type 2 diabetes and chronic kidney disease (CKD), because they showed beneficial effects on the renal end‐points8, albeit as secondary outcomes, in large cardiovascular outcomes trials, such as Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients Removing Excess Glucose, Canagliflozin Cardiovascular Assessment Study and Dapagliflozin Effect on Cardiovascular Events ‐ Thrombolysis in Myocardial Infarction 589, 10, 11, 12. The protective effects of SGLT2i on renal function were observed in albuminuria and in the renal hard end‐points including a ≥40% decrease in eGFR to <60 mL/min/1.73 m2, new ESKD, or death from renal or cardiovascular causes, and the effects were consistent in all three SGLT2i trials. The beneficial effects on renal end‐points were likely to be class effects of SGLT2i based on the functional mechanism and the study results. These studies have predominantly included patients with normal renal function (eGFR ≥60 mL/min/1.73 m2) and just 25.8, 20.1 and 7.4% of patients in each study had an eGFR of 30–60 mL/min/1.73 m2; patients with an eGFR <30 mL/min/1.73 m2 were excluded9, 10, 11.
The glucose‐lowering efficacy of SGLT2i in the patients with an eGFR <45 mL/min/1.73 m2 is diminished because of their reduced glucose filtration, and thus, treatment with SGLT2i is not currently recommended when eGFR is significantly reduced. The safety of SGLT2i in patients with more severely impaired kidney function (i.e., eGFR of 15–45 mL/min/1.73 m2) and its effect on renal function is not fully understood. Therefore, we investigated the effects of SGLT2i on renal function in ESKD high‐risk patients with type 2 diabetes whose eGFR was <60 mL/min/1.73 m2 and had declined >20% over 2 years.
Methods
Study population
Patients were eligible for inclusion in the treatment cohort if they had type 2 diabetes mellitus, were at high risk of ESKD (eGFR <60 mL/min/1.73 m2 and eGFR decline rate ≥20%/2 years) and had been treated with SGLT2i for >2 years. As a control, patients without SGLT2i administration who were at high risk of ESKD were also included. The exclusion criteria included patients with the following: type 1 diabetes, type 2 diabetes mellitus who were aged <20 years, did not provide consent, poor adherence or interruption of the medication, lack of eGFR data at 2 years before SGLT2i initiation and eGFR ≥60 mL/min/1.73 m2. The %ΔeGFR−2y was calculated using the following formula: %ΔeGFR−2y = (eGFR at starting SGLT2i) − (eGFR before 2 years of the starting) / (eGFR before 2 years of the starting); %ΔeGFR+2y = (eGFR after starting SGLT2i) − (eGFR at the starting) / (eGFR at the starting).
Protocol
This was a retrospective, longitudinal study of patients with type 2 diabetes mellitus and CKD, who initiated SGLT2i with a minimum of 2 years of follow up after initiation. Data were collected between April 2014 and December 2018 at the outpatient center at the Kurihara Clinic. Two periods of medical history were reported: before and after the SGLT2i initiation date. The baseline was defined as the date that SGLT2i was initiated. Outcome data were collected from the patients’ medical records as follows: 2 years before and after baseline, as well as 1–2 months and 1 year after baseline. Baseline data for age, sex, duration of diabetes, and medications for diabetes and hypertension were also collected. Common measurements, such as bodyweight, body mass index, systolic and diastolic blood pressure, glycated hemoglobin (HbA1c), plasma glucose, fasting insulin or C‐peptide, kidney and liver function tests, and the urinary albumin creatinine ratio (UACR) at each clinic visit were also collected. Because the eGFR data were variable at each clinic visit, the average of three eGFR test results just before −2, 0 and + 2 years after initiating SGLT2i were used. The eGFR 1–2 months after initiating SGLT2i was also used as an eGFR initial data point.
