Abstract
Aims/Introduction
To evaluate the differences in the results of 75‐g oral glucose tolerance tests (OGTTs) according to gestational age in Japan.
Materials and Methods
In this prospective cohort study, 2,578 pregnant women were divided into three categories based on their gestational age during the 75‐g OGTT: <14 weeks’ gestation, 14–23 weeks’ gestation and 24–32 weeks’ gestation. The association between gestational age and the results of the 75‐g OGTT were evaluated using multivariable analysis.
Results
Early gestational age was associated with high fasting plasma glucose levels at the time of the 75‐g OGTT, and low corresponding 1‐h and 2‐h plasma glucose levels. Compared with women with a gestational age of 24–32 weeks, women who had undergone the 75‐g OGTT at <14 weeks’ gestation had significantly higher odds of gestational diabetes mellitus diagnosis based on the currently used criteria in Japan (adjusted odds ratio 1.42, 95% confidence interval 1.07–1.90).
Conclusions
The results of the 75‐g OGTT varied by gestational age. The use of the same 75‐g OGTT cut‐off values for the diagnosis of gestational diabetes mellitus, regardless of gestational age, might lead to increases in the prevalence of gestational diabetes mellitus diagnosis in Japan.
Keywords: 75‐g Oral glucose tolerance test, Gestational age, Gestational diabetes mellitus
Introduction
Gestational diabetes mellitus (GDM) is a common complication during pregnancy, and it is a risk factor for adverse perinatal outcomes1, 2, 3. However, the diagnostic criteria for GDM vary across countries. The Hyperglycemia and Adverse Pregnancy Outcome study showed continuous relationships between carrying out a 75‐g oral glucose tolerance test (OGTT) at 24–32 weeks’ gestation and perinatal outcomes4. Based on the results of the Hyperglycemia and Adverse Pregnancy Outcome study, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) published the diagnostic criteria for GDM in 20105. At <24 weeks’ gestation, the IADPSG does not recommend carrying out a 75‐g OGTT due to insufficient data on the association between 75‐g OGTTs and perinatal outcomes6. Alternatively, fasting plasma glucose (PG) is used to diagnose GDM at <24 weeks’ gestation. At 24–28 weeks’ gestation, GDM is diagnosed based on a 75‐g OGTT5. The Japan Society of Obstetrics and Gynecology (JSOG) recommends that GDM screening be carried out for all pregnant women at ≤28 weeks’ gestation, including those in the first trimester of pregnancy, and that a 75‐g OGTT be carried out regardless of gestational age to diagnose GDM, in Japan7, 8, 9, 10.
The IADPSG defines GDM as a fasting PG level of 92–125 mg/dL at <24 weeks’ gestation5. This cut‐off fasting PG value is extrapolated from that at 24–28 weeks’ gestation11. In Japan, since June 2010, the JSOG has been confirming the presence of GDM using the following three criteria during 75‐g OGTTs: fasting PG level 92–125 mg/dL; 1‐h PG level ≥180 mg/dL; and 2‐h PG level ≥153 mg/dL, regardless of the gestational age9, 10. This means that the IADPSG criteria for GDM at 24–28 weeks’ gestation are applied to other gestational ages in Japan.
However, there is a lack of sufficient evidence justifying the application of the same 75‐g OGTT cut‐off values for GDM diagnosis, regardless of gestational age. Furthermore, the results of previous studies focusing on the differences in the PG levels or 75‐g OGTT results across gestational ages are inconsistent12, 13, 14, 15. If the results of 75‐g OGTTs vary across gestational ages, the likelihood of GDM diagnosis would differ. However, few studies have investigated this16. The present study was carried out to evaluate the differences in the results of 75‐g OGTTs, in terms of fasting PG levels, 1‐h PG, 2‐h PG levels and GDM diagnosis, by gestational age, in Japan.
Methods
Study design and participants
The present study was carried out as part of the Japan Assessment of Gestational Diabetes Mellitus Screening (JAGS) trial – a prospective cohort study. The JAGS trial was carried out between 2001 and 2004 in Japan. The primary aims of the JAGS trial were to examine the prevalence of GDM and investigate the GDM screening rates in Japan. The ethics committees of all the hospitals described in the supporting information approved the study protocol. Written informed consent was obtained from all participants. The study was planned such that participants would undergo 75‐g OGTTs at both <24 weeks’ gestation and ≥24 weeks’ gestation. Participants with hyperemesis gravidarum symptoms did not undergo 75‐g OGTTs regardless of the degree of hyperemesis gravidarum. Participants and medical staff were not blinded to the results of the 75‐g OGTTs. When the JAGS trial was carried out, GDM was diagnosed when two or more of the following values during a 75‐g OGTT were met, regardless of the gestational age: fasting PG level ≥100 mg/dL; 1‐h PG level ≥180 mg/dL; and 2‐h PG level ≥150 mg/dL – namely, the 1984 JSOG criteria8. Details of the JAGS trial have been described previously17.
In the present study, only the results of the initial 75‐g OGTT (i.e., a 75‐g OGTT at <24 weeks’ gestation) were analyzed for participants who had undergone the 75‐g OGTT twice. This was because the results of the second 75‐g OGTT (i.e., a 75‐g OGTT at ≥24 weeks’ gestation) were only obtained from the participants who were not diagnosed with GDM based on the 1984 JSOG criteria at <24 weeks’ gestation.
