Abstract
Background
Personal cancer diagnosis and family cancer history factor into which individuals should undergo genetic testing for hereditary breast and ovarian cancer (HBOC) syndrome. Family history is often determined in the research setting through kindreds with disease clusters, or clinically from self‐report. The population prevalence of individuals with diagnostic characteristics and/or family cancer history meeting criteria for HBOC testing is unknown.
Methods
Utilizing Surveillance, Epidemiology, and End Results (SEER) cancer registry data and a research resource linking registry records to genealogies, the Utah Population Database, the population‐based prevalence of diagnostic and family history characteristics meeting National Comprehensive Cancer Network (NCCN) criteria for HBOC testing was objectively assessed.
Results
Among Utah residents with an incident breast cancer diagnosis 2010‐2015 and evaluable for family history, 21.6% met criteria for testing based on diagnostic characteristics, but the proportion increased to 62.9% when family history was evaluated. The proportion of cases meeting testing criteria at diagnosis was 94% for ovarian cancer, 23% for prostate cancer, and 51.1% for pancreatic cancer. Among an unaffected Utah population of approximately 1.7 million evaluable for family history, 197,601 or 11.6% met testing criteria based on family history.
Conclusions
This study quantifies the population‐based prevalence of HBOC criteria using objectively determined genealogy and cancer incidence data. Sporadic breast cancer likely represents a portion of the high prevalence of family cancer history seen in this study. These results underline the importance of establishing presence of a deleterious mutation in an affected family member, per NCCN guidelines, before testing unaffected relatives.
Keywords: BRCA1/2, cancer registry, epidemiology, genetic counseling, hereditary cancer
Using diagnostic and family history data, almost 63% of individuals with breast cancer diagnoses meet criteria for genetic testing based on diagnostic and family history. Utilizing the Utah Population Database, a research resource that links of four decades of cancer registry records to genealogies, almost 12% of the unaffected Utah population meets criteria for genetic testing. Given the high proportion of the population, targeting affected cases can maximize family impact while minimizing cost.
1. INTRODUCTION
Hereditary breast and ovarian cancer (HBOC) caused by pathogenic variants in BRCA1 or BRCA2 represent approximately 2% of breast cancers1 and 10%‐15% of ovarian cancers.2, 3 Clinical counseling and testing services for hereditary breast and ovarian cancer have been available since the 1990s and criteria for identifying breast and ovarian cancer cases due to hereditary causes have been developed over time. Several characteristics associated with an increased likelihood of a pathogenic BRCA1 or BRCA2 variant include young age of breast cancer onset (<45 years), epithelial ovarian cancer, triple negative breast cancer, or multiple relatives with breast and/or ovarian cancer. Women with a BRCA1 or BRCA2 pathogenic variant have a 50%‐80% risk of developing breast cancer and a 20%‐40% risk of developing ovarian cancer. Men with BRCA1 or BRCA2 pathogenic variants have a 15%‐30% risk of prostate cancer and a 2%‐6% risk of male breast cancer.4 When HBOC pathogenic variant status is known, the risk of cancer mortality can be reduced by following established management guidelines including cancer screening at younger ages or risk‐reducing mastectomy and salpingo‐oophorectomy.
Given the opportunity for reduced morbidity and mortality, The Centers for Disease Control and Prevention (CDC)'s Office of Public Health Genomics has identified BRCA1/2 testing as a genomic application that has potential for positive impact on the public's health.5, 6 Guidelines on who should receive genetic counseling and testing have been compiled by expert committees,7, 8, 9 and are repeatedly revised and expanded. Recent guidelines have suggested more widespread genetic testing given that people with pathogenic variants may not meet referral criteria.10, 11 A widely used set of criteria are disseminated by the National Comprehensive Cancer Network (NCCN). However, there is limited understanding of the prevalence of individuals who meet various testing criteria and thus of the influence of criteria on number of people who might be recommended for testing. Cancer registry data can be used to evaluate patients who meet NCCN criteria based on the diagnostic characteristics of their cancer such as age at diagnosis, epithelial ovarian cancer histology, and triple‐negative breast cancer. HER2 status, a variable needed to define triple‐negative breast cancer, has been available in cancer registry data only since 2010. To the best of our knowledge, the population‐based prevalence of cancers meeting testing criteria due to diagnostic characteristics has not recently been summarized.
Family cancer history information is a key component of testing criteria both for individuals affected with cancer and for unaffected patients. Cancer registry databases do not include information about family history of cancer.6 Family history is often determined in the research setting through kindreds with recognized clusters of HBOC diagnoses. In the clinical setting, family history is obtained from self‐report. The validity of self‐report of family history is a concern and may vary based on subject characteristics.12 Population‐based data on family history of cancer has been compiled from self‐report on national surveys; however, the detail needed to apply HBOC testing criteria is not available.13 Collection of high‐quality self‐reported family history data requires specialized tools and is not widely implemented.14 The Utah Population Database (UPDB) is a research resource that links over four decades of state‐wide central cancer registry records to extensive genealogies, allowing for objective assessment of family history of cancer for both affected and unaffected individuals. This study seeks to estimate the population prevalence of individuals meeting criteria for HBOC and genetic testing based on the characteristics of a personal diagnosis of cancer, and based on the combination of personal and family cancer history.
