Abstract
To investigate the incidence and risk factors as well as prognosis of autoimmune hemolytic anemia (AIHA) following allogeneic hematopoietic stem cell transplantation (allo‐HSCT), a total of 1377 adult hematological malignancies at three institutions were enrolled in this study. The 3‐year cumulative incidence of AIHA was 2.2 ± 0.4%. Multivariate analysis showed that haploidentical donors (HRDs) and chronic graft vs host disease (cGVHD) were the independent risk factors for AIHA. Patients with AIHA treated initially with corticosteroids combined with cyclosporine A (CsA) had a higher complete response rate than those with corticosteroids monotherapy (66.7% vs 11.1%; P = .013). The 3‐year cumulative incidence of malignant diseases relapse was 4.4 ± 4.3% and 28.0 ± 1.3% (P = .013), treatment‐related mortality (TRM) was 8.9 ± 6.3% and 17.4 ± 1.2% (P = .431), disease‐free survival (DFS) was 56.1 ± 1.5% and 86.7 ± 7.2% (P = .011), and overall survival (OS) was 86.3 ± 7.4% and 64.1 ± 1.5% (P = .054), respectively, in the patients with AIHA and those without AIHA. Our results indicate that HRDs and cGVHD are risk factors for AIHA and corticosteroids combined with CsA are superior to corticosteroids as initial treatment for AIHA. Autoimmune hemolytic anemia does not contribute to increase TRM and could reduce the malignant diseases relapse and increase DFS.
Keywords: autoimmune hemolytic anemia, hematopoietic stem cell transplantation, risk factors, treatment
Autoimmune hemolytic anemia (AIHA) occurs more frequently than other autoimmune hematological diseases (AHDs) after allogeneic hematopoietic stem cell transplantation. Haploidentical donors and chronic graft vs host disease are risk factors for AIHA and corticosteroids combined with cyclosporine A are superior to corticosteroids as initial treatment for AIHA. AIHA does not contribute to increase treatment‐related mortality and could reduce the malignant diseases relapse and increase disease‐free survival.

1. INTRODUCTION
Autoimmune hematological diseases (AHDs) have been reported to occur more frequently than other autoimmune complications after allogeneic hematopoietic stem cell transplantation (allo‐HSCT).1, 2, 3, 4, 5, 6, 7, 8 Autoimmune hematological diseases may affect a single lineage of blood cells, for example, autoimmune hemolytic anemia (AIHA) and immune thrombocytopenia (ITP), or 2 and/or 3 lineages, for example, Evans syndrome. In these AHDs, AIHA is the most common with estimates of the incidence between 2% and 6%7, 9, 10, 11 in recipients of allo‐HSCT. AIHA posttransplants have been proved to be associated with many factors, such as human leukocyte antigen (HLA)‐mismatched transplants, chronic graft vs host disease (cGVHD), using antithymocyte globulin (ATG), and so on.5, 12, 13, 14 In recent years, an increasing incidence of AIHA has been observed. Haploidentical and unrelated transplants have been widely used,12, 15, 16 whether haploidentical transplants could cause AIHA is rarely reported. Corticosteroids are usually used as first‐line treatment for AIHA, but the effective rate is approximately 10%‐40% in patients with AIHA posttransplants.3, 10, 17, 18 In our multicenter report, we retrospectively analyzed the incidence and risk factors, and the outcomes of corticosteroids combined with cyclosporine A (CsA) or corticosteroids monotherapy as initial treatment in the patients developed AIHA after allo‐HSCT.
2. PATIENTS AND METHODS
2.1. Study design and patients
All consecutive adult patients with hematological malignancies who underwent first allo‐HSCT between December 2011 and December 2016 at Nanfang Hospital, Xiangya Hospital, and First Affiliated Hospital of Guangxi Medical University were analyzed in this retrospective study. Medical records for all patients were reviewed for demographic data, primary diseases, transplant‐related parameters, and AIHA including information on the history and treatment of AIHA pretransplantation. If the patients had a history of AIHA pretransplantation, they were excluded. This study was performed in accordance with the Declaration of Helsinki and was approved by the Institution Review Board of our institution.
2.2. Transplant procedures
High‐resolution molecular techniques were used to detect HLA typing of recipients and donors.11 All patients received myeloablative conditioning regimens. The selection of conditioning regimens was as follows: (a) BuCY (busulfan + cyclophosphamide) or BuF (busulfan + Fludarabine) was applied to patients who had myeloid malignancies with complete remission (CR); (b) TBI + CY (total body irradiation + cyclophosphamide) were applied to patients who had lymphoid malignancies with CR; (c) fludarabine + cytarabine + TBI + CY + etoposide (VP‐16) were applied to patients with no CR (NR).11, 16, 19 Grafts from peripheral blood stem cells (PBSCs) were used in HLA‐matched sibling transplantation patients and unrelated donor transplantation patients. Grafts from PBSCs combined with bone marrow (BM) were used in haploidentical transplantation patients. The selection of graft vs host disease (GVHD) prophylaxis regimens was as follows: (a) CsA + methotrexate (MTX) (at days + 1, 3, and 6) were applied to patients who received transplants from HLA‐matched sibling donor (MSD); (b) CsA + MTX + ATG (7.5 mg/kg) were applied to patients who received transplants from matched unrelated donor (MUD); (c) CsA + MTX + ATG (7.5‐10 mg/kg) + mycophenolate mofetil (MMF) (0.5 g, 2/d × 28 days) were applied to patients who received transplants from haploidentical donor (HRD).11, 16
2.3. Diagnosis and response criteria of AIHA
As previously reported,14, 18, 20 the diagnostic criteria of AIHA and Evans syndrome diagnoses were described below. The diagnostic criteria of AIHA included: (a) a positive direct antiglobulin test (DAT); (b) a positive indirect antiglobulin test with broad reactivity to red blood cells in the serum and eluate; (c) clinical and laboratory evidence of hemolysis (increase in lactate dehydrogenase and bilirubin levels, decrease in hemoglobin [Hb] and haptoglobin levels, or increase in transfusion requirements); and (d) a differentiation diagnosis. Patients of DAT positivity caused by ABO (blood group of ABO) antibodies and patients had history of AIHA or a positive DAT before HSCT were excluded. Patients who never had a DAT positivity were not presumed to have clinically significant AIHA. Patients who had a positive DAT but had evidence of nonimmune hemolysis, for example microangiopathic hemolytic anemia, were also excluded. Furthermore, the primary and secondary poor graft function posttransplants were also excluded from the diagnosis of AIHA after allo‐HSCT. The diagnostic criteria of Evans syndrome: either a simultaneous combination or a sequential combination of ITP and AIHA with DAT positivity.