The primary objective was to assess the clinical effectiveness of SGLT2i on renal function by analyzing the change in eGFR at 2 years after initiating SGLT2i (%ΔeGFR+2y) compared with that at 2 years before its initiation (%ΔeGFR−2y). A secondary objective was to investigate the variables associated with %ΔeGFR+2y, to identify the preferred type 2 diabetes mellitus patients with a high risk of ESKD in whom SGLT2i should be initiated and to compare %ΔeGFR+2y between the groups with or without SGLT2i administration. Another secondary objective was safety of SGLT2i in these patients.
The opt‐out consent procedure was used in the study. This study was registered with the University Hospital Medical Information Network (UMIN) Center (#000035263), approved by the institutional review board of Japan Clinicians Diabetes Association, and carried out in accordance with the Declaration of Helsinki.
Statistical analysis
The paired t‐test, Wilcoxon rank‐sum test or the χ2‐test was used for the comparison between two paired groups. The results were expressed as the mean ± standard deviation for normally distributed data, and as the median and 95% confidence (95% CI) interval for non‐normally distributed data. To compare multiple groups, we used the Kruskal–Wallis test followed by the post‐hoc Dunnett's test. To examine which factors are associated with %ΔeGFR+2y, we carried out Spearman's rank correlation coefficient test. Additionally, to examine which factors independently determined %ΔeGFR+2y, we carried out multiple regression analysis, and P‐values <0.05 were considered to denote statistical significance. Statistical analyses were carried out using jmp Pro version 14.0.0 (SAS Inc., Cary, NC, USA).
Results
The reduction in %ΔeGFR−2y was >20% in 17 patients (18.5%), who were defined as the patients with a rapid decline in renal function (rapid eGFR decliners), among 92 type 2 diabetes mellitus patients with CKD who were treated with SGLT2i for >2 years7 (Figure 1). The average age, body mass index and HbA1c were 68.5 years, 30.1 kg/m2 and 7.3%, respectively, in the 17 participants. The control group comprised 58 rapid eGFR decliners without SGLT2i administration (Figure 1).
Figure 1.

Flow diagram of the study. eGFR, estimated glomerular filtration rate; SGLT2, sodium–glucose cotransporter 2.
For CKD staging among the 17 patients, six were G3a (45–60 mL/min/1.73 m2), seven were G3b (30–45 mL/min/1.73 m2) and four were G4 (<30 mL/min/1.73 m2). Diabetic nephropathy, based on the Japan Diabetes Society guidelines, was as follows: six were stage 1, three were stage 2, four were stage 3 and four were stage 4. Half of the rapid eGFR decliners had normo‐ or microalbuminuria (i.e., stage 1 or 2). eGFR was 38.3 ± 12.1 mL/min/1.73 m2, and %ΔeGFR−2y was −31.7% (95% CI −64.4 to 20.2) at baseline. There were no significant differences in baseline parameters between the groups with and without SGLT2i administration, except baseline age, body mass index and the prevalence of diuretics administration (Table 1).
Table 1.