2. MATERIALS AND METHODS
2.1. Research design and study population
We used data from the US National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) program SEER 18 Registries15 to calculate the prevalence of diagnostic characteristics meeting NCCN HBOC testing criteria among a set of incident cancers representing a broad sample of the US diagnosed during the period 2010‐2015. Testing criteria defined in the National Comprehensive Cancer Network (NCCN) Familial Breast/Ovarian Risk Assessment guidelines was used (V2.2015, Figure 1). Cancer‐specific variables including cancer site, histology, SEER summary stage, year of diagnosis, diagnostic information, ethnicity, and age at diagnosis were queried from SEER. NCCN criteria evaluable from SEER data include male breast cancer, young age at diagnosis, triple‐negative breast cancer (estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 amplification negative), second primary diagnosis of breast cancer, and diagnosis of ovarian, primary peritoneal, or fallopian tube cancer. Breast cancers considered included all invasive diagnoses and ductal carcinoma in situ (DCIS). Other cancer sites were limited to invasive diagnoses. Specific cancer site and histology codes that were used to establish diagnoses meeting criteria can be found in Table S1.
Figure 1.
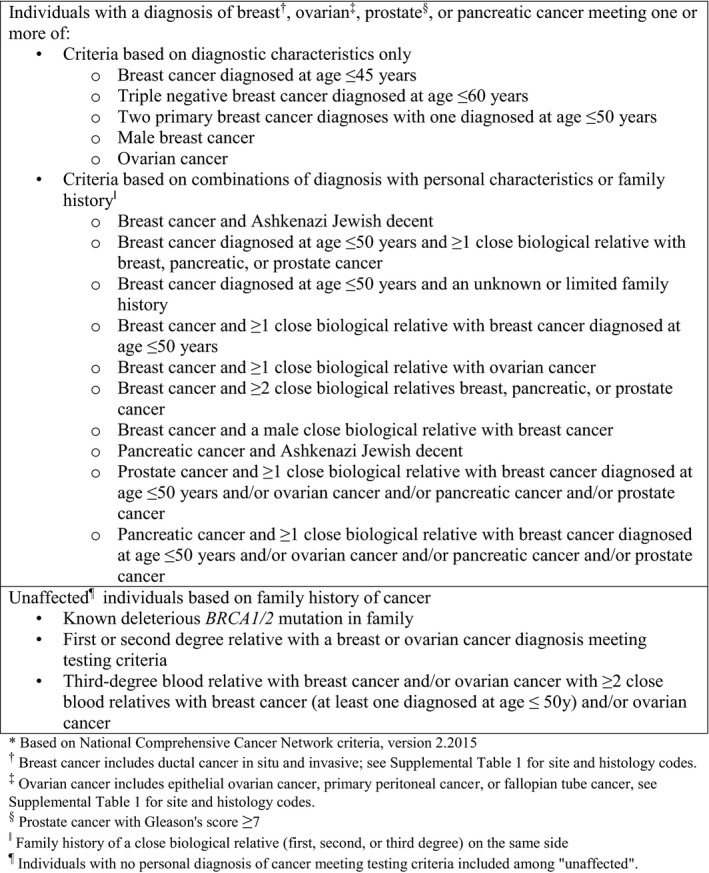
Hereditary breast and ovarian cancer syndrome testing criteria*
In order to estimate the prevalence of family history meeting HBOC testing criteria, we used data from the Utah Cancer Registry, a state‐wide registry since 1966 and a participating registry in the SEER program since 1973, linked to the UPDB. UPDB is a unique research data resource that includes extensive multi‐generation genealogies linked to individual health data from state‐wide resources.16, 17, 18 We assessed the prevalence of meeting HBOC criteria at the time of diagnosis for individuals with a cancer diagnosis. Cases included in the analysis were those diagnosed in 2010‐2015 with invasive cancer of HBOC sites or DCIS of the breast. We further limited the analysis to individuals who were linked through UPDB to enough family members in the state to evaluate family cancer history. This was defined as at least three adult relatives living in Utah after 1966. We used registry variables as described above to evaluate whether the case met criteria for HBOC testing based on diagnostic characteristics at the time of diagnosis. We used UPDB information about cancer diagnoses among relatives, querying relevant diagnoses among first, second, and third degree relatives as shown in Figure 1, to evaluate whether the case met criteria for HBOC testing based on family cancer history. We evaluated whether Utah residents without a personal diagnosis of cancer (unaffected) meeting HBOC criteria met criteria for testing based on family history. We limited the analysis to individuals who were living in Utah in 2018 and who were linked through UPDB to at least three adult relatives living in Utah after 1966. We again used UPDB information about cancer diagnoses among first, second, and third degree relatives to assess eligibility for HBOC testing as shown in Figure 1 under "unaffecteds." (We had no data source to evaluate the criterion "known deleterious BRCA 1/2 pathogenic variant in family".)