Responses were mainly evaluated at 4 and 12 weeks after initial treatment. Thresholds for determining response were based on standard and previous studied outcome criteria for AIHA and Evans syndrome.11, 21 The criteria for effectiveness were as follows: (a) CR: Hb level of 12 g/dL and a platelet (PLT) level of 100 g/L or more in the absence of a transfusion without features of hemolysis (normal bilirubin and lactate dehydrogenase levels ± normal haptoglobin level if performed); (b) partial response (PR): Hb level of at least 10 g/dL with an increase of at least 2 g from baseline and a PLT level of at least 50 g/L; (c) NR: failure to meet the above two criteria; and (d) the overall response (OR) rate included both CR and PR.
2.4. Evaluation points and definitions
This study mainly focused on the incidence and risk factors of AIHA, treatment response, treatment‐related mortality (TRM), malignant diseases relapse, disease‐free survival (DFS), and overall survival (OS). In patients with AIHA in CR or PR, relapse of AIHA was defined as the loss of CR or PR status, respectively. Relapse of malignant diseases was defined by reappearance of blasts in the peripheral blood, recurrence of BM blasts >5%, or development of extramedullary disease infiltrates at any site. Treatment‐related mortality was defined as death from any cause except malignant diseases relapse. Disease‐free survival was defined as survival in a state of continuous CR. Overall survival was defined as the earliest time from AIHA diagnosis to death from all causes.
2.5. Statistical analysis
Patient follow‐up was updated on December 2017. Data were presented as the mean ± SD or median (range) for continuous variables, depending on the distribution. Categorical variables were presented as numbers (%). The Chi‐squared or Fisher exact tests were used to compare proportions. Disease‐free survival and OS were analyzed with the Kaplan‐Meier method, comparing groups using the log rank test (Mantel‐Haenszel). Treatment‐related mortality and relapse were calculated using reciprocal cumulative incidence estimates to account for competing risks (Gray test). Risk factors achieving statistical significance upon univariate analysis underwent additional multivariate analysis using Cox regression to identify the most significant independent risk factors. All P values were two‐sided and considered significant with P < .05. Statistical analyses were performed with SPSS Version 19.0.
3. RESULTS
3.1. Patients' demographics and baseline characteristics
Of the 1381 patients with hematological malignancies enrolled in this retrospective study, 1377 were retained for analysis, and four were excluded due to the history of AIHA before transplantation. Among the 1377 patients retained for analysis, 651 patients came from Nanfang Hospital, 266 patients came from Xiangya Hospital, and 460 patients came from First Affiliated Hospital of Guangxi Medical University. These patients had a median age of 30 years (range, 13‐78 years), with 752 males and 625 females. The underlying diseases included myelogenous leukemia (n = 782) and lymphoid leukemia (n = 595). Nine hundred and ninety‐three patients achieved CR and 384 patients were in PR or NR at the time of transplantation. Seven hundred and sixty‐six patients received MSD, 328 MUD, and 283 HRD transplants.
3.2. Incidence and risk factors of AIHA
Twenty‐six patients had AIHA, including 19 with AIHA and seven with AIHA accompanied with thrombocytopenia (Evans syndrome). Eleven were females and 15 males with a median age of 23.5 years (range, 15‐46) at transplants. The median time of AIHA onset was 215 days (range, 34‐756 days) posttransplants. The median number of white blood cell count, Hb count, and PLT count at the time of AIHA diagnosis was 3.13 G/L (range, 1.32‐7.23), 57.5 g/L (range, 34‐75), and 133 G/L (range, 11‐187), respectively. These patients all had complete donor chimerism at time of diagnosis of AIHA. At the time of AIHA onset, 15 patients were treated with immunosuppressive agents, including six cases with GVHD prophylaxis that gradually tapered and nine with GVHD treatment (Table 1). The baseline and transplant characteristics of patients with and without AIHA are shown in Table 2.
Table 1.