Patients’ baseline characteristics
| SGLT2i (+) n = 17 | SGLT2i (−) n = 58 | P‐value | |
|---|---|---|---|
| Sex | Male 6/female 11 | Male 34/female 24 | 0.105 |
| Age (years) | 68.5 ± 7.0 | 74.5 ± 7.8 | 0.010 |
| Duration of diabetes (years) | 12.7 ± 6.3 | 16.9 ± 9.3 | 0.118 |
| BMI (kg/m2) | 30.1 ± 5.6 | 24.8 ± 3.7 | 0.000 |
| Bodyweight (kg) | 73.3 ± 11.4 | 67.0 ± 10.2 | 0.002 |
| HbA1c (%) | 7.3 ± 1.0 | 6.8 ± 0.7 | 0.069 |
| eGFR (mL/min/1.73 m2) | 37.3 ± 12.1 | 41.5 ± 10.9 | 0.502 |
| %ΔeGFR−2y (%) | −31.7 (−64.4 to −20.2) | −27.1 (−55.2 to −20.0) | 0.502 |
| Stage of diabetic nephropathy | I 6/II 3/III 3/IV 5 | I 26/II 10/III 14/IV 8 | 0.305 |
| ACR, mg/g (n = 11 and 40) | 19.4 ± 13.9 | 81.3 ± 183.8 | 0.614 |
| Urine protein creatinine ratio, g/g (n = 6 and 18) | 1.4 ± 1.2 | 2.2 ± 2.6 | 0.941 |
| Systolic BP (mmHg) | 126 ± 22 | 127 ± 13 | 0.854 |
| Diastolic BP (mmHg) | 71 ± 14 | 69 ± 9 | 0.339 |
| ARB or ACE‐I (%) | 88.2 (15/17) | 75.9 (44/58) | 0.336 |
| Diuretics (%) | 70.6 (12/17) | 31.0 (18/58) | 0.010 |
| Furosemide | 29.4 (5/17) | 6.9 (4/58) | 0.024 |
| Torasemide | 0 (0/17) | 1.7 (1/58) | 1.000 |
| Azosemide | 11.8 (2/17) | 5.2 (3/58) | 0.317 |
| Trichlormethiazide | 17.6 (3/17) | 10.3 (6/58) | 0.415 |
| Hydrochlorothiazide | 29.4 (5/17) | 12.1 (7/58) | 0.128 |
| Spironolactone | 23.5 (4/17) | 3.4 (2/58) | 0.074 |
| SGLT2i (%) | |||
| Empagliflozin | 5.9 (1/17) | – | – |
| Ipragliflozin | 5.9 (1/17) | – | – |
| Luseogliflozin | 23.5 (4/17) | – | – |
| Dapagliflozin | 11.8 (2/17) | – | – |
| Tofogliflozin | 47.1 (8/17) | – | – |
| Canagliflozin | 5.9 (1/17) | – | – |
| Other OHA (%) | |||
| Sulfonylurea | 47.1 (8/17) | 36.2 (21/58) | 0.160 |
| Biguanide | 64.7 (11/17) | 58.6 (34/58) | 0.781 |
| Thiazolidine | 5.9 (1/17) | 5.2 (3/58) | 0.207 |
| α‐GI | 11.8 (2/17) | 20.7 (12/58) | 0.502 |
| Glinide | 0 (0/17) | 1.7 (1/58) | 1.000 |
| DPP4i | 76.5 (13/17) | 67.2 (39/58) | 0.364 |
| Insulin | 11.8 (2/17) | 13.8 (8/58) | 0.186 |
Data are presented as the mean ± standard deviation or as the median (95% confidence interval). %ΔeGFR−2y, estimated glomerular filtration rate was <60 mL/min/1.73 m2 and had declined >20% over 2 years; α‐GI, alpha‐glucosidase inhibitor; ACE‐I, angiotensin‐converting enzyme inhibitor; ACR, albumin‐to‐creatinine ratio; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; DPP4i, dipeptidyl peptidase‐4 inhibitors; HbA1c, glycated hemoglobin; OHA, oral hypoglycemic agents; SGLT2i, sodium–glucose cotransporter 2 inhibitors.
The median eGFR at 1 and 2 years after administering SGLT2i was 39.0 and 39.6 mL/min/1.73 m2, respectively, and they were comparable with the baseline eGFR (38.3 mL/min/1.73 m2). The overall %ΔeGFR+2y was +2.3% (95% CI −48.8 to 56.9), and it was significantly higher compared with the %ΔeGFR+2y (−21.7%) in the control group (P < 0.0001; Figures 2,S1). HbA1c, UACR and systolic/diastolic blood pressure did not change with the administration of SGLT2i for 2 years (from 7.29 ± 0.96% to 7.16 ± 0.62%, from 76.6 to 60.4 mg/g and from 126.2 ± 22.3/70.9 ± 14.2 to 130.6 ± 20.0/73.6 ± 13.7 mmHg, respectively). Bodyweight was decreased from 73.4 ± 11.2 to 70.3 ± 13.0 kg with SGLT2i administration, but the difference was not significant. There were no significant differences in the changes in HbA1c, UACR and systolic/diastolic blood pressure between the groups except for bodyweight (Figure S2).