The study was approved by both the Institutional Review Boards of the University of Utah and Intermountain Healthcare.
2.2. Statistical analysis
We conducted descriptive analysis tabulating incident breast and ovarian cancer cases by the presence or absence of cancer characteristics meeting specific NCCN criteria and according to characteristics of the case at diagnosis including race, ethnicity, and place of residence. SEER*Stat software was used. For analyses incorporating family history criteria, a data set consisting of Utah Cancer Registry data linked by UPDB to family history variables was created and analyzed using SAS v9.2.
3. RESULTS
3.1. Breast and ovarian cancer cases meeting testing criteria based on diagnostic characteristics
From 2010 to 2015, 426,972 breast cancers (including invasive cancers and ductal carcinoma in situ) were diagnosed in the 18 SEER regions (Table 1). Based on diagnostic characteristics in the SEER data, overall 18.1% (n = 77 487) of breast cancer cases met one or more NCCN criteria for genetic testing. Among the female cases (n = 423 795), 12.2% (n = 51 631) met criteria due to being diagnosed at or below the age of 45. Young age at diagnosis was most prevalent among Hispanics (20.4%), followed by Asian or Pacific Islander (17.7%), with non‐Hispanic whites having the lowest proportion diagnosed under 45. The criterion of triple negative (ER/PR/Her2) breast cancer diagnosed in a woman younger than 60 years of age was met by 4.9% of female cases overall, and at a higher proportion (10.0%) in black or African American cases. Only 2.8% of cases met testing criteria based on a second breast cancer diagnosis with one at or before age 50. A diagnosis of male breast cancer occurred in 3,177 individuals, accounting for 0.7% of breast cancer cases.
Table 1.
Population‐based prevalence of cancer cases with diagnostic characteristics meeting criteria for hereditary breast and ovarian cancer (HBOC) testing at the time of diagnosis, 18 SEER registries 2010‐2015
| Total | Race and ethnicitya | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non‐Hispanic White | Hispanic | Black or African American | Asian or Pacific Islander | American Indian or Alaska native | Other or unknown | |||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Breast cancer, total | 426 972 | 291 144 | 46 320 | 47 442 | 36 813 | 2303 | 2950 | |||||||
| Meets any criterion for testingb | ||||||||||||||
| Yes | 77 487 | 18.1 | 43 865 | 15.1 | 12 404 | 26.8 | 11 766 | 24.8 | 8473 | 23.0 | 459 | 19.9 | 520 | 17.6 |
| No | 349 485 | 81.9 | 247 279 | 84.9 | 33 916 | 73.2 | 35 676 | 75.2 | 28 340 | 77.0 | 1844 | 80.1 | 2430 | 82.4 |
| Sex | ||||||||||||||
| Male | 3177 | 0.7 | 2307 | 0.8 | 230 | 0.5 | 461 | 1.0 | 146 | 0.4 | 7 | 0.3 | 26 | 0.9 |
| Female | 423 795 | 99.3 | 288 837 | 99.2 | 46 090 | 99.5 | 46 981 | 99.0 | 36 667 | 99.6 | 2296 | 99.7 | 2 924 | 99.1 |
| Among female breast cancer cases | ||||||||||||||
| Age at dx | ||||||||||||||
| ≤45 | 51 631 | 12.2 | 28 019 | 9.7 | 9405 | 20.4 | 6962 | 14.8 | 6492 | 17.7 | 335 | 14.6 | 418 | 14.3 |
| 46‐50 | 43 205 | 10.2 | 26 116 | 9.0 | 6360 | 13.8 | 5 267 | 11.2 | 4866 | 13.3 | 265 | 11.5 | 331 | 11.3 |
| 51+ | 328 959 | 77.6 | 234 702 | 81.3 | 30 325 | 65.8 | 34 752 | 74.0 | 25 309 | 69.0 | 1696 | 73.9 | 2175 | 74.4 |
| Triple negative | ||||||||||||||
| Yes | 38 235 | 9.0 | 23 043 | 8.0 | 4587 | 10.0 | 7634 | 16.2 | 2601 | 7.1 | 215 | 9.4 | 155 | 5.3 |