Data collection
Classification of gestational age at 75‐g OGTT
Data on the gestational week at the time the 75‐g OGTT was carried out were collected from medical records. Participants who had undergone a 75‐g OGTT at <24 weeks’ gestation were subdivided into two categories: (i) <14 weeks’ gestation (the first trimester); and (ii) 14–23 weeks’ gestation. Among participants who had undergone a 75‐g OGTT at ≥24 weeks’ gestation, those who had undergone a 75‐g OGTT at ≥33 weeks’ gestation were excluded, as the number of participants was too small to be analyzed. Therefore, participants were classified into three categories based on their gestational age during the 75‐g OGTT (i.e., <14 weeks’ gestation, 14–23 weeks’ gestation and 24–32 weeks’ gestation).
75‐g OGTT and definition of GDM diagnosis
The outcomes in the present study were the PG levels during the 75‐g OGTT, and GDM diagnosis. After an overnight fast, blood was drawn from patients’ veins. SRL, Inc, Tokyo, Japan – a subcontractor – carried out all blood sample measurements. The 75‐g OGTT results were collected as continuous variables and were used to diagnose GDM. When the levels of fasting PG, and 1‐h and 2‐h PG during a 75‐g OGTT were treated as categorical variables, 92–125 mg/dL, ≥180 mg/dL and ≥153 mg/dL, respectively, were treated as the outcomes, considering their clinical significance.
In the present study, GDM was defined based on several criteria, such as the 1984 JSOG criteria, 2010 JSOG criteria and 2010 IADPSG criteria. The 1984 JSOG criteria were used until June 2010 in Japan. After the completion of the JAGS trial in 2004, both the 2010 JSOG criteria and 2010 IADPSG criteria were published5, 9. Therefore, we redefined GDM based on the 2010 JSOG criteria and 2010 IADPSG criteria in the present study. GDM, based on the 2010 JSOG criteria, was defined when at least one of the following three values during a 75‐g OGTT, regardless of the gestational age, was met: fasting PG level of 92‐125 mg/dL, 1‐h PG level ≥180 mg/dL and 2‐h PG level ≥153 mg/dL10. As per the 2010 IADPSG criteria, GDM is defined by only a fasting PG level of 92–125 mg/dL, but not by 1‐h and 2‐h PG at <24 weeks’ gestation. At 24–28 weeks’ gestation, GDM was also defined when at least one of the following three values during a 75‐g OGTT was met: fasting PG level of 92–125 mg/dL, 1‐h PG level ≥180 mg/dL and 2‐h PG level ≥153 mg/dL. At ≥28 weeks’ gestation, GDM was not defined in the 2010 IADPSG criteria5. Therefore, when GDM diagnosis – confirmed based on the 2010 IADPSG criteria – was treated as an outcome, participants who had undergone a 75‐g OGTT at 24–32 weeks’ gestation were restricted to those who had undergone a 75‐g OGTT at 24–28 weeks’ gestation.
Other variables used in this study
All background data were obtained from patients’ medical records, including maternal age, parity, family history of diabetes mellitus, month at 75‐g OGTT, bodyweight (BW) at delivery, delivery week and infant birthweight. A family history of diabetes mellitus was defined as the presence of a second‐degree relative with diabetes mellitus. The seasons during which the 75‐g OGTTs were carried out were: spring (March, April or May), summer (June, July or August), fall (September, October or November) and winter (December, January or February). Self‐reported maternal height and pre‐pregnancy BW were also obtained from medical records. Pre‐pregnancy body mass index was calculated as pre‐pregnancy BW in kilograms / (height in meters)2. Gestational weight gain was calculated as follows: BW at delivery – pre‐pregnancy BW.
Statistical analysis
We applied the general linear model to evaluate the associations between gestational age at 75‐g OGTT (i.e., <14 weeks’ gestation, 14–23 weeks’ gestation and 24–32 weeks’ gestation) and the corresponding PG levels as continuous variables. In the analyses of the associations between gestational age at 75‐g OGTT and the corresponding PG levels as categorical variables (i.e., fasting PG level of 92–125 mg/dL, 1‐h PG level ≥180 mg/dL and 2‐h PG level ≥153 mg/dL), we used a multiple logistic regression model to calculate the odds ratio (OR). We also applied a multiple logistic regression model to evaluate the association between gestational age and GDM diagnosis based on the 1984 JSOG criteria, 2010 JSOG criteria and 2010 IADPSG criteria. Details on the statistical analyses are described in the supporting information.
Results
Maternal and neonatal characteristics of the study participants
Figure 1 shows the flow chart of study participant inclusion. Of the 2,993 study participants, cases of twin pregnancies (18 women), triplet pregnancies (one woman), abortions (16 women) and overt diabetes mellitus in pregnancy – defined as a fasting PG level ≥126 mg/dL (6 women) – and those with missing data on the gestational week at the time of the 75‐g OGTT (328 women) were excluded. In addition, women who had undergone a 75‐g OGTT at ≥32 weeks’ gestation (44 women) and those with improbable values (i.e., 0 mg/dL) in the 75‐g OGTT (2 women) were also excluded. Finally, 2,578 women were analyzed in the present study.
Figure 1.