Characteristics of patients with AIHA
| No | Gender | Age | Diagnosis | Donor type | Type of AIHA | Time to AIHA | cGVHD | Immunosuppressive therapies at the onset of AIHA | Initial treatment | AIHA relapse | Underlying disease relapse |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 46 | AML | HRD | Evans | 245 | Yes | MMF(0.25 g2/d) + tacrolimus (0.5 mg 2/d) | GC + CsA | No | No |
| 2 | Male | 21 | MDS | HRD | Evans | 203 | Yes | MMF(0.25 g 2/d) + CsA (150 mg 1/d) + methylprednisolone (32 mg 1/d) | GC + CsA | No | No |
| 3 | Male | 18 | T‐LBL | MUD | AIHA | 312 | No | CsA (50 mg 2/d) + methylprednisolone (32 mg 1/d) | GC + CsA | No | No |
| 4 | Male | 25 | AML | HRD | Evans | 133 | Yes | CsA (50 mg 2/d) + methylprednisolone (20 mg 1/d) | GC + CsA | No | No |
| 5 | Male | 37 | ALL | HRD | AIHA | 189 | No | Methylprednisolone (60 mg 1/d) | —a | —a | No |
| 6 | Male | 38 | AUL | MSD | AIHA | 375 | Yes | Methylprednisolone (40 mg 1/d) + prograf (1.5 mg 2/d) | GC + CsA | —b | No |
| 7 | Female | 29 | ALL | MSD | AIHA | 756 | No | methylprednisolone(32 mg 1/d) | GC + CsA | No | No |
| 8 | Female | 46 | MDS | HRD | Evans | 60 | No | MMF (0.5 g 2/d) + CsA (50 mg 2/d) + methylprednisolone (40 mg 3/d) | GC + CsA | No | No |
| 9 | Female | 20 | ALL | HRD | AIHA | 140 | No | MMF (0.25 g 1/d) + CsA (50 mg 2/d) + methylprednisolone (40 mg 1/d) | GC + CsA | —b | No |
| 10 | Male | 18 | ALL | HRD | Evans | 104 | No | MMF (0.2 g 2/d) + tacrolimus (0.5 mg 2/d) + methylprednisolone (40 mg 1/d) | GC + CsA | —b | No |
| 11 | Male | 16 | ALL | MUD | AIHA | 154 | Yes | CsA (50 mg 2/d) + methylprednisolone (32 mg 1/d) | GC + CsA | No | No |
| 12 | Male | 19 | ALL | HRD | Evans | 203 | No | MMF(0.5 g 2/d) + CsA (50 mg 2/d) + methylprednisolone (40 mg 3/d) | GC + CsA | No | No |
| 13 | Male | 22 | ALL | MUD | AIHA | 252 | Yes | — | GC + CsA | No | No |
| 14 | Male | 22 | AML | HRD | AIHA | 215 | Yes | — | GC | No | No |
| 15 | Male | 44 | AML | MUD | Evans | 111 | Yes | CsA (25 mg 2/d) | GC | Yes | No |
| 16 | Male | 44 | ALL | MSD | AIHA | 258 | Yes | — | GC | —b | No |
| 17 | Female | 15 | ALL | HRD | AIHA | 34 | No | MMF (0.25 g 2/d) + tacrolimus (0.5 mg 2/d) | GC + CsA | No | No |
| 18 | Female | 36 | AML | HRD | AIHA | 134 | Yes | MMF (0.25 g 2/d) + CsA (150 mg 1/d) + methylprednisolone (32 mg 1/d) | GC | Yes | No |
| 19 | Female | 31 | ALL | HRD | AIHA | 139 | Yes | CsA (50 mg 2/d) + methylprednisolone (32 mg 1/d) | GC | —b | No |
| 20 | Male | 20 | ALL | MSD | AIHA | 254 | Yes | — | GC | Yes | No |
| 21 | Female | 27 | ALL | HRD | AIHA | 349 | Yes | — | GC | Yes | No |
| 22 | Female | 26 | ALL | HRD | AIHA | 263 | Yes | — | GC | —c | No |
| 23 | Male | 18 | AML | HRD | AIHA | 250 | Yes | — | GC | No | No |
| 24 | Female | 22 | AML | MUD | AIHA | 220 | Yes | MMF (0.5 g 2/d) + CsA (50 mg 2/d) + methylprednisolone (40 mg 3/d) | GC + CsA | No | No |
| 25 | Female | 34 | AML | MSD | AIHA | 197 | No | — | GC + CsA | No | No |
| 26 | Female | 21 | AML | MUD | AIHA | 228 | Yes | — | GC | No | Yes |
Abbreviations: AIHA, autoimmune hematological diseases; ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; AUL, acute undifferentiated leukemia; CsA, cyclosporine A; GC, glucocorticoid; HRD, haploidentical‐related donor; MDS, myelodysplastic syndrome; MMF, mycophenolate mofetil; MSD, matched sibling donor; MUD, matched unrelated donor; T‐LBL, T‐lymphoblastic lymphoma.
This patient died of infectious shock 2 days after the diagnosis of AIHA and was excluded from AIHA treatment and relapse analyses.
These patients received corticosteroids combined CsA treatment who obtained no response after the 4 weeks' initial treatment changed their regimen by adding second‐line treatments so that was excluded from AIHA relapse analyses.
This patient received corticosteroids monotherapy who did not response to the 2 weeks' initial treatment changed their regimen by adding CsA so that was excluded from AIHA relapse analyses.
Table 2.