| Yes and dx ≤ age 60 | 20 926 | 4.9 | 11 243 | 3.9 | 3231 | 7.0 | 4683 | 10.0 | 1547 | 4.2 | 132 | 5.7 | 90 | 3.1 |
| Yes and dx >age 60 | 17 309 | 4.1 | 11 800 | 4.1 | 1 356 | 2.9 | 2 951 | 6.3 | 1054 | 2.9 | 83 | 3.6 | 65 | 2.2 |
| No | 355 402 | 83.9 | 246 098 | 85.2 | 37 886 | 82.2 | 35 842 | 76.3 | 31 536 | 86.0 | 1932 | 84.1 | 2108 | 72.1 |
| Missing | 30 158 | 7.1 | 19 696 | 6.8 | 3617 | 7.8 | 3505 | 7.5 | 2530 | 6.9 | 149 | 6.5 | 661 | 22.6 |
| Second breast primary | ||||||||||||||
| Yes | 40 412 | 9.5 | 28 690 | 9.9 | 3504 | 7.6 | 4 577 | 9.7 | 3350 | 9.1 | 207 | 9.0 | 84 | 2.9 |
| Yes First primary ≤ age 50 | 11 761 | 2.8 | 7366 | 2.6 | 1423 | 3.1 | 1 639 | 3.5 | 1251 | 3.4 | 60 | 2.6 | 22 | 0.8 |
| Yes First primary >age 50 | 28 651 | 6.8 | 21 324 | 7.4 | 2081 | 4.5 | 2938 | 6.3 | 2099 | 5.7 | 147 | 6.4 | 62 | 2.1 |
| No | 383 383 | 90.5 | 260 147 | 90.1 | 42 586 | 92.4 | 42 404 | 90.3 | 33 317 | 90.9 | 2089 | 91.0 | 2 840 | 97.1 |
| Ovarian, fallopian tube, or primary peritoneal cancer, total | 43 476 | 30 062 | 5638 | 3744 | 3579 | 275 | 178 | |||||||
| Meets any criterion for testingc | ||||||||||||||
| Yes | 35 479 | 81.6 | 25 141 | 83.6 | 4334 | 76.9 | 2788 | 74.5 | 2870 | 80.2 | 223 | 81.1 | 123 | 69.1 |
| No | 7997 | 18.4 | 4921 | 16.4 | 1304 | 23.1 | 956 | 25.5 | 709 | 19.8 | 52 | 18.9 | 55 | 30.9 |
| Ovarian cancer histology | ||||||||||||||
| Epithelial, mucinous | 373 | 0.9 | 234 | 0.8 | 60 | 1.1 | 24 | 0.6 | 50 | 1.4 | <5 | 0.4 | <5 | 2.2 |
| Epithelial, non‐mucinous | 29 045 | 66.8 | 20 405 | 67.9 | 3620 | 64.2 | 2338 | 62.4 | 2403 | 67.1 | 182 | 66.2 | 97 | 54.5 |
| Non‐epithelial | 7 997 | 18.4 | 4921 | 16.4 | 1304 | 23.1 | 956 | 25.5 | 709 | 19.8 | 52 | 18.9 | 55 | 30.9 |
| Fallopian tube or primary peritoneal | 6 061 | 13.9 | 4502 | 15.0 | 654 | 11.6 | 426 | 11.4 | 417 | 11.7 | 40 | 14.5 | 22 | 12.4 |
Includes Hispanic or Latino, of any race.
"Yes" if male, diagnosed ≤age 45, triple negative and diagnosed ≤age 60, or second breast primary. Rows may not sum to total because a case may meet more than one criterion.
"Yes" if epithelial ovarian or any fallopian tube, or primary peritoneal cancer.
During the same 2010‐2015 timeframe, 43 476 women were diagnosed with ovarian, fallopian tube, or primary peritoneal cancer. Of these, only non‐epithelial ovarian cancers do not meet criteria for genetic testing, and the remaining 35 479 (81.6%) do meet NCCN criteria. Non‐Hispanic White had the highest rate of ovarian cancers that met testing criteria (83.6%), followed by American Indian or Alaska Native (81.1%) and Asian or Pacific Islander (80.2%).
3.2. Utah cancer cases meeting diagnostic criteria for genetic testing based on family history
There were 5590 total breast cancer cases diagnosed in Utah from 2010 to 2015 with evaluable family history. Breast cancer cases who met criteria for family history and/or diagnostic criteria comprised the majority, 62.9% of breast cancers in the population (Table 2). Of the total breast cancer cases, 21.6% (n = 1207) met diagnostic criteria for genetic testing. When UPDB pedigree information was included, 54.9% (n = 3068) of breast cancer cases met NCCN testing family history criteria. Breast cancer cases with a family history of solely breast cancer comprised 13% (n = 398) of the cases meeting family history criteria (Figure 2A). Others met criteria based on ovarian cancer family history only or combinations of breast or ovarian with male breast, prostate (Gleason ≥7), and pancreatic cancers.
Table 2.