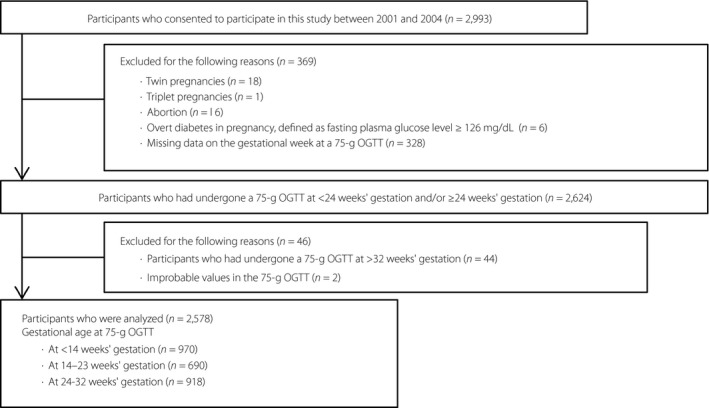
Flow diagram of study participant inclusion. OGTT, oral glucose tolerance test.
Table 1 shows the maternal and neonatal characteristics of the study participants. The numbers of women who had undergone a 75‐g OGTT at <14, 14–23 and 24–32 weeks’ gestation were 970, 690 and 918, respectively. Table 2 shows the results of the 75‐g OGTT of the study participants. The percentages and numbers of GDM cases based on the 1984 JSOG criteria and 2010 JSOG criteria were 2.4% (61 women) and 10.1% (261 women), respectively. Of the 2,357 women who had undergone a 75‐g OGTT at ≤28 weeks’ gestation, the percentage (number) of GDM cases based on the 2010 IADPSG criteria was 6.3% (149 women).
Table 1.
Maternal and neonatal characteristics of the study participants
| Characteristics | Total (n = 2,578) | Gestational age at 75‐g OGTT | P‐value | ||
|---|---|---|---|---|---|
| <14 weeks’ gestation (n = 970) | 14–23 weeks’ gestation (n = 690) | 24‐32 weeks’ gestation (n = 918) | |||
| Gestational week at a 75‐g OGTT, weeks (median, IQR) | – | 12.7, 11.7–13.3 | 15.3, 14.6–16.4 | 28.0, 26.9–28.9 | – |
| Age (years) | 30.2 (4.7) | 30.3 (4.6) | 30.2 (4.7) | 30.3 (4.4) | 0.5 |
| <25 years, n (%) | 255 (9.9) | 118 (12.2) | 58 (8.4) | 79 (8.6) | <0.0001 |
| 25–29.9 years, n (%) | 816 (31.7) | 317 (32.7) | 192 (27.8) | 307 (33.4) | |
| 30–34.9 years, n (%) | 902 (35.0) | 307 (31.7) | 226 (32.8) | 369 (40.2) | |
| ≥35 years, n (%) | 427 (16.6) | 129 (13.3) | 149 (21.6) | 149 (16.2) | |
| Missing, n (%) | 178 (6.9) | 99 (10.2) | 65 (9.4) | 14 (1.5) | |
| Pre‐pregnancy BMI (kg/m2) | 21.2 (3.4) | 21.3 (3.4) | 21.2 (3.6) | 21.0 (3.4) | 0.1 |
| <18.5 kg/m2 (underweight), n (%) | 405 (15.7) | 147 (15.2) | 104 (15.4) | 152 (16.6) | <0.0001 |
| 18.5‐24.9 kg/m2 (normal range), n (%) | 1643 (63.7) | 585 (60.3) | 417 (60.4) | 641 (69.8) | |
| 25.0‐29.9 kg/m2 (overweight), n (%) | 190 (7.4) | 82 (8.5) | 37 (5.4) | 71 (7.7) | |
| ≥30 kg/m2 (obese), n (%) | 62 (2.4) | 21 (2.2) | 20 (2.9) | 21 (2.3) | |
| Missing, n (%) | 278 (10.8) | 135 (13.9) | 110 (15.9) | 33 (3.6) | |
| Primipara, n (%) | 1493 (57.9) | 567 (58.5) | 359 (52.0) | 567 (61.8) | 0.0004 |
| Family history of diabetes mellitus, n (%) | |||||
| Yes | 440 (17.1) | 611 (19.4) | 342 (14.6) | 669 (16.5) | <0.0001 |
| No | 1622 (62.9) | 188 (63.0) | 101 (49.6) | 151 (72.9) | |
| Missing | 516 (20.0) | 171 (17.6) | 247 (35.8) | 98 (10.7) | |
| Season at 75‐g OGTT, n (%) | |||||
| Spring | 824 (32.0) | 360 (37.1) | 210 (30.4) | 254 (27.7) | <0.0001 |
| Summer | 598 (23.2) | 219 (22.6) | 151 (21.9) | 228 (24.8) | |
| Fall | 498 (19.3) | 146 (15.1) | 159 (23.0) | 193 (21.0) | |
| Winter | 658 (25.5) | 245 (25.3) | 170 (24.6) | 243 (26.5) | |
| Gestational weight gain (kg)† | 9.7 (4.1) | 9.5 (3.8) | 9.7 (4.4) | 9.8 (4.2) | 0.1 |
| Gestational age at delivery (weeks)† | 39.2 (1.7) | 39.2 (1.8) | 39.1 (2.2) | 39.4 (1.4) | 0.03 |
| Infant birthweight (g)† | 3012 (420) | 2998 (404) | 3043 (466) | 3008 (408) | 0.7 |
Continuous variables are shown as the mean (standard deviation) unless otherwise noted. Categorical variables are shown as numbers (percentages). †The percentages of missing data were 35.3% for gestational weight gain, 31.2% for delivery week and 29.8% for infant birthweight. BMI, body mass index; IQR, interquartile range; OGTT, oral glucose tolerance test.