Characteristics of patients with and without AIHA
| Characteristic | Patients with AIHA | Patients without AIHA | P |
|---|---|---|---|
| Gender, n (%) | .457 | ||
| Male | 15 (58) | 737 (55) | |
| Female | 11 (42) | 614 (45) | |
| Median age at HSCT, y (range) | 23.5 (15‐46) | 30 (13‐78) | .466 |
| Type of underlying disease, n (%) | .318 | ||
| Myelogenous | 12(46) | 770 (57) | |
| Lymphoid | 14 (54) | 581 (43) | |
| Disease status at HSCT, n (%) | .741 | ||
| CR | 18 (69) | 975 (72) | |
| Non‐CR | 8 (31) | 376 (28) | |
| Donor source, n (%) | <.001* | ||
| MSD | 6 (23) | 760 (56) | |
| MUD | 5 (19) | 323 (24) | |
| HRD | 15 (58) | 268 (20) | |
| HLA disparity, n (%) | <.001* | ||
| Matched | 11 (42) | 1045 (77) | |
| Mismatched | 15 (58) | 306 (23) | |
| ABO matched, n (%) | .985 | ||
| Yes | 13 (50) | 678 (50) | |
| No | 13 (50) | 673 (50) | |
| Sex matched, n (%) | .232 | ||
| Yes | 15 (58) | 620 (46) | |
| No | 11 (42) | 731 (54) | |
| Conditioning regimens, n (%) | .443 | ||
| TBI used | 17 (65) | 782 (58) | |
| TBI non‐used | 9 (35) | 569 (42) | |
| GVHD prophylaxis, n (%) | .001* | ||
| ATG used | 21 (81) | 651 (48) | |
| ATG non‐used | 5 (19) | 700 (52) | |
| Source of stem cell, n (%) | <.001* | ||
| Bone marrow + PBSCs | 15 (58) | 294 (22) | |
| PBSCs | 11 (42) | 1057 (78) | |
| CMV viremia posttransplants, n (%) | .991 | ||
| Yes | 14 (54) | 726 (54) | |
| No | 12 (46) | 625 (46) | |
| aGVHD, n (%) | .711 | ||
| Yes | 11 (42) | 621 (46) | |
| No | 15 (58) | 730 (54) | |
| cGVHD, n (%) | .009* | ||
| Yes | 18 (69) | 582 (43) | |
| No | 8 (31) | 769 (57) |
Abbreviations: aGVHD, acute graft vs host disease; AIHA, autoimmune hematological diseases; ATG, antithymocyte globulin; cGVHD, chronic graft vs host disease; CMV, cytomegalovirus; CR, complete remission; GVHD, graft vs host disease; HLA, human leukocyte antigen; HRD, haploidentical‐related donor; HSCT, hematopoietic stem cell transplantation; MSD, matched sibling donor; MUD, matched unrelated donor; TBI, total body irradiation; PBSCs, peripheral blood stem cells.
P < .05.
The overall 3‐year incidence of AIHA posttransplantation was 2.2 ± 0.4%, and the 3‐year incidence of AIHA in HRD, MUD, and MSD was 6.3 ± 1.6%, 1.8 ± 0.8% and 1.0 ± 0.4%, respectively. HRD had higher incidence than MUD (P < .001) and MSD (P = .004), but there was no difference between MUD and MSD transplants (P = .222) (Figure 1). Univariate analysis showed that donor source, HLA mismatched, ATG, and cGVHD were risk factors for AIHA (Table 2), but multivariate analysis showed that only HRD and cGVHD were risk factors for AIHA (Table 3).
Figure 1.
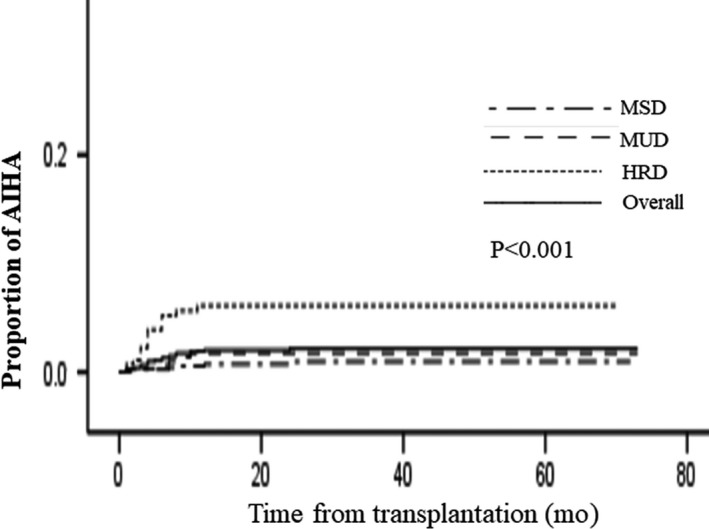
Cumulative incidence of autoimmune hemolytic anemia (AIHA) according to type of donor
Table 3.
Multivariate analysis for risk factors of AIHA
| Variable | Multivariate (HR) |
|---|---|
| Male vs female | P = .860 |
| Patient age, >30 y old, ≤30 y old | P = .956 |
| Myelogenous vs lymphoid | P = .079 |
| MSD vs HRD |
P < .001 (7.076) 95% CI: 2.741‐18.265 |
| MUD vs HRD |
P = .012 (3.679) 95% CI: 1.336‐10.132 |
| MSD vs MUD | P = .276 |
| CR vs non‐CR | P = .576 |
| PBSCs vs PBSCs + BM | P = .957 |
| HLA matched vs mismatched | P = .802 |
| ABO matched vs mismatched | P = .667 |
| Sex matched vs mismatched | P = .374 |
| ATG used vs non‐used | P = .332 |
| TBI used vs non‐used | P = .297 |
| CMV viremia positive vs negative | P = .581 |
| aGVHD vs non‐aGVHD | P = .468 |
| cGVHD vs non‐cGVHD |
P = .028 (2.554) 95% CI: 1.109‐5.884 |
Abbreviations: aGVHD, acute graft vs host disease; AIHA, autoimmune hematological diseases; ATG, antithymocyte globulin; BM, bone marrow; cGVHD, chronic graft vs host disease; CI, confidence interval; CMV, cytomegalovirus; CR, complete remission; HLA, human leukocyte antigen; HR, hazard ratio; HRD, haploidentical‐related donor; MSD, matched sibling donor; MUD, matched unrelated donor; PBSCs, peripheral blood stem cells; TBI, total body irradiation.