Prevalence of cancer cases with diagnostic characteristics or family history meeting hereditary breast and ovarian cancer (HBOC) testing criteria at the time of diagnosis, Utaha 2010‐2015
| Total | Race and Ethnicityb | |||||||
|---|---|---|---|---|---|---|---|---|
| Non‐Hispanic White | Hispanic | Other | ||||||
| n | % | n | % | n | % | n | % | |
| Breast cancer, total | 5590 | 5353 | 144 | 93 | ||||
| Meets any criterion for testingc | ||||||||
| Yes | 3515 | 62.9 | 3409 | 63.7 | 70 | 48.6 | 36 | 38.7 |
| No | 2075 | 37.1 | 1944 | 36.3 | 74 | 51.4 | 57 | 61.3 |
| Meets criteria based on diagnostic characteristicsd | ||||||||
| Yes | 1207 | 21.6 | 1137 | 21.2 | 49 | 34.0 | 21 | 22.6 |
| No | 4383 | 78.4 | 4216 | 78.8 | 95 | 66.0 | 72 | 77.4 |
| Meets criteria based on family history | ||||||||
| Yes | 3068 | 54.9 | 3006 | 56.2 | 39 | 27.1 | 23 | 24.7 |
| No | 2522 | 45.1 | 2347 | 43.8 | 105 | 72.9 | 70 | 75.3 |
| Ovarian, primary peritoneal, or fallopian tube cancer, total | 539 | 516 | 19 | <5 | ||||
| Meets any criterion for testinge | ||||||||
| Yes | 506 | 93.9 | 485 | 94.0 | 17 | 89.5 | <5 | 100.0 |
| No | 33 | 6.1 | 31 | 6.0 | <5 | 10.5 | 0 | 0.0 |
| Prostate cancer, total | 5335 | 4997 | 106 | 232 | ||||
| Meets criteria based on Gleason ≥7 and family history | ||||||||
| Yes | 1297 | 24.3 | 1248 | 25.0 | 9 | 8.5 | 40 | 17.2 |
| No, Gleason ≥7 but no family history | 1590 | 29.8 | 1492 | 29.9 | 42 | 39.6 | 56 | 24.1 |
| No, Gleason <7 | 2448 | 45.9 | 2257 | 45.2 | 55 | 51.9 | 136 | 58.6 |
| Pancreatic cancer, total | 888 | 824 | 45 | 19 | ||||
| Meets criteria based on family history | ||||||||
| Yes | 453 | 51.0 | 439 | 53.3 | 10 | 22.2 | <5 | 21.1 |
| No | 435 | 49.0 | 385 | 46.7 | 35 | 77.8 | 15 | 78.9 |
Table limited to cases who had ≥3 adult relatives living in Utah for evaluation of family history.
Hispanic includes Hispanic or Latino, of any race. Other includes Black or African American, Asian or Pacific Islander, American Indian or Alaska Native, and other or unknown.
"Yes" if meets diagnostic criteria or family history criteria. Rows may not add to total because a case may meet more than one criterion.
"Yes" if male, diagnosed ≤age 45, triple negative and diagnosed ≤age 60, or second breast primary.
"Yes" if epithelial ovarian or any fallopian tube or primary peritoneal cancer.
Figure 2.
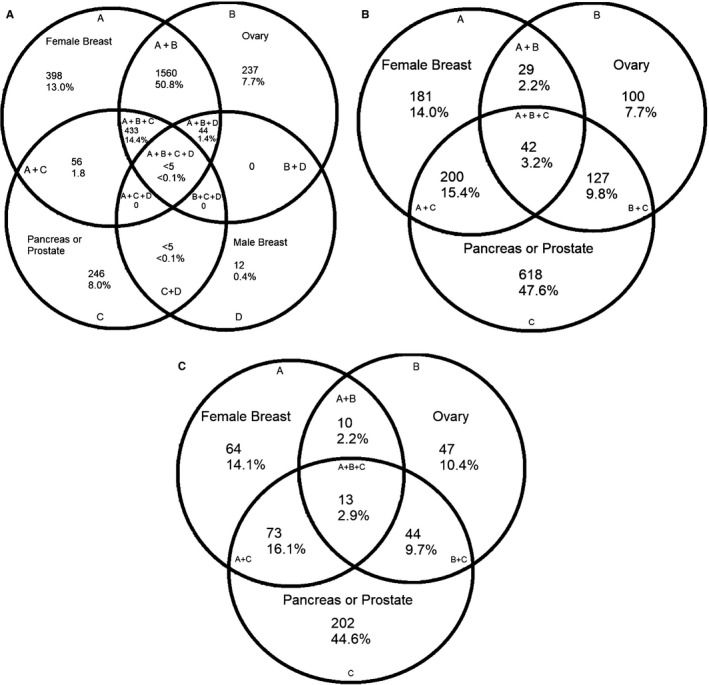
Cancer sites among relatives who contribute to family cancer history for incident cancer cases with a diagnosis of breast (A), prostate (B), or pancreatic (C) cancer, Utah 2010‐2015
Family history criteria may also indicate that genetic testing for HBOC is appropriate for individuals with a diagnosis of prostate or pancreatic cancer. The Utah data indicate that 24.3% of prostate cancers and 51.0% of pancreatic cancers met any NCCN testing criteria at the time of diagnosis (Table 2). Although NCCN guidelines now have broader diagnostic criteria for prostate and pancreatic cancer referral, family history of pancreas or prostate cancer was the largest contributor to meeting criteria for both of these cancer sites because diagnostic criteria for these cancers were not included in the version of the guidelines used for this study. (Figure 2C,D).