Table 2.
Results of the 75‐g oral glucose tolerance test of the study participants
| Results of 75‐g OGTT | Total (n = 2,578) | Gestational age at 75‐g OGTT | P‐value | ||
|---|---|---|---|---|---|
| <14 weeks’ gestation (n = 970) | 14–23 weeks’ gestation (n = 690) | 24–32 weeks’ gestation (n = 918) | |||
| Fasting PG (mg/dL) | 80.4 (6.3) | 82.0 (6.2) | 80.7 (6.3) | 78.6 (6.0) | <0.0001 |
| 1‐h PG (mg/dL) | 122.8 (29.7) | 120.1 (31.0) | 120.3 (29.3) | 127.4 (28.1) | <0.0001 |
| 2‐h PG (mg/dL) | 110.0 (25.5) | 108.6 (24.7) | 109.5 (30.4) | 111.8 (21.9) | 0.006 |
| GDM (JSOG criteria 1984), n (%) | 61 (2.4) | 29 (3.0) | 13 (1.9) | 19 (2.1) | 0.3 |
| Fasting PG ≥100 mg/dL and 1‐h PG ≥180 mg/dL and 2‐h PG <150 mg/dL, n (%) | 1 (0.04) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0.6 |
| Fasting PG ≥100 mg/dL and 1‐h PG <180 mg/dL and 2‐h PG ≥150 mg/dL, n (%) | 1 (0.04) | 0 (0.0) | 1 (0.1) | 0 (0.0) | 0.3 |
| Fasting PG <100 mg/dL and 1‐h PG ≥180 mg/dL and 2‐h PG ≥150 mg/dL, n (%) | 57 (2.2) | 27 (2.8) | 12 (1.7) | 18 (2.0) | 0.3 |
| Fasting PG ≥100 mg/dL and 1‐h PG ≥180 mg/dL and 2‐h PG ≥150 mg/dL, n (%) | 2 (0.1) | 2 (0.2) | 0 (0.0) | 0 (0.0) | 0.3 |
| GDM (2010 JSOG criteria), n (%) | 261 (10.1) | 121 (12.5) | 61 (8.8) | 79 (8.6) | 0.009 |
| Fasting PG (92–125 mg/dL) | 112 (4.4) | 58 (6.0) | 30 (4.4) | 24 (2.6) | 0.002 |
| 1‐h PG (≥180 mg/dL) | 99 (3.8) | 43 (4.4) | 20 (2.9) | 36 (3.9) | 0.3 |
| 2‐h PG (≥153 mg/dL) | 124 (4.9) | 55 (5.7) | 27 (3.9) | 42 (4.6) | 0.2 |
| GDM (2010 IADPSG criteria†), n (%) | 149 (6.3)‡ | 58 (6.0) | 30 (4.4) | 61 (8.8)§ | 0.1 |
| Fasting PG (92–125 mg/dL) | 105 (4.5)‡ | 58 (6.0) | 30 (4.4) | 17 (2.4)§ | 0.003 |
| 1‐h PG (≥180 mg/dL) | 29 (4.2)§ | – | – | 29 (4.2)§ | – |
| 2‐h PG (≥153 mg/dL) | 33 (4.7)§ | – | – | 33 (4.7)§ | – |
Continuous variables are shown as mean (standard deviation) unless otherwise noted. Categorical variables are shown as numbers (percentages). †At <24 weeks’ gestation, gestational diabetes mellitus (GDM) based on the 2010 International Association of Diabetes and Pregnancy Study Groups (IADPSG) was diagnosed using fasting plasma glucose (PG) levels. At 24–28 weeks’ gestation, GDM based on the 2010 IADPSG was diagnosed using a 75‐g oral glucose tolerance test (OGTT). ‡Number and percentage among 2,357 women who had received a 75‐g OGTT at ≤28 weeks’ gestation. §Number and percentage among 697 women who had received a 75‐g OGTT at 24–28 weeks’ gestation. JSOG, Japan Society of Obstetrics and Gynecology.
Differences in the PG levels during a 75‐g OGTT and GDM diagnosis based on the 1984 JSOG criteria and 2010 JSOG criteria according to gestational age at 75‐g OGTT
Table 3 shows the differences in the results of the 75‐g OGTTs and GDM diagnosis based on the 1984 JSOG criteria and 2010 JSOG criteria across gestational ages, at ≤32 weeks’ gestation. When the PG level in each 75‐g OGTT was treated as a continuous variable, the earlier the gestational age at 75‐g OGTT, the higher the fasting PG level (P‐value for trend <0.0001). In contrast, the earlier the gestational age at 75‐g OGTT, the lower the 1‐h and 2‐h PG levels (P‐values for trend were <0.0001 and 0.005, respectively).
Table 3.