Figure 2.
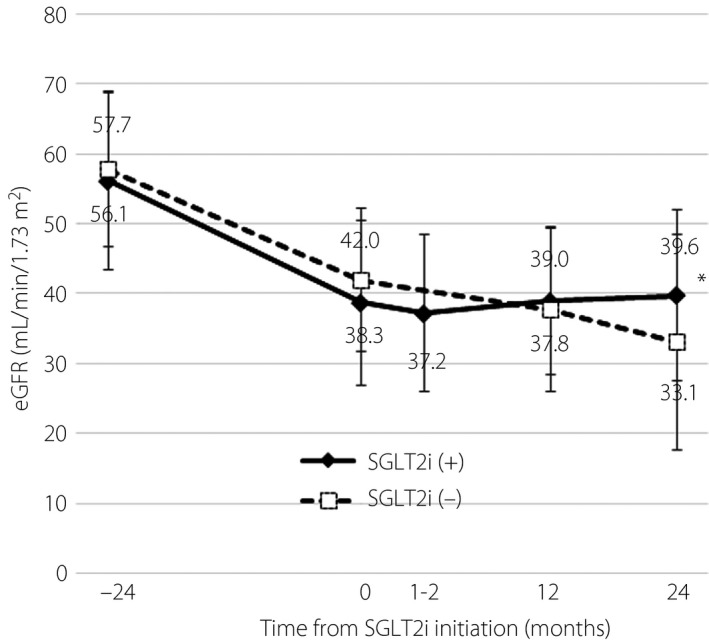
Changes in estimated glomerular filtration rate (eGFR) in patients with or without sodium–glucose cotransporter 2 inhibitor (SGLT2i) administration (n = 17 and 58, respectively). The change in eGFR 2 years after initiation in the 2 years after baseline. Data are presented as the mean ± standard deviation (SD). *P < 0.05 between the groups.
Next, the associated variables with %ΔeGFR+2y in the patients with SGLT2i administration were investigated in a bivariate analysis (age, sex, duration of diabetes, baseline kidney and liver function tests, baseline UACR, baseline and change in HbA1c, blood pressure, or medications). %ΔeGFR−2y, baseline alanine transaminase and baseline systolic/diastolic blood pressure were inversely correlated with %ΔeGFR+2y (P = 0.024, 0.029 and 0.018/0.007, respectively). Based on the simple linear regression analysis and multiple regression analysis for %ΔeGFR+2y as the dependent variable, only %ΔeGFR−2y was an associated (Table S1) and an independent determinant of the %ΔeGFR+2y (P = 0.024; Table 2). A similar tendency for association was observed when only data from patients with eGFR <45 mL/min/1.73 m2 were analyzed (Figure S3).
Table 2.
Multiple regression analysis for change in eGFR 2 years after initiation as the dependent variable
| Parameter | Standardized partial regression coefficient | P‐value |
|---|---|---|
| Δ%eGFR–2y | −0.64046 | 0.0452 |
| Δ%HbA1c–2y | 0.39936 | 0.2353 |
| Δ%Bodyweight–2y | −0.02883 | 0.9197 |
| Δ%SBP–2y | 0.30235 | 0.2741 |
Δ%Bodyweight–2y, change in bodyweight over 2 years before sodium–glucose cotransporter 2 inhibitor initiation; Δ%eGFR–2y, estimated glomerular filtration rate was <60 mL/min/1.73 m2 and had declined >20% over 2 years; Δ%HbA1c–2y, change in glycated hemoglobin over 2 years before sodium–glucose cotransporter 2 inhibitor initiation; Δ%SBP–2y, change in systolic blood pressure over 2 years before sodium–glucose cotransporter 2 inhibitor initiation; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure.