3.3. Unaffected individuals in Utah who meet criteria for genetic evaluation
Individuals with no personal cancer diagnosis meeting HBOC criteria can be recommended for testing if they are a relative of an individual(s) with certain cancer diagnoses. In UPDB, about 1.7 million living individuals (from a state population of approximately 3 million) were evaluable for family history. In total, 11.6% (n = 197 601) of the unaffected, evaluated Utah population met HBOC criteria for genetic testing based on family history (Table 3). The most frequent contributing factor was a second‐degree relative with a cancer diagnosis meeting a criterion.
Table 3.
Prevalence of unaffected individuals with family history meeting hereditary breast and ovarian cancer (HBOC) testing criteria, Utah
| n | % | |
|---|---|---|
| Total population evaluateda | 1 702 300 | |
| Total unaffecteds meeting testing criteria based on family historyb | 197 601 | |
| First degree relative with breast or ovarian cancer meeting testing criteriac | 66 660 | 33.7 |
| Second degree relative with breast or ovarian cancer meeting testing criteriac | 111 161 | 56.3 |
| ≥3 relatives with breast or ovarian cancerd | 41 051 | 20.8 |
Adults living in Utah with no personal diagnosis of cancer meeting testing criteria and who have>=3 adult relatives in Utah to assess family cancer history.
Total who meet testing criteria based on one or more of the family history criteria below. Rows may not add to total because a case can meet more than one criterion.
Relative has a cancer diagnosis meeting testing criteria: breast cancer diagnosed ≤age 45, triple negative and diagnosed ≤age 60, second breast primary if first was diagnosed ≤age 50, or male; epithelial ovarian cancer or any primary peritoneal or fallopian tube cancer.
At least 3 close blood relatives with breast and/or ovarian cancer including one relative with breast cancer diagnosed ≤age 50.
4. DISCUSSION
Given the limited availability of objectively determined family cancer history data,12 predicting the prevalence of individuals in the general population who will meet genetic testing criteria is a significant challenge. In this study, utilizing a database that incorporates cancer registry data with extensive genealogies allows the first estimate of a state's prevalence of individuals in the general population who meet HBOC criteria. This was measured using both a snapshot approach for individuals with a diagnosis of cancer in 2010‐2015, and by identifying living individuals who meet HBOC criteria. We found that more than 50% of individuals diagnosed with breast, ovarian, or pancreatic cancer meet criteria at the time of diagnosis. This is higher than previous studies that have shown approximately 35.6% of breast cancer patients meeting NCCN criteria;19 however, these were based on in‐home family interviews, not objective cancer data. We estimate that 11.6% of the unaffected Utah population meets criteria for genetic testing for BRCA1 and BRCA2 pathogenic variants based on family history extended out to second degree relatives.
Although NCCN guidelines present testing criteria for unaffected individuals, they recommend that genetic testing begin with an affected case as feasible.20 This both minimizes potentially unnecessary testing expense and provides the most accurate risk assessment for a family. Given that family members (with the exception of monozygotic twins) are at most 50% genetically similar, testing an affected patient has the highest likelihood of identifying a genetic susceptibility for their cancer diagnosis. Negative genetic testing in an unaffected first‐degree relative can be uninformative, as it will remain unknown whether the affected patient had a genetic susceptibility to their cancer diagnosis. Furthermore, unaffected relatives with negative germline testing often undergo increased cancer screening that may unnecessary if they were found to be “true negatives” when an affected relative has a pathogenic variant identified. If an affected individual tests negative, genetic testing is not indicated for their relatives, which allows for optimal usage of health care resources. A limitation of the present study is that genetic testing status and results were not available. Therefore, it is presumed a portion of those found to meet criteria for testing in this study do not need genetic testing given previous family member testing and/or previous testing for themselves.
Quantifying the need for genetic testing can also have implications for clinician staffing and conversations with patients during appointments. Identifying cases that need genetic evaluation lies in the oncologists’ realm, however, other clinicians can oversee the risk assessment and genetic testing portion. Genetic counselors can be utilized to facilitate the genetic testing process for this large population, notably in cancer institutes who see high volumes of HBOC‐related cancers that would meet criteria for genetic testing.