Differences in the plasma glucose levels during a 75‐g oral glucose tolerance test and gestational diabetes mellitus diagnosis based on the 1984 Japan Society of Obstetrics and Gynecology criteria and 2010 Japan Society of Obstetrics and Gynecology criteria according to gestational age at 75‐g oral glucose tolerance test
| Outcomes | Gestational age at 75‐g OGTT (n = 2,578) | P‐value for trend | ||
|---|---|---|---|---|
| <14 weeks’ gestation (n = 970) | 14–23 weeks’ gestation (n = 690) | 24‐32 weeks’ gestation (n = 918) | ||
| PG levels during a 75‐g OGTT as continuous variables |
Estimate (95% CI) P‐value |
Estimate (95% CI) P‐value |
Reference | |
| Model 1† | ||||
| Fasting PG (mg/dL) |
3.4 (2.8–4.0) <0.0001 |
2.1 (1.5–2.7) <0.0001 |
Reference | <0.0001 |
| 1‐h PG (mg/dL) |
−7.3 (−10.0 to −4.7) <0.0001 |
−7.2 (−10.1 to −4.3) <0.0001 |
Reference | <0.0001 |
| 2‐h PG (mg/dL) |
−3.2 (−5.5 to −0.9) 0.006 |
−2.3 (−4.8 to 0.2) 0.08 |
Reference | 0.005 |
| Model 2‡ | ||||
| Fasting PG (mg/dL) |
3.4 (2.8–3.9) <0.0001 |
2.0 (1.4–2.6) <0.0001 |
Reference | <0.0001 |
| 1‐h PG (mg/dL) |
−7.1 (−9.6 to −4.5) <0.0001 |
−8.1 (−10.9 to −5.3) <0.0001 |
Reference | <0.0001 |
| 2‐h PG (mg/dL) |
−2.8 (−5.0 to −0.6) 0.01 |
−3.0 (−5.5 to −0.6) 0.02 |
Reference | 0.005 |
| PG levels during a 75‐g OGTT as categorical variables |
OR (95% CI) P‐value |
OR (95% CI) P‐value |
Reference | |
| Model 1† | ||||
| Fasting PG (92–125 mg/dL) |
2.37 (1.46–3.85) 0.0005 |
1.69 (0.98–2.92) 0.06 |
Reference | 0.001 |
| 1‐h PG (≥180 mg/dL) |
1.14 (0.72–1.79) 0.6 |
0.73 (0.42–1.28) 0.3 |
Reference | 0.9 |
| 2‐h PG (≥153 mg/dL) |
1.25 (0.83–1.89) 0.3 |
0.85 (0.52–1.39) 0.5 |
Reference | 0.5 |
| Model 2‡ | ||||
| Fasting PG (92–125 mg/dL) |
1.53 (1.19–1.96) 0.001 |
1.23 (0.93–1.63) 0.2 |
Reference | 0.002 |
| 1‐h PG (≥180 mg/dL) |
1.08 (0.85–1.36) 0.6 |
0.81 (0.61–1.08) 0.2 |
Reference | 1.0 |
| 2‐h PG (≥153 mg/dL) |
1.15 (0.93–1.43) 0.2 |
0.86 (0.67–1.11) 0.3 |
Reference | 0.5 |
| GDM |
OR (95% CI) P‐value |
OR (95% CI) P‐value |
Reference | |
| Model 1† | ||||
| 1984 JSOG criteria |
1.46 (0.81–2.62) 0.2 |
0.91 (0.45–1.85) 0.8 |
Reference | 0.4 |
| 2010 JSOG criteria |
1.51 (1.12–2.04) 0.007 |
1.03 (0.73–1.46) 0.9 |
Reference | 0.03 |
| Model 2‡ | ||||
| 1984 JSOG criteria |
1.24 (0.92–1.68) 0.2 |
0.90 (0.63–1.29) 0.6 |
Reference | 0.4 |
| 2010 JSOG criteria |
1.25 (1.07–1.46) 0.005 |
0.96 (0.80–1.15) 0.7 |
Reference | 0.04 |
†Crude. ‡Adjusted by maternal age, pre‐pregnancy body mass index, parity (primipara or not), family history of diabetes mellitus and season at 75‐g oral glucose tolerance test (OGTT). CI, confidence interval; GDM, gestational diabetes mellitus based on the 1984 Japan Society of Obstetrics and Gynecology criteria and 2010 Japan Society of Obstetrics and Gynecology criteria; JSOG, Japan Society of Obstetrics and Gynecology; OR, odds ratio; PG, plasma glucose.
When each PG value in the 75‐g OGTT was treated as a categorical variable, the earlier the gestational age at 75‐g OGTT, the higher the adjusted OR of fasting PG levels of 92–125 mg/dL (P‐value for trend = 0.001). The linear associations between gestational age and 1‐h PG levels ≥180 mg/dL or 2‐h PG levels ≥153 mg/dL were not statistically significant (P‐values for trend were 0.9 and 0.5, respectively).
The differences in the diagnosis of GDM based on the 1984 JSOG criteria, by gestational age, were not statistically significant. Women who had undergone a 75‐g OGTT at <14 weeks’ gestation had significantly higher odds of GDM diagnosis based on the 2010 JSOG criteria than those who had undergone a 75‐g OGTT at 24–32 weeks’ gestation. The adjusted OR for GDM diagnosis based on the 2010 JSOG criteria was 1.25 (95% confidence interval 1.07–1.46).