An obvious initial decrease in eGFR (≥1.0 mL/min/1.73 m2) was shown in seven patients among the 17 patients with SGLT2i administration, and the average of the overall decrease was −1.48 mL/min/1.73 m2. Diuretics were discontinued in seven patients when SGLT2i was initiated, and in three patients during the following 2 years of treatment. Of the 17 patients, 10 discontinued diuretics, and an initial decrease was not seen in seven of the 10 patients. The initial decrease in the eGFR was significantly smaller in patients who had discontinued diuretics (n = 10) compared with those who continued diuretics (n = 7; P = 0.004; Figure 3).
Figure 3.

Comparison of initial decrease in the estimated glomerular filtration rate (eGFR) after sodium–glucose cotransporter 2 inhibitor initiation among patients who had their doses of diuretics reduced or withdrawn and those who did not change their diuretic dose. The Wilcoxon rank‐sum test was used for statistical analysis. **P < 0.01.
There were no serious adverse effects in the 17 patients for ≥2 years after initiation of SGLT2i. There was no increase in the overall adverse renal events including acute kidney injury (AKI) in the patients who initiated SGLT2i at this center.
Discussion
Diabetic kidney disease (DKD) is the leading cause of ESKD in Japan and worldwide13, 14. More than a 20% decline in eGFR over a 2‐year period was reported to be a candidate surrogate marker in a Japanese cohort study7. Despite optimal diabetes care, including good glycemic control and treatment of hypertension through renin–angiotensin system inhibition, a large residual risk for developing DKD and its progression remains15, and there is a great unmet need for treatment. In clinical trials that investigated the cardiovascular safety of SGLT2i9, 10, 11, secondary effects of preventing or reducing albuminuria and the decline in eGFR spurred further investigation into their potential application in DKD16. The following ongoing clinical trials are currently testing the efficacy and safety of SGLT2i on primary outcomes in CKD patients with a lower eGFR, with and without type 2 diabetes mellitus: Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation, Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease (NCT03036150), and Study of Heart and Kidney Protection With Empagliflozin17, 18. Changes to the recommendations could be forthcoming, depending on these results.
In the present study, we initiated SGLT2i treatment in patients with type 2 diabetes mellitus who were at high risk of ESKD. Although there have been several studies including patients with type 2 diabetes mellitus and stage 3 CKD19, 20, 21, 22 and one study including patients with type 2 diabetes mellitus and stage 3–4 CKD23, this is the first report on the effects of SGLT2i in rapid eGFR decliners with type 2 diabetes mellitus and stage 3–4 CKD (i.e., eGFR 15–59 mL/min/1.73 m2). We also showed that SGLT2i prevents a further decline in eGFR and that it has a good safety profile. Treatment with SGLT2i is not currently recommended when eGFR is significantly reduced (<30 or 45 mL/min/1.73 m2)23, 24. These indications resulted from a concern about reduced glycemic efficacy and a lack of safety data, such as information on AKI in the DKD population. The US Food and Drug Administration has strengthened the existing warning about the risk of AKI for SGLT2i. However, recent studies reported that SGLT2 use in type 2 diabetes mellitus patients was not associated with an increased risk of AKI22, 25.
We showed, in the present study, that eGFR was not decreased in the 2 years after initiating SGLT2i (ΔeGFR+2y), although eGFR in the control group continued to decrease during this time. We showed that SGLT2i maintained its beneficial effect on renal function, even in type 2 diabetes mellitus patients with stage G3 and G4 CKD. Besides the benefits resulting from lowering blood pressure and weight loss, SGLT2i promotes anti‐inflammatory and antifibrotic pathways, and reduces glomerular hypertension and hyperfiltration through restoring tubuloglomerular feedback26, 27, 28, 29. In addition to modifying these abovementioned factors, SGLT2i might improve renal oxygenation through erythropoietin‐mediated effects on hematocrit30. Each residual nephron in the kidney of patients with DKD is overloaded because of the small number of nephrons. Less energy expenditure in the proximal tubule through blockade of glucose and sodium reabsorption, and a greater oxygen and energy (increased ketone body) supply for each residual nephron might be more beneficial for severely damaged kidneys in patients with DKD. In the present study, we identified that the improvement of %ΔeGFR+2y was more significant in the patients with a more severe decline in eGFR within the past 2 years (%ΔeGFR−2y). This might be related to the mechanism of the residual nephron overload. Although there was no information regarding renal decline before initiating SGLT2i in the previous prospective studies23, 31, %ΔeGFR−2y should be measured in all patients with type 2 diabetes mellitus at clinical follow up to determine the benefit of kidney protection that results from SGLT2i administration.