Since our initial analysis, NCCN guidelines have expanded to recommend genetic testing for all patients with pancreatic cancer or metastatic prostate cancer regardless of family history.20, 21 Given these ever‐expanding guidelines, it is expected that analysis performed using updated guidelines would show pancreatic cancer having similar case proportions to the ovarian cancer analyses performed. Similarly, the proportion of prostate cancer cases meeting criteria will be significantly increased. There is also evidence that patients who do not meet current referral criteria may also have pathogenic variants and would benefit from testing. This results in an expected increase in individuals meeting criteria for genetic services, which must be addressed on multiple levels.
In general, identifying the most appropriate unaffected patients to maximize genetic testing impact is typically a responsibility of primary care providers and other physicians. Given that over 10% of primary care providers' patient cohort is indicated for genetic testing, and up to 60% of an oncologists' patient cohort, family health history remains an important part of the initial intake. Asking patients about cancers in the family across all specialties, along with type and age of diagnosis, can allow for a comprehensive health history to facilitate genetic testing in appropriate patients.
Ultimately, better identification of individuals who meet genetic testing criteria, paired with expanded guidelines, will dramatically drive demand for genetic services. Considering options for genetic counseling and information service delivery will be crucial to meet this demand. From training a growing genetic counseling workforce, to partnering with non‐genetics providers to provide collaborative genetics education, providers across various specialties, not limited to primary care and genetic counselors, must come together to increase appropriate identification and provision of genetic services to individuals. Policy‐level considerations that address geographic and socioeconomic disparities will also be imperative to ensure widespread access to genetic services.
This study is a snapshot of one state and may be an over or underestimate of the true national population that meets HBOC criteria. Utah has the highest average number of children per household in the country (2.21, national median 1.85), and with larger family sizes, the proportion of unaffected individuals who have a relative with a cancer diagnosis may be somewhat larger than in other US states and therefore the Utah estimates may overestimate the number of at‐risk relatives compared to other geographic areas. UPDB captures cancer diagnoses only for Utah residents; to the extent that Utah residents have relatives who reside in other states, the family history may be underestimated. In addition, prevalence may be higher in states with larger populations of Ashkenazi Jewish descent given their increased incidence of BRCA1/2 pathogenic variants. Limitations aside, this is the first study to utilize objective family cancer history and diagnostic criteria from a SEER cancer registry to quantify the percentage of individuals in a state meeting NCCN HBOC criteria.
Overall, this study highlights the large proportion of a state's population that may qualify for genetic testing, although it is limited in that it does not account for those who have undergone genetic evaluation. Efforts focused on identifying affected cases can have the greatest impact on resource utilization and efficiently evaluating this population. Given the high proportion of breast, pancreatic, prostate, and ovarian cases that meet criteria due to family history, there is a need for heightened awareness of family health history. In families where testing the affected case is not possible, evaluation of the unaffected population to identify high‐risk individuals who may benefit from HBOC genetic testing is necessary.
ACKNOWLEDGMENTS
The study was supported by the Enhancing Cancer Genomic Best Practices through Education, Surveillance and Policy Grant, DP005360‐01, funded by the Center for Disease Control and Prevention; contents are solely the responsibility of the authors and may not represent the official views of the Centers for Disease Control and Prevention or the Department of Health and Human Services. The Utah Cancer Registry is funded by the National Cancer Institute's SEER Program, Contract No. HHSN261201800016I, the US Center for Disease Control and Prevention's National Program of Cancer Registries, Cooperative Agreement No. NU58DP0063200‐01, with additional support from the University of Utah and Huntsman Cancer Foundation. The UPDB and Genetic Counseling Shared Resource are supported in part by grant P30 CA2014 by the National Cancer Institute and awarded to the Huntsman Cancer Institute and the Huntsman Cancer Foundation. The study sponsor was involved in the manuscript preparation as it relates to contributing to the background and facilitating meetings amongst the team. The UPDB is also supported by the University of Utah's Program in Personalized Health and Center for Clinical and Translational Science. The authors thank all stakeholders across multiple Utah institutions who have played a key role in this grant's mission and activities.
CONFLICT OF INTEREST
Samantha Greenberg reports one‐time consulting fees from Tempus laboratories, outside of the scope of submitted work. The rest of the authorship reports no conflict of interests.