3.3. Treatment and response
Among the 26 patients diagnosed AIHA, only 25 patients had access to treatment because one patient with AIHA died of infectious shock 2 days after the diagnosis of AIHA. Of the 25 patients who received treatment on the basis of their original immunosuppressive agents, 15 had corticosteroids (1‐2 mg/kg) combined with CsA as initial treatment and the remaining 10 patients had corticosteroids (1‐2 mg/kg) monotherapy as initial treatment. Baseline information between the two treatment groups is shown in Table 4.
Table 4.
Baseline information between the two treatment groups
| CsA + GC | GC | P‐value | |
|---|---|---|---|
| Number | 15 | 10 | |
|
Age (y) Median (range) |
22 (15‐46) | 26.5 (18‐44) | .478 |
|
Disease type Myelogenous/Lymphoid |
6/9 | 5/5 | .697 |
|
Transplant type HRD/MSD/MUD |
8/3/4 | 6/2/2 | .924 |
| Sex ratio (men/women) | 9/6 | 5/5 | .466 |
|
Pretransplant disease state CR/Non‐CR |
11/4 | 7/3 | 1.000 |
|
Stem cell source PBSC/PBSC + BM |
7/8 | 4/6 | .742 |
|
HLA matched Yes/No |
7/8 | 4/6 | .742 |
|
ABO matched Yes/No |
8/7 | 5/5 | 1.000 |
|
Sex matched Yes/No |
7/8 | 7/3 | .414 |
|
ATG/CD25 used Yes/No |
11/4 | 9/1 | .615 |
|
TBI used Yes/No |
10/5 | 6/4 | .734 |
|
CMV viremia Positive/Negative |
10/5 | 3/7 | .111 |
|
aGVHD Yes/No |
7/8 | 4/6 | .742 |
|
cGVHD Yes/No |
7/8 | 10/0 | .008 |
|
AHDs type AIHA/Evans |
9/6 | 9/1 | .179 |
Abbreviations: aGVHD, acute graft vs host disease; AHDs, autoimmune hematological diseases; AIHA, autoimmune hemolytic anemia; ATG, antithymocyte globulin; BM, bone marrow; cGVHD, chronic graft vs host disease; CMV, cytomegalovirus; CsA, cyclosporine A; CR, complete remission; Evans, Evans syndrome; GC, glucocorticoid; HLA, human leukocyte antigen; HRD, haploidentical‐related donor; MSD, matched sibling donor; MUD, matched unrelated donor; PBSCs, peripheral blood stem cells; TBI, total body irradiation.
After 4 weeks of initial treatment, one patient who received corticosteroids treatment was excluded from effect analysis because the patient did not respond to the 2‐week initial corticosteroids treatment and received corticosteroids combined with CsA. The OR rate was 80.0% and 77.7% (P = .635), and the CR rate was 66.7% and 11.1% (P = .013), respectively, in patients who received corticosteroids combined with CsA and corticosteroids monotherapy. Five patients who had no response to treatment after 4 weeks all received second‐line treatments, including rituximab (n = 4) and CsA + MMF (n = 1). Within 12 weeks of treatment, all patients had response. One patient (6.7%) experienced AIHA relapse who received corticosteroids combined with CsA treatment, while five (50.0%) experienced relapse who received corticosteroids monotherapy (P = .023) at a median follow‐up of 22 months (range, 6‐56 months). Fortunately, all of the relapsed patients achieved remission again after immunosuppressive therapy.
3.4. Survival
Twenty‐three patients were alive and three were dead at a median follow‐up of 662 days (range, 2‐1726 days) after AIHA. The causes of death included infections (n = 2) and leukemia relapse (n = 1). To avoid the effect of the patients who died or relapsed before onset of AIHA, we chose the earliest date of AIHA onset (on 34 day posttransplants) as a landmark to calculate the outcome (relapse, OS, TRM). Of the 1351 patients without AIHA, 1335 cases were enrolled in analysis for malignant disease relapse, TRM, DFS, and OS based on the above contents. The 3‐year cumulative incidence of malignant diseases relapse, DFS, and OS posttransplant were 27.5 ± 1.2%, 56.1 ± 1.5%, and 64.5 ± 1.5%, respectively. The 3‐year cumulative incidence of malignant diseases relapse was 4.4 ± 4.3% and 28.0 ± 1.3% (P = .013) (Figure 2A), TRM posttransplants was 8.9 ± 6.3% and 17.4 ± 1.2% (P = .431) (Figure 2B), DFS was 56.1 ± 1.5% and 86.7 ± 7.2% (P = .011) (Figure 2C), and OS was 86.3 ± 7.4% and 64.1 ± 1.5% (P = .054) (Figure 2D), respectively, for patients with AIHA and those without AIHA. Risk factors for malignant diseases relapse and survival are presented in Table 5. In multivariate analysis for malignant diseases relapse and DFS, myelogenous disease, CR at transplantation, cGVHD, and AIHA were beneficial factors. In multivariate analysis for OS, CR at transplantation and cGVHD were beneficial factors.
Figure 2.