SGLT2 inhibition causes a characteristic decrease in eGFR soon after initiation of therapy by 4–6 mL/min/1.73 m2 because of its putative afferent vasoconstrictive effect32. Half of the participants (n = 10) did not show an initial decrease in eGFR in the present study (Figure 3). The mechanism is not fully understood, but glomerular blood flow could be maintained, rather than increased, by discontinuing diuretics. SGLT2i provides better control of heart congestion by reducing interstitial fluid volume to a greater extent compared with blood volume (i.e., preservation of intravascular volume and reduced volume overload), whereas loop diuretics reduced intravascular volume more than interstitial fluid volume33. Patients allocated to the empagliflozin group in the Empagliflozin, Cardiovascular Outcomes and Mortality in Type 2 Diabetes trial had a loop diuretic added to their regimen less often, suggesting that empagliflozin had a “loop diuretic–sparing” agent34.
A limitation of the present study is that it was carried out retrospectively. The primary objective of this study was to assess the 2 years before, as well as the change of eGFR at 2 years after, initiating SGLT2i. This is too difficult to investigate prospectively. Another limitation of this study was the relatively small study population. There was a limitation in the assessment of SGLT2i adverse effects in stage 4 CKD, because the population was too small in this study. The present study included 553 type 2 diabetes mellitus patients who received SGLT2i for more than 2 years, and a larger study in multiple facilities should be carried out to address these limitations.
Rapid eGFR decliners comprised 18.5% of the patients with type 2 diabetes mellitus and CKD. Almost all failed to notice the decline, and half of them had no macroalbuminuria. We elucidated in the present study that SGLT2i protected kidney function in rapid renal function decliners with type 2 diabetes mellitus and CKD, and the extent of improvement was more significant in the patients with a more severe decline in renal function before the initiation. In conclusion, the decline in the rate of eGFR, other than albuminuria, should be measured in all patients with type 2 diabetes mellitus, and SGLT2i is a promising agent that might resolve many unmet needs to prevent DKD development and progression.
Disclosure
There was no financial support for this study. H Miyoshi, A Nakamura and Y Kurihara have received honoraria for lectures, and received research funding from organizations follows. H Miyoshi has received honoraria for lectures from Astellas Pharma Inc., Dainippon Pharma Co, Eli Lilly, Mitsubishi Tanabe Pharma Co., MSD, Novartis Pharma, Novo Nordisk Pharma, Kowa Pharmaceutical Co., Ltd., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co., Ltd. and Sanofi; and has received research funding from Astellas Pharma Inc., Daiichi Sankyo, Dainippon Pharma Co., Eli Lilly, Mitsubishi Tanabe Pharma Co., Novo Nordisk Pharma, Kowa Pharmaceutical Co., Abbott Japan Co., Nippon Boehringer Ingelheim Co., Ono Pharmaceutical Co., Ltd. and Taisho Toyama Pharmaceutical Co., Ltd. A Nakamura has received research funding from Mitsubishi Tanabe Pharma Co. and Ono Pharmaceutical Co., Ltd. N Manda has received honoraria for lectures from Ono Pharmaceutical Co., Ltd. Y Kurihara has received honoraria for lectures from Astellas Pharma Inc., AstraZeneca, Mitsubishi Tanabe Pharma Co., Ltd., MSD, Ono Pharmaceutical Co., Ltd., Sanofi, Shionogi & Co., Ltd., Taisho Toyama Pharmaceutical Co., Ltd. and Takeda Pharmaceutical Co., Ltd. The other authors declare no conflict of interest.