Differences in the fasting PG levels during a 75‐g OGTT and GDM diagnosis based on the 2010 IADPSG criteria according to gestational age at 75‐g OGTT
Table 4 shows the differences in the fasting PG levels at 75‐g OGTT and GDM diagnosis based on the 2010 IADPSG criteria, by gestational age, at ≤28 weeks’ gestation. The results pertaining to the differences in the fasting PG levels as continuous variables or categorical variables, according to gestational age, were similar to the results observed in women who had undergone a 75‐g OGTT at ≤32 weeks’ gestation. The earlier the gestational age, the lower the adjusted OR for GDM diagnosis based on the 2010 IADPSG criteria (P‐value for trend = 0.002). Women whose fasting PG levels were examined at <14 and 14‐23 weeks’ gestation had significantly lower odds of GDM diagnosis based on the 2010 IADPSG criteria than those who had undergone a 75‐g OGTT at 24–28 weeks’ gestation, with adjusted ORs of 0.80 (95% confidence interval 0.66–0.97) and 0.65 (95% confidence interval 0.51–0.82), respectively.
Table 4.
Differences in the fasting plasma glucose levels during a 75‐g oral glucose tolerance test and gestational diabetes mellitus diagnosis based on the 2010 International Association of Diabetes and Pregnancy Study Groups criteria according to gestational age at 75‐g oral glucose tolerance test
| Outcomes | Gestational age at 75‐g OGTT (n = 2,357) | P‐value for trend | ||
|---|---|---|---|---|
| <14 weeks’ gestation (n = 970) | 14–23 weeks’ gestation (n = 690) | 24–28 weeks’ gestation (n = 697) | ||
| Fasting PG levels as continuous variables (mg/dL) |
Estimate (95% CI) P‐value |
Estimate (95% CI) P‐value |
Reference | |
| Model 1† |
3.8 (3.2–4.4) <0.0001 |
2.5(1.8–3.1) <0.0001 |
Reference | <0.0001 |
| Model 2‡ |
3.7 (3.1–4.3) <0.0001 |
2.3 (1.7–3.0) <0.0001 |
Reference | <0.0001 |
| Fasting PG levels as categorical variables (92–125 mg/dL) |
OR (95% CI) P‐value |
OR (95% CI) P‐value |
Reference | |
| Model 1† |
2.54 (1.47–4.41) 0.001 |
1.82 (0.99–3.33) 0.05 |
Reference | 0.001 |
| Model 2‡ |
1.58 (1.20–2.10) 0.001 |
1.27 (0.93–1.73) 0.1 |
Reference | 0.003 |
| GDM (2010 IADPSG criteria) |
OR (95% CI) P‐value |
OR (95% CI) P‐value |
Reference | |
| Model 1† |
0.68 (0.48–0.97) 0.03 |
0.50 (0.33–0.76) 0.001 |
Reference | 0.049 |
| Model 2‡ |
0.80 (0.66–0.97) 0.03 |
0.65 (0.51–0.82) 0.0002 |
Reference | 0.002 |
†Crude. ‡Adjusted by maternal age, pre‐pregnancy body‐mass index, parity (primipara or not), family history of diabetes mellitus, and season at 75‐g oral glucose tolerance test (OGTT). CI, confidence interval; GDM, gestational diabetes mellitus based on the 2010 International Association of Diabetes and Pregnancy Study Groups criteria; IADPSG, International Association of Diabetes and Pregnancy Study Groups; OGTT, oral glucose tolerance test; OR, odds ratio; PG, plasma glucose.
Discussion
To the best of our knowledge, this is the first study to show the difference in the prevalence of GDM diagnosis based on several diagnostic criteria in Japan by gestational age. Participants who had undergone a 75‐g OGTT at <14 weeks’ gestation had significantly higher odds of GDM diagnosis based on the 2010 JSOG criteria than those who had undergone the test at 24–32 weeks’ gestation. In contrast, the differences in the prevalence of GDM diagnosis based on the 1984 JSOG criteria, by gestational age, were not statistically significant. These results indicate that the implementation of a 75‐g OGTT at <14 weeks’ gestation might increase the prevalence of GDM, based on the currently used criteria in Japan.
Participants in whom the fasting PG levels during a 75‐g OGTT were examined at <14 weeks’ and 14–23 weeks’ gestation had significantly lower odds of GDM diagnosis based on the 2010 IADPSG criteria than those who had undergone the test at 24–28 weeks’ gestation. Therefore, the prevalence of GDM, especially at <24 weeks’ gestation, might decrease if the 2010 IADPSG criteria are adopted in Japan.
The findings of the present study pertaining to the differences in the fasting PG levels during a 75‐g OGTT, by gestational age, are similar to those of previous studies. Although the underlying mechanism is unclear, Mills et al.14 reported that fasting PG levels decrease during pregnancy. They also showed that a decline in the fasting PG levels begins in the first trimester14. Hagiwara et al.15 showed that the fasting PG level in pregnant women with a median gestational age of 13 weeks at GDM diagnosis was higher than that in those with a median gestational age of 29 weeks at GDM diagnosis, in Japan. However, the results of several other studies are not consistent with the present findings12, 13. Siegmund et al.12 reported that fasting PG levels did not significantly change during pregnancy. Froslie et al.13 showed that the fasting PG levels at 30–32 weeks’ gestation were higher than that those at 14–16 weeks’ gestation. These differences could be attributed to differences in the sample size, ethnicity and other unmeasured confounding factors.