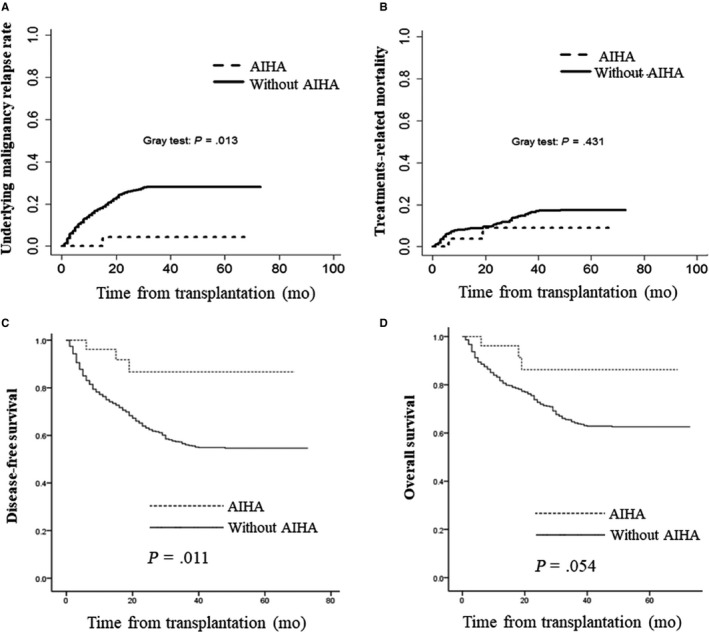
Relapse, treatment‐related mortality, disease‐free survival, and overall survival in patients with and without autoimmune hematological diseases (AIHA). A, Accumulation underlying malignancy relapse rate in patients with and without AIHA. B, Accumulation of treatment‐related mortality in patients with and without AIHA. C, Accumulation of disease‐free survival function in patients with and without AIHA. D, Accumulation of survival function in patients with and without AIHA
Table 5.
Univariate and multivariate analyses for malignant diseases relapse, DFS, and OS
| Variable | Relapse | DFS | OS | |||
|---|---|---|---|---|---|---|
| Univariable | Multivariable (HR) | Univariable | Multivariable (HR) | Univariable | Multivariable (HR) | |
| Male vs female | P = .773 | P = .925 | P = .747 | P = .727 | P = .422 | P = .475 |
| Patient age, >30 y old, ≤30 y old | P = .300 | P = .965 | P = .770 | P = .649 | P = .511 | P = .512 |
| Myelogenous vs lymphoid | P = .005 |
P = .004 (0.732) 95% CI: 0.592‐0.904 |
P = .086 |
P = .026 (0.824) 95% CI: 0.694‐0.978 |
P = .667 | P = .295 |
| Donor type | P = .041 | P = .021 | P = .080 | |||
| MSD vs HRD | P = .365 | P = .431 | P = .264 | |||
| MUD vs HRD | P = .113 | P = .733 | P = .147 | |||
| MSD vs MUD | P = .312 | P = .778 | P = .556 | |||
| CR vs non‐CR | P < .001 |
P < .001 (0.668) 95% CI: 0.536‐0.833 |
P < .001 |
P = .004 (0.765) 95% CI: 0.639‐0.916 |
P < .001 |
P < .001 (0.678) 95% CI: 0.554‐0.829 |
| PBSCs vs PBSCs + BM | P = .062 | P = .531 | P = .022 | P = .584 | P = .047 | P = 309 |
| HLA matched vs mismatched | P = .016 | P = .263 | P = .010 | P = .411 | P = .032 | P = 242 |
| ABO matched vs mismatched | P = .817 | P = .651 | P = .911 | P = .713 | P = .745 | P = 911 |
| Sex matched vs mismatched | P = .216 | P = .136 | P = .250 | P = .159 | P = .337 | P = 227 |
| ATG used vs non‐used | P = .705 | P = .958 | P = .263 | P = .706 | P = .083 | P = .190 |
| TBI uses vs non‐used | P < .001 | P = .798 | P = .001 | P = .795 | P < .001 | P = .214 |
| CMV viremia positive vs negative | P = .341 | P = .484 | P = .880 | P = .618 | P = .068 | P = .444 |
| aGVHD vs non‐aGVHD | P = .186 | P = .131 | P = .989 | P = .432 | P = .361 | P = .849 |
| cGVHD vs non‐cGVHD | P < .001 |
P < .001 (0.667) 95% CI: 0.532‐0.838 |
P < .001 |
P = .001 (0.738) 95% CI: 0.617‐0.883 |
P < .001 |
P < .001 (0.666) 95% CI: 0.540‐0.821 |
| AIHA vs non‐AIHA | P = .011 |
P = .049 (0.139) 95% CI: 0.020‐0.999 |
P = .003 |
P = .026 (0.275) 95% CI: 0.088‐0.856 |
P = .036 | P = .074 |
Abbreviations: aGVHD, acute graft vs host disease; AIHA, autoimmune hematological diseases; ATG, antithymocyte globulin; BM, bone marrow; cGVHD, chronic graft vs host disease; CI, confidence interval; CMV, cytomegalovirus; CR, complete remission; DFS, disease‐free survival; HLA, human leukocyte antigen; HR, hazard ratio; HRD, haploidentical‐related donor; MSD, matched sibling donor; MUD, matched unrelated donor; OS, overall survival; PBSCs, peripheral blood stem cells; TBI, total body irradiation.
4. DISCUSSION
In this study, we retrospectively reviewed the incidence of AIHA in a multicenter from southern China. Our result showed that the 3‐year incidence of AIHA in HRD, MUD, and MSD was 6.3 ± 1.6%, 1.8 ± 0.8%, and 1.0 ± 0.4%, respectively, which was consistent with our single‐center report.11 Multivariate analysis demonstrated that HRD and cGVHD were risk factors of AIHA posttransplants. It has been reported that the most common AHDs posttransplant was AIHA.7, 10, 13, 14 In our group of 26 patients with AIHA, our result showed that seven patients diagnosed with Evans syndrome were accompanied with thrombocytopenia and they mainly occurred in HRD patients (n = 6).