Supporting information
Figure S1 | Changes in (a) glycated hemoglobin (HbA1c), (b) bodyweight, (c) urinary albumin creatinine ratio (UACR), (d) systolic blood pressure and (e) diastolic blood pressure. Data are presented as the mean ± standard deviation, *P < 0.05. n.s., not significant.
Figure S2 | Changes in HbAlc (A), body weight (B), urinary albumin creatinine ratio (UACR) (C), systolic blood pressure (D), and diastolic blood pressure (E). n.s., not significant. Data are presented as the mean ± SD. P < 0.05.
Figure S3 | The association between those whose estimated glomerular filtration rate (eGFR) was <60 mL/min/1.73 m2 and had declined >20% over 2 years (%ΔeGFR−2y) and change in eGFR 2 years after initiation (%ΔeGFR+2y) in the patients with type 2 diabetes and (a–c) stage G3 and G4 chronic kidney disease or (d–f) stage G4 chronic kidney disease in the subgroup of patients (c,f) with or (b,e) without rapid renal function decline.
Table S1 | Simple linear regression analysis versus change in eGFR 2 years after initiation.
Acknowledgments
We thank the participating patients at the Kurihara Clinic. We also thank Jodi Smith, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
J Diabetes Investig 2019; 10: 1510–1517
Clinical Trial Registry
University Hospital Medical Information Network
UMIN000035263
References
- 1. Tuttle KR, Bakris GL, Bilous RW, et al Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care 2014; 37: 2864–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Masakane I, Nakai S, Ogata S, et al An Overview of regular dialysis treatment in Japan (As of 31 December 2013). Ther Apher Dial 2015; 19: 540–574. [DOI] [PubMed] [Google Scholar]
- 3. Registry CoRD . An overview of regular dialysis treatment in Japan. Jan Soc Dialysis Ther 2018. [Google Scholar]
- 4. Wada T, Haneda M, Furuichi K, et al Clinical impact of albuminuria and glomerular filtration rate on renal and cardiovascular events, and all‐cause mortality in Japanese patients with type 2 diabetes. Clin Exp Nephrol 2014; 18: 613–620. [DOI] [PubMed] [Google Scholar]
- 5. Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015; 38: 954–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levey AS, Stevens LA, Schmid CH, et al A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oshima M, Toyama T, Haneda M, et al Estimated glomerular filtration rate decline and risk of end‐stage renal disease in type 2 diabetes. PLoS ONE 2018; 13: e0201535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davies MJ, D'Alessio DA, Fradkin J, et al Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neal B, Perkovic V, Mahaffey KW, et al Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377: 644–657. [DOI] [PubMed] [Google Scholar]
- 10. Wiviott SD, Raz I, Bonaca MP, et al Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2018. [DOI] [PubMed] [Google Scholar]
- 11. Zinman B, Wanner C, Lachin JM, et al Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 12. Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 13. Nakai S, Watanabe Y, Masakane I, et al Overview of regular dialysis treatment in Japan (as of 31 December 2011). Ther Apher Dial 2013; 17: 567–611. [DOI] [PubMed] [Google Scholar]
- 14. Kidney Disease: Improving Global Outcomes CKDMBDWG . KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease‐Mineral and Bone Disorder (CKD‐MBD). Kidney Int Suppl 2009; S1–S130. [DOI] [PubMed] [Google Scholar]
- 15. Levin A, Tonelli M, Bonventre J, et al Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–1917. [DOI] [PubMed] [Google Scholar]
- 16. Alicic RZ, Johnson EJ, Tuttle KR. SGLT2 Inhibition for the Prevention and Treatment of Diabetic Kidney Disease: a Review. Am J Kidney Dis 2018; 72: 267–277. [DOI] [PubMed] [Google Scholar]