Women who had undergone a 75‐g OGTT at <14 weeks’ gestation had higher odds of GDM diagnosis based on the 2010 JSOG criteria than those who had undergone the test at 24–32 weeks’ gestation; this could be attributed to the high fasting PG levels rather than the 1‐h or 2‐h PG levels. In the present study, women whose fasting PG levels were measured at <14 weeks’ gestation had higher odds of having levels of 92–125 mg/dL than those in whom the measurement was carried out at 24–32 weeks’ gestation. Therefore, the use of the same cut‐off fasting PG value for GDM diagnosis, regardless of gestational age, might increase the odds of GDM diagnosis based on the 2010 JSOG criteria. Opinions on the cut‐off fasting PG value for GDM diagnosis at <24 weeks’ gestation are conflicting. The IADPSG stated that at <24 weeks’ gestation, GDM is diagnosed when the fasting PG level is 92–125 mg/dL5. Riskin‐Mashiah et al. also proposed that the same cut‐off fasting PG value of 92–125 mg/dL could be used for GDM diagnosis throughout pregnancy18. However, Cosson et al. proposed that the cut‐off fasting PG value for GDM diagnosis at <24 weeks’ gestation was 100–125 mg/dL, rather than 92–125 mg/dL19.
The implementation of a 75‐g OGTT at <14 or 14–23 weeks’ gestation was associated with low 1‐h and 2‐h PG levels compared with those observed in 75‐g OGTTs carried out at 24–28 weeks’ gestation. This finding was consistent with those of previous studies. Froslie et al.13 showed that the 2‐h PG level during a 75‐g OGTT carried out at 14–16 weeks’ gestation was lower than that observed at 30–32 weeks’ gestation. Hagiwara et al.15 also showed that both the 1‐h and 2‐h PG levels during a 75‐g OGTT in pregnant women with a median gestational age of 13 weeks at GDM diagnosis were lower than those in women with a median gestational age of 29 weeks at GDM diagnosis. Siegmund et al.12 reported that both 1‐h and 2‐h PG levels do not significantly change during pregnancy. One reason for the inconsistency in the findings could be variations in the sample size. An increase in maternal insulin resistance might be the reason for high 1‐h and 2‐h PG levels during a 75‐g OGTT at 24–32 weeks’ gestation20. However, the associations between gestational age at 75‐g OGTT and the presence of corresponding 1‐h PG levels ≥180 mg/dL or 2‐h PG levels ≥153 mg/dL were not statistically significant in the present study. There is a lack of studies focusing on the association between the presence of 1‐h PG levels ≥180 mg/dL or 2‐h PG levels ≥153 mg/dL during a 75‐g OGTT at <24 weeks’ gestation and perinatal outcomes. Therefore, the clinical significance of the differences in the 1‐h and 2‐h PG levels during a 75‐g OGTT, at different gestational ages, should be considered based on further studies investigating the association between changes in the 1‐h PG and 2‐h PG levels at 75‐g OGTT during pregnancy and perinatal outcomes.
The present study had several limitations. First, the perinatal outcomes associated with GDM, which was diagnosed at different gestational ages, could not be evaluated, because we did not collect enough data on adverse maternal and neonatal outcomes in this study. Therefore, the present study could not identify the cut‐off fasting PG value for GDM diagnosis at <24 weeks’ gestation. Second, the present study did not obtain enough data for discussions to be held on the justification of the implementation of a 75‐g OGTT to diagnose GDM at <24 weeks’ gestation. The European Board and College of Obstetrics and Gynaecology proposed screening for overt diabetes, rather than GDM using fasting PG or glycated hemoglobin levels, or a random PG level at <24 weeks’ gestation21. The American Diabetes Association also recommends the performance of a test for undiagnosed diabetes mellitus, rather than GDM at <24 weeks’ gestation. The American Diabetes Association proposed the criteria for the diagnosis of diabetes mellitus using fasting PG levels or a 75‐g OGTT, or glycated hemoglobin or random PG measurements at <24 weeks’ gestation21, 22. In the European Board and College of Obstetrics and Gynaecology and American Diabetes Association recommendations, GDM is not defined at <24 weeks’ gestation21, 22. It is not clear if GDM diagnosis and intervention initiation at <24 weeks’ gestation can improve perinatal outcomes23, 24. Third, data on the levels of glycated hemoglobin – which are used to diagnose overt diabetes in pregnancy – were not collected in the present study. Therefore, the degree of the exclusion of study participants due to the presence of overt diabetes in pregnancy might be insufficient.
In conclusion, the fasting PG, and 1‐h and 2‐h PG levels at 75‐g OGTT differed by gestational age. The likelihood of GDM diagnosis based on the 2010 JSOG criteria and 2010 IADPSG criteria also differed by gestational age. Healthcare providers should be aware that the implementation of a 75‐g OGTT at <14 weeks’ gestation was associated with a higher odds of GDM being diagnosed based on the 2010 JSOG criteria in Japan. Further studies are required to clarify the association between the performance of a 75‐g OGTT at <24 weeks’ gestation and perinatal outcomes. In addition, there is a need for studies that evaluate the benefit of GDM diagnosis and intervention initiation at <24 weeks’ gestation, and justify the application of a 75‐g OGTT at <24 weeks’ gestation.
Disclosure
The authors declare no conflict of interest.
Supporting information
Data S1 | Supplementary material.
Acknowledgments
This work was supported by Grants‐in‐Aid (200100372A, 200200370A and 200300296A) from the Ministry of Health, Labor and Welfare, Health and Labor Sciences Research Grants, Japan. We acknowledge the contributions of the hospitals described in the supporting information.
