Abstract
Obesity is a substantial public health challenge across the globe. The use of resistant starch has been proposed as a probable management strategy for complications of obesity. We investigated the effects of resistant starch intake on lipid profiles, glucose metabolism, antioxidant status, lipid peroxidation marker, blood pressure, and anthropometric variables in subjects with overweight or obesity. In this 12-week, randomized, double-blind, placebo-controlled, 2 × 2 crossover trial, 21 Participants (mean age, 35 ± 7.0 years; body mass index, 32.4 ± 3.5 kg/m2) were given 13.5 g Hi-Maize 260 or placebo daily for 4 weeks, separated by a 4-week washout period. Changes in total antioxidant status (p = 0.04) and serum concentrations of insulin in 52.4% participants with insulin levels above 16 µIU/mL at the baseline (p = 0.04) were significantly different in the three phases. In addition, the mean of serum high-density lipoprotein cholesterol after the intervention was significantly higher than after baseline value (p = 0.04). We found no significant differences in serum concentrations of total cholesterol, triacylglycerol, low-density lipoprotein cholesterol, fasting blood sugar, insulin, homeostatic model assessment of insulin resistance, quantitative insulin sensitivity check index, superoxide dismutase activity, malondialdehyde, blood pressure, and anthropometric variables in the three phases of baseline, after intervention with resistant starch and after placebo. Resistant starch consumption improved serum insulin concentrations, lipid profiles, and antioxidant status in subjects with overweight or obesity.
Trial Registration
ClinicalTrials.gov Identifier: NCT01992783
Keywords: Dietary fiber, Insulin resistance, Superoxide dismutase, Malondialdehyde, Overweight, Obesity
INTRODUCTION
Overweight and obesity are the world's fifth cause of mortality, and represent a substantial public health challenge across the globe [1]. According to the results of the third national surveillance of risk factors of non-communicable diseases, the prevalence rates of central and general adiposity were about 53.6% and 22.3% respectively, among Iranian adults in 2007 [2]. Obesity is a multifactorial disorder that has been linked to chronic oxidative stress, dyslipidemia, hypertension, and hyperinsulinemia [3,4]. Management modalities for obesity include pharmacotherapy, bariatric surgery and lifestyle modification, adherence to a healthy diet especially the functional food and regular physical activity [5].
Resistant starch, as alimentary prebiotic, can alter the composition and activity of the gastrointestinal microbiota [6]. Resistant starch escapes hydrolysis in the small intestine, but can be fermented by resident microflora after passing into the large intestine and produces short chain fatty acids (SCFAs). The potential health benefits ascribed to resistant starch seem to be mediated via SCFAs [7]. It was demonstrated that dietary intervention with resistant starch reduces fat accumulation, increases antioxidant enzyme activity and improves glucose homeostasis and lipid metabolism [5].
A few studies investigated the effects of resistant starch supplementation on metabolic status. An animal study has shown the effects of resistant starch on body weight [6] as well as its effects on insulin sensitivity [7] and lipid profile [8]. Few studies have been conducted in humans, though findings from small pilot studies indicate favorable effects of resistant starch supplementation on insulin sensitivity [9], lipid level, glucose metabolism [10,11], and oxidative stress [12] in men and women with metabolic syndrome [13].
Resistant starch supplementation is low cost and safer than pharmacotherapy and bariatric surgery and seems to be a promising dietary fiber for the management of obesity and related risk factor [14]. To the best of our knowledge, a few randomized controlled trials have investigated the effect of resistant starch on antioxidant status [12,15]. The present study aimed to evaluate the effects of resistant starch supplementation on lipid profiles, glucose metabolism, lipid peroxidation marker, antioxidant status, blood pressure, and anthropometric variables in subjects with overweight or obesity.
MATERIALS AND METHODS
Recruitment and eligibility screening
In total, 126 healthy overweight and obese subjects were assessed for participation eligibility through advertisements in the local newspaper and at the Shahid Motahari Medical Teaching Center (one of the main teaching hospitals of Urmia University of Medical Sciences). A total of 27 subjects met the inclusion criteria, defined as follows: body mass index (BMI; in kg/m2) of 27–40 and aged 20–50 years. The exclusion criteria were as follows: weight loss of ≥ 10% of body weight within the 6 months and ≥ 5% of body weight within the 1 month before enrollment in the study; subjects were changed their routine physical activity during the 12-week intervention; pregnancy; lactation; menopause; history of acute disease; acute or chronic inflammatory diseases; cardiovascular disease; renal disease; liver disease; endocrine disease; abnormal thyroid hormone concentrations; cancer or chemotherapy/radiotherapy; use of drugs potentially affecting glucose and lipid metabolism; use of antihypertensive drugs; taking antibiotics, anti-obesity drugs, and medications that could affect energy expenditure, prebiotics, probiotics, within 2 weeks before the intervention or during the intervention; if they had a fiber intake of > 30 g/day; taking antioxidant; vitamin and/or mineral supplements; smoking; and inability to give informed consent.