- 17. Jardine MJ, Mahaffey KW, Neal B, et al The Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) Study Rationale, Design, and Baseline Characteristics. Am J Nephrol 2017; 46: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herrington WG, Preiss D, Haynes R, et al The potential for improving cardio‐renal outcomes by sodium‐glucose co‐transporter‐2 inhibition in people with chronic kidney disease: a rationale for the EMPA‐KIDNEY study. Clin Kidney J 2018; 11: 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnett AH, Mithal A, Manassie J, et al Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 20. Yale JF, Bakris G, Cariou B, et al Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 2014; 16: 1016–1027. [DOI] [PubMed] [Google Scholar]
- 21. Fioretto P, Del Prato S, Buse JB, et al Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes Metab 2018; 20: 2532–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Petrykiv S, Sjostrom CD, Greasley PJ, et al Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol 2017; 12: 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kohan DE, Fioretto P, Tang W, et al Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 2014; 85: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kelly MS, Lewis J, Huntsberry AM, et al Efficacy and renal outcomes of SGLT2 inhibitors in patients with type 2 diabetes and chronic kidney disease. Postgrad Med 2018; 1–12. [DOI] [PubMed] [Google Scholar]
- 25. Nadkarni GN, Ferrandino R, Chang A, et al Acute kidney injury in patients on SGLT2 inhibitors: a propensity‐matched analysis. Diabetes Care 2017; 40: 1479–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kashiwagi A, Maegawa H. Metabolic and hemodynamic effects of sodium‐dependent glucose cotransporter 2 inhibitors on cardio‐renal protection in the treatment of patients with type 2 diabetes mellitus. J Diabetes Investig 2017; 8: 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kawanami D, Matoba K, Takeda Y, et al SGLT2 inhibitors as a therapeutic option for diabetic nephropathy. Int J Mol Sci 2017; 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Terami N, Ogawa D, Tachibana H, et al Long‐term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014; 9: e100777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cherney DZ, Perkins BA. Sodium‐glucose cotransporter 2 inhibition in type 1 diabetes: simultaneous glucose lowering and renal protection? Can J Diabetes 2014; 38: 356–363. [DOI] [PubMed] [Google Scholar]
- 30. Sano M, Takei M, Shiraishi Y, et al Increased hematocrit during sodium‐glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J Clin Med Res 2016; 8: 844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dekkers CCJ, Wheeler DC, Sjostrom CD, et al Effects of the sodium‐glucose co‐transporter 2 inhibitor dapagliflozin in patients with type 2 diabetes and Stages 3b‐4 chronic kidney disease. Nephrol Dial Transplant 2018; 33: 2005–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heerspink HJL, Kosiborod M, Inzucchi SE, et al Renoprotective effects of sodium‐glucose cotransporter‐2 inhibitors. Kidney Int 2018; 94: 26–39. [DOI] [PubMed] [Google Scholar]
- 33. Hallow KM, Helmlinger G, Greasley PJ, et al Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018; 20: 479–487. [DOI] [PubMed] [Google Scholar]
- 34. Fitchett D, Zinman B, Wanner C, et al Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME(R) trial. Eur Heart J 2016; 37: 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 | Changes in (a) glycated hemoglobin (HbA1c), (b) bodyweight, (c) urinary albumin creatinine ratio (UACR), (d) systolic blood pressure and (e) diastolic blood pressure. Data are presented as the mean ± standard deviation, *P < 0.05. n.s., not significant.
Figure S2 | Changes in HbAlc (A), body weight (B), urinary albumin creatinine ratio (UACR) (C), systolic blood pressure (D), and diastolic blood pressure (E). n.s., not significant. Data are presented as the mean ± SD. P < 0.05.
Figure S3 | The association between those whose estimated glomerular filtration rate (eGFR) was <60 mL/min/1.73 m2 and had declined >20% over 2 years (%ΔeGFR−2y) and change in eGFR 2 years after initiation (%ΔeGFR+2y) in the patients with type 2 diabetes and (a–c) stage G3 and G4 chronic kidney disease or (d–f) stage G4 chronic kidney disease in the subgroup of patients (c,f) with or (b,e) without rapid renal function decline.
Table S1 | Simple linear regression analysis versus change in eGFR 2 years after initiation.