The treatment of AIHA posttransplants is no consensus. This AIHA is more refractory to corticosteroids as a first‐line treatment compared with primary AIHA.18, 22, 23 To those who failed in response to corticosteroids treatment, second‐ and third‐line treatments, such as rituximab, CsA, and MMF, were administered, with an effective rate of approximately 60%‐85%.3, 10, 17, 18 In this report, we compared the efficacy of corticosteroids combined with CsA to corticosteroids monotherapy as initial treatment for AIHA after allo‐HSCT. Our results demonstrated that corticosteroids combined with CsA were superior to corticosteroids monotherapy as initial treatment for AIHA. Rituximab is frequently used as second‐line therapy for AIHA, with effective rate of above 80%.7, 10, 24, 25 In our study, the four patients who failed to respond to initial therapy all had a response to rituximab. Relapse occurred in approximately 50% of patients with primary AIHA, but the relapse rate for AIHA posttransplants had rarely been reported.26, 27 In our study, the relapse rate was higher in the patients received corticosteroids monotherapy compared with those received corticosteroids combined with CsA treatment. Whether AIHA contributes to increase TRM has not yet been defined.7, 12, 18 Daikeler et al suggested that AIHA was not attributable to TRM.7, 12 In contrast, Sokol et al28 and Meng et al18 reported that AIHA contributed to increase TRM. Our results demonstrated that AIHA did not contribute to increase mortality instead of increasing DFS. The good survival of AIHA patients was attributed to good therapeutic responses of AIHA and a lower rate of relapse for primary malignancies. Interestingly, only one patient experienced primary malignancy relapse in 26 patients with AIHA in our study and the 3‐year cumulative incidence of malignant diseases relapse was 4.4 ± 4.3% while the incidence of patients without AIHA was 28.0 ± 1.3% (P = .013). Our result showed that patients with AIHA had low primary malignancy relapse which was consistent with Sanz J's study.14 Sanz reported that none of the 12 cases with AIHA died of primary malignancy relapse. A reasonable interpretation of low primary malignancy relapse rate in patients with AIHA might be grafts vs malignancy (GVM) effects in these patients.29, 30 In this report, our result showed that cGVHD was a risk factor of AIHA and it was a protective factor for primary malignancy relapse, which supported our above interpretation. Whether AIHA‐induced immune responses contributed to GVM effects is worth further study.
A major limitation of the study was that this is a retrospective analysis of data. Some relevant factors might not be deeply discussed and found because of incomplete data records and the data records might not be accurate enough which could increase the error of the acquired data.
5. CONCLUSION
In conclusion, our results suggest that HRD transplants and cGVHD are risk factors for AIHA and corticosteroids combined with CsA are superior to corticosteroids as initial treatment for AIHA. Notably, AIHA does not contribute to increase TRM instead of contributing to reduce primary diseases relapse.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
W.‐R.L., H.Q., and M.‐Q.W. performed investigations, analyzed data, and wrote the paper; Z.‐P.F. and F.H. analyzed data; N.X., L.X., R.L., K.Z., and J.S. performed investigations; Y.‐R.L, Y.‐J.X., and Q.‐F.L. designed study. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant nos. U1401221, 81470349, 81621001), the Natural Science Foundation of Guangdong Province (Grant No. 2014B020226004, No. 2016B030230003), the Guangzhou Science and Technology Program Key Projects (Grant No. 201707010213).
Lv W, Qu H, Wu M, et al. Autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation in adults: A southern China multicenter experience. Cancer Med. 2019;8:6549–6558. 10.1002/cam4.2539
Weiran Lv, Hong Qu and Meiqing Wu contributed equally to this work.
Yongrong Lai, Yajing Xu and Qifa Liu are co‐corresponding authors.