J Diabetes Investig 2019; 10: 1576–1585
References
- 1. Meek CL, Lewis HB, Patient C, et al Diagnosis of gestational diabetes mellitus: falling through the net. Diabetologia 2015; 58: 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Billionnet C, Mitanchez D, Weill A, et al Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia 2017; 60: 636–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hosseini E, Janghorbani M, Aminorroaya A. Incidence, risk factors, and pregnancy outcomes of gestational diabetes mellitus using one‐step versus two‐step diagnostic approaches: A population‐based cohort study in Isfahan. Iran. Diabetes Res Clin Pract 2018; 140: 288–294. [DOI] [PubMed] [Google Scholar]
- 4. HAPO Study Cooperative Research Group ; Metzger BE, Lowe LP, Dyer AR, et al Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 5. International Association of Diabetes Pregnancy Study Groups Consensus Panel ; Metzger BE, Gabbe SG, Persson B, et al International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McIntyre HD, Sacks DA, Barbour LA, et al Issues with the diagnosis and classification of hyperglycemia in early pregnancy. Diabetes Care 2016; 39: 53–54. [DOI] [PubMed] [Google Scholar]
- 7. Maegawa Y, Sugiyama T, Kusaka H, et al Screening tests for gestational diabetes in Japan in the 1st and 2nd trimester of pregnancy. Diabetes Res Clin Pract 2003; 62: 47–53. [DOI] [PubMed] [Google Scholar]
- 8. Kuzuya T, Nakagawa S, Satoh J, et al ; Committee of the Japan Diabetes Society on the diagnostic criteria of diabetes mellitus . Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002;55:65–85. [DOI] [PubMed] [Google Scholar]
- 9. Minakami H, Hiramatsu Y, Koresawa M, et al ; Japan Society of Obstetrics and Gynecology; Japan Association of Obstetricians and Gynecologists . Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2011 edition. J Obstet Gynaecol Res 2011;37:1174–1197. [DOI] [PubMed] [Google Scholar]
- 10. Minakami H, Maeda T, Fujii T, et al Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res 2014;40:1469–1499. [DOI] [PubMed] [Google Scholar]
- 11. Benhalima K, Devlieger R, Van Assche A. Screening and management of gestational diabetes. Best Pract Res Clin Obstet Gynaecol 2015; 29: 339–349. [DOI] [PubMed] [Google Scholar]
- 12. Siegmund T, Rad NT, Ritterath C, et al Longitudinal changes in the continuous glucose profile measured by the CGMS in healthy pregnant women and determination of cut‐off values. Eur J Obstet Gynecol Reprod Biol 2008; 139: 46–52. [DOI] [PubMed] [Google Scholar]
- 13. Froslie KF, Roislien J, Qvigstad E, et al Shape information in repeated glucose curves during pregnancy provided significant physiological information for neonatal outcomes. PLoS ONE 2014; 9: e90798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mills JL, Jovanovic L, Knopp R, et al Physiological reduction in fasting plasma glucose concentration in the first trimester of normal pregnancy: the diabetes in early pregnancy study. Metabolism 1998; 47: 1140–1144. [DOI] [PubMed] [Google Scholar]
- 15. Hagiwara Y, Kasai J, Nakanishi S, et al Should the IADPSG criteria be applied when diagnosing early‐onset gestational diabetes? Diabetes Res Clin Pract 2018; 140: 154–161. [DOI] [PubMed] [Google Scholar]
- 16. Seshiah V, Balaji V, Balaji MS, et al Gestational diabetes mellitus manifests in all trimesters of pregnancy. Diabetes Res Clin Pract 2007; 77: 482–484. [DOI] [PubMed] [Google Scholar]
- 17. Iwama N, Sugiyama T, Metoki H, et al Maternal body mass index is a better indicator of large‐for‐gestational‐age infants compared with a 75‐g oral glucose tolerance test in early pregnancy: The JAGS trial. Diabetes Res Clin Pract 2017; 132: 10–18. [DOI] [PubMed] [Google Scholar]
- 18. Riskin‐Mashiah S, Damti A, Younes G, et al Normal fasting plasma glucose levels during pregnancy: a hospital‐based study. J Perinat Med 2011; 39: 209–211. [DOI] [PubMed] [Google Scholar]
- 19. Cosson E, Carbillon L, Valensi P. High fasting plasma glucose during early pregnancy: a review about early gestational diabetes mellitus. J Diabetes Res 2017; 2017: 8921712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr 2000; 71: 1256s–1261s. [DOI] [PubMed] [Google Scholar]
- 21. Benhalima K, Mathieu C, Damm P, et al A proposal for the use of uniform diagnostic criteria for gestational diabetes in Europe: an opinion paper by the European Board & College of Obstetrics and Gynaecology (EBCOG). Diabetologia 2015; 58: 1422–1429. [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes A. 2 . Classification and diagnosis of diabetes: standards of medical care in diabetes‐2018. Diabetes Care 2018;41:S13–S27. [DOI] [PubMed] [Google Scholar]
- 23. Colagiuri S, Falavigna M, Agarwal MM, et al Strategies for implementing the WHO diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Diabetes Res Clin Pract 2014; 103: 364–372. [DOI] [PubMed] [Google Scholar]
- 24. Shinar S, Berger H. Early diabetes screening in pregnancy. Int J Gynaecol Obstet 2018; 142: 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 | Supplementary material.