AUTHOR CONTRIBUTIONS
Samantha Greenberg contributed to data curation, formal analysis, writing‐original draft, and writing‐review, and editing. Saundra S. Buys contributed to conceptualization, methodology, project administration, supervision, validation, writing‐review, and editing. Sandie L. Edwards contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, validation, writing‐original draft, writing‐review and editing. Whitney Espinel contributed to supervision, writing‐original draft, writing‐review and editing. Alison Fraser contributed to conceptualization, data curation, formal analysis, software. Amanda Gammon contributed to conceptualization, data curation, writing‐review and editing. Brent Hafen contributed to conceptualization, methodology, supervision. Kim A. Herget contributed to data curation, formal analysis, methodology, validation, writing‐review and editing. Wendy Kohlmann contributed to conceptualization, data curation, supervision, writing‐review and editing. Camille Roundy contributed to conceptualization, project administration, resources, supervision, writing‐original draft, writing‐review and editing. Carol Sweeney contributed to conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, writing‐original draft, writing‐review and editing.
Supporting information
Greenberg S, Buys SS, Edwards SL, et al. Population prevalence of individuals meeting criteria for hereditary breast and ovarian cancer testing. Cancer Med. 2019;8:6789–6798. 10.1002/cam4.2534
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Bayraktar S, Arun B. BRCA mutation genetic testing implications in the United States. Breast. 2017;31:224‐232. [DOI] [PubMed] [Google Scholar]
- 2. Møller P, Hagen AI, Apold J, et al. Genetic epidemiology of BRCA mutations–family history detects less than 50% of the mutation carriers. Eur J Cancer. 2007;43:1713‐1717. [DOI] [PubMed] [Google Scholar]
- 3. Risch HA, McLaughlin JR, Cole D, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin‐cohort study in Ontario, Canada. J Natl Cancer Inst. 2006;98:1694‐1706. [DOI] [PubMed] [Google Scholar]
- 4. Mano R, Tamir S, Kedar I, et al. Malignant abnormalities in male BRCA mutation carriers: results from a prospectively screened cohort. JAMA Oncol. 2018;4:872‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bowen MS, Kolor K, Dotson WD, Ned RM, Khoury MJ. Public health action in genomics is now needed beyond newborn screening. Public Health Genomics. 2012;15:327‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez JL, Thomas CC, Massetti GM, et al. Family history and genomics as tools for cancer prevention and control. MMWR Morb Mortal Wkly Rep. 2016;65:1291‐1294. [DOI] [PubMed] [Google Scholar]
- 7. Daly MB, Pilarski R, Axilbund JE, et al. Genetic/familial high-risk assessment: breast and ovarian, version 2.2015. J Natl Compr Canc Netw. 2016;14:153–162. [DOI] [PubMed] [Google Scholar]
- 8. Zagouri F, Liakou P, Bartsch R, et al. Discrepancies between ESMO and NCCN breast cancer guidelines: an appraisal. Breast. 2015;24:513‐523. [DOI] [PubMed] [Google Scholar]
- 9. Moyer VA; USPSTF . Assessment genetic counseling, and genetic testing for BRCA‐related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:271‐281. [DOI] [PubMed] [Google Scholar]
- 10. Nicolosi P, Ledet E, Yang S, et al. Prevalence of germline variants in prostate cancer and implications for current genetic testing guidelines. JAMA Oncol. 2019;5(4):523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang S, Axilbund JE, O'Leary E, et al. Underdiagnosis of hereditary breast and ovarian cancer in medicare patients: Genetic testing criteria miss the mark. Ann Surg Oncol. 2018;25:2925‐2931. [DOI] [PubMed] [Google Scholar]
- 12. Tehranifar P, Wu HC, Shriver T, Cloud AJ, Terry MB. Validation of family cancer history data in high‐risk families: the influence of cancer site, ethnicity, kinship degree, and multiple family reporters. Am J Epidemiol. 2015;181:204‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population‐based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006;8:571‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu RR, Himmel TL, Buchanan AH, et al. Quality of family history collection with use of a patient facing family history assessment tool. BMC Fam Pract. 2014;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Surveillance Epidemiology and End Results (SEER) Program . Surveillance, Epidemiology, and End Results (SEER) Program Research Data, SEER 18 Regions Public Use 2000–2013, released April 2016, based on the November 2015 submission. National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, 2016.
- 16. Cannon Albright LA. Utah family‐based analysis: past, present and future. Hum Hered. 2008;65:209‐220. [DOI] [PubMed] [Google Scholar]
- 17. Wylie JE, Mineau GP. Biomedical databases: protecting privacy and promoting research. Trends Biotechnol. 2003;21:113‐116. [DOI] [PubMed] [Google Scholar]
- 18. Skolnick M. The Utah genealogical database: a resource for genetic epidemiology In: Cairns JJ, Lyon JL, Skolnick M, eds. Cancer Incidence in Defined Populations. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1980:285‐296. [Google Scholar]
- 19. Childers CP, Childers KK, Maggard‐Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800‐3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daly MB, Pilarski R, Berry M, et al. Guidelines insights: genetic/familial high‐risk assessment: breast and ovarian, version 2.2017. J Natl Compr Canc Netw. 2017;15:9‐20. [DOI] [PubMed] [Google Scholar]
- 21. Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479‐505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