REFERENCES
- 1. Chang YJ, Zhao XY, Xu LP, et al. Donor‐specific anti‐human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drobyski WR, Potluri J, Sauer D, et al. Autoimmune hemolytic anemia following T cell‐depleted allogeneic bone marrow transplantation. Bone Marrow Transplant. 1996;17:1093‐1099. [PubMed] [Google Scholar]
- 3. O'Brien TA, Eastlund T, Peters C, et al. Autoimmune haemolytic anaemia complicating haematopoietic cell transplantation in paediatric patients: high incidence and significant mortality in unrelated donor transplants for non‐malignant diseases. Br J Haematol. 2004;127:67‐75. [DOI] [PubMed] [Google Scholar]
- 4. Daikeler T, Tyndall A. Autoimmunity following haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2007;20:349‐360. [DOI] [PubMed] [Google Scholar]
- 5. Loh Y, Oyama Y, Statkute L, et al. Development of a secondary autoimmune disorder after hematopoietic stem cell transplantation for autoimmune diseases: role of conditioning regimen used. Blood. 2007;109:2643‐2648. [DOI] [PubMed] [Google Scholar]
- 6. Page KM, Mendizabal AM, Prasad VK, et al. Posttransplant autoimmune hemolytic anemia and other autoimmune cytopenias are increased in very young infants undergoing unrelated donor umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2008;14:1108‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daikeler T, Labopin M, Ruggeri A, et al. New autoimmune diseases after cord blood transplantation: a retrospective study of EUROCORD and the Autoimmune Disease Working Party of EBMT. Blood. 2013;121:1059‐1064. [DOI] [PubMed] [Google Scholar]
- 8. Tivol E, Komorowski R, Drobyski WR. Emergent autoimmunity in graft‐versus‐host disease. Blood. 2005;105:4885‐4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holbro A, Abinun M, Daikeler T. Management of autoimmune diseases after haematopoietic stem cell transplantation. Br J Haematol. 2012;157:281‐290. [DOI] [PubMed] [Google Scholar]
- 10. Faraci M, Zecca M, Pillon M, et al. Autoimmune hematological diseases after allogeneic hematopoietic stem cell transplantation in children: an Italian multicenter experience. Biol Blood Marrow Transplant. 2014;20(2):272‐278. [DOI] [PubMed] [Google Scholar]
- 11. Lv WR, Fan ZP, Huang F, et al. Autoimmune hematological diseases following haploidentical donor hematopoietic stem cell Transplant compared with matched sibling and unrelated donor. Oncotarget. 2017;8(16):26505‐26514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Daikeler T, Labopin M, Di Gioia M, et al. Secondary autoimmune diseases occurring after HSCT for an autoimmune disease: a retrospective study of the EBMT Autoimmune Disease Working Party. Blood. 2011;118:1693‐1698. [DOI] [PubMed] [Google Scholar]
- 13. Sanz J, Arango M, Carpio N, et al. Autoimmune cytopenias after umbilical cord blood transplantation in adults with hematological malignancies: a single‐center experience. Bone Marrow Transplant. 2014;49:1084‐1088. [DOI] [PubMed] [Google Scholar]
- 14. Sanz J, Arriaga F, Montesinos P, et al. Autoimmune hemolytic anemia following allogeneic hematopoietic stem cell transplantation in adult patients. Bone Marrow Transplant. 2007;39:555‐561. [DOI] [PubMed] [Google Scholar]
- 15. Huang XJ. Current status of haploidentical stem cell transplantation for leukemia. J Hematol Oncol. 2008;1:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu S, Fan Q, Sun J, et al. Haploidentical transplantation without in vitro t‐cell depletion results in outcomes equivalent to those of contemporaneous matched sibling and unrelated donor transplantation for acute leukemia. Medicine. 2016;95(11):e2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crowther M, Chan Y, Garbett IK, Lim W, Vickers MA, Crowther MA. Evidence‐based focused review of the treatment of idiopathic warm immune hemolytic anemia in adults. Blood. 2011;118:4036‐4040. [DOI] [PubMed] [Google Scholar]
- 18. Wang M, Wang W, Abeywardane A, et al. Autoimmune hemolytic anemia after allogeneic hematopoietic stem cell transplantation: analysis of 533 adult patients who underwent transplantation at King's College Hospital. Biol Blood Marrow Transplant. 2015;21:60‐66. [DOI] [PubMed] [Google Scholar]
- 19. Xuan LI, Huang F, Fan Z, et al. Effects of intensified conditioning on Epstein‐Barr virus and cytomegalovirus infections in allogeneic hematopoietic stem cell transplantation for hematological malignancies. J Hematol Oncol. 2012;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norton A, Roberts I. Management of Evans syndrome. Br J Haematol. 2006;132(2):125‐137. [DOI] [PubMed] [Google Scholar]
- 21. Kotb R, Pinganaud C, Trichet C, et al. Efficacy of mycophenolate mofetil in adult refractory auto‐immune cytopenias: a single center preliminary study. Eur J Haematol. 2005;75(1):60‐64. [DOI] [PubMed] [Google Scholar]
- 22. Petz LD. Treatment of autoimmune haemolytic anaemias. Curr Opin Hematol. 2001;8:411‐416. [DOI] [PubMed] [Google Scholar]
- 23. Rovira J, Cid J, Gutiérrez‐García G, et al. Fatal immune hemolytic anemia following allogeneic stem cell transplantation: report of 2 cases and review of literature. Transfus Med Rev. 2013;27(3):166‐170. [DOI] [PubMed] [Google Scholar]
- 24. Hongeng S, Tardtong P, Worapongpaiboon S, Ungkanont A, Jootar S. Successful treatment of refractory autoimmune haemolytic anaemia in a post‐unrelated bone marrow transplant paediatric patient with rituximab. Bone Marrow Transplant. 2002;29:871‐872. [DOI] [PubMed] [Google Scholar]
- 25. Raj K, Narayanan S, Augustson B, et al. Rituximab is effective in the management of refractory autoimmune cytopenias occurring after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;35:299‐301. [DOI] [PubMed] [Google Scholar]
- 26. Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930‐2936. [DOI] [PubMed] [Google Scholar]
- 27. Rattarittamrong E, Eiamprapai P, Tantiworawit A, et al. Clinical characteristics and long‐term outcomes of warm‐type autoimmune hemolytic anemia. Hematology. 2016;21(6):368‐374. [DOI] [PubMed] [Google Scholar]
- 28. Sokol R, Stamps R, Booker D, et al. Posttransplant immune‐mediated hemolysis. Transfusion. 2002;42:198‐204. [DOI] [PubMed] [Google Scholar]
- 29. Weisdorf D, Zhang M‐J, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft‐versus‐host disease induced graft‐versus‐leukemia effect: greater impact on relapse and disease‐free survival after reduced intensity conditioning. Biol Blood Marrow Transplant. 2012;18(11):1727‐1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mo X‐D, Xu L‐P, Zhang X‐H, et al. Chronic GVHD induced GVL effect after unmanipulated haploidentical hematopoietic SCT for AML and myelodysplastic syndrome. Bone Marrow Transplant. 2015;50(1):127‐133. [DOI] [PubMed] [Google Scholar]