Study design
This randomized, double-blind, placebo-controlled, 2 × 2 crossover trial was conducted from September 2013 to December 2013. Written informed consent was obtained from all participants before enrollment. The study protocol was approved by the Urmia University of Medical Science Ethics Committee and was registered at ClinicalTrials.gov as NCT01992783.
At baseline, subjects were randomly assigned to receive either resistant starch supplements or the identical-appearing placebo (maltodextrin). Follow-up assessments were performed every 4 weeks at weeks 4, 8, and 12 after randomization. Randomization lists were computer-generated by a statistician. Subjects, investigators, statistician, and laboratory staff were blind to the intervention assignment until the end of the study.
Sample size
In this study, we used the formula suggested for crossover trials sample size calculation. With α = 0.05, β = 5% (power = 95%), S = 0.82, and Δ = 0.46 (based on a study conducted by Mutlu-Türkoğlu et al. [16] and mean change in malondialdehyde), the sample size needed was estimated to be 21 participants. Considering a drop-out rate of 30%, the final sample size needed was estimated to be 27 participants.
Intervention
The participants in the first period were given 13.5 g Hi-Maize 260 containing up to 60% resistant starch (Ingredion Inc., Manchester, United Kingdom), while the participants in second period were given an identical appearing placebo (13.5 g maltodextrin) daily for 4 weeks, separated by a 4-week washout period. The appearance of the placebo was indistinguishable in shape, color, size, packaging, smell, and taste from the resistant starch supplement. Following the washout, the groups were crossed over to receive the opposite intervention for 4 weeks. The participants were instructed to consistently take the supplements with the main meals. At the onset of the study, subjects were requested to maintain their regular diet and levels of physical activity throughout the trial period. Adherence was assessed by supplement counts confirmed at each visit. Compliance with the supplements was monitored every week by a telephone call. None of the subjects completing the study had any serious adverse events such as gastrointestinal, central nervous system, skin symptoms, and hypoglycemia [17,18].
Biochemical measurements
Biochemical testing was performed at baseline and at weeks 4, 8, and 12 to determine serum lipid profiles, glycemic variables, lipid peroxidation marker, and antioxidant status. Blood (10 mL) was drawn at the Urmia University of Medical Sciences reference laboratory after 12 hours of fasting and was centrifuged at 1,465 ×g at room temperature for 10 minutes into 4 aliquot microtubes. Microtubes of each serum sample were frozen immediately at −80°C until analysis. Serum concentrations of total cholesterol, triacylglycerols (TGs), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and fasting blood sugar (FBS) were measured by using routine enzymatic assays with commercial kits (Pars Azmoon, Tehran, Iran) with the use of an autoanalyzer (Hitachi 902 Auto analyzer; HiTAShi Ltd., Tokyo, Japan). Serum concentrations of insulin were quantified using an immunoradiometric assay kit (DIAsource INS-IRMA Kit; DIAsource, Louvain-la-Neuve, Belgium). The measurement of superoxide dismutase (SOD) was based on the principle that xanthine reacts with xanthine oxidase to generate superoxide radicals, which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye which is read at 505 nm [19]. The procedure was done based on spectrophotometric method (Perkin-Elmer, D-7770 Uberlingen, Germany) using a Biorex kit (Biorex, Antrim, UK). Total antioxidant status (TAS) was measured by using the determination ferric reducing ability power (FRAP) [20]. In this method ferric, present in FRAP reagent, is reduced to the ferrous form at low pH and the absorbance of resultant color is read at 593 nm using a spectrophotometer (Perkin-Elmer, D-7770 Uberlingen, Germany). Malondialdehyde (MDA) concentration was measured using Thiobarbituric Acid Reactive Substances method [21]. The method is based on the MDA reaction with thiobarbituric acid at temperature of 90°C–100°C for 30 minutes and pH = 2–3 to obtain a pink pigment that absorbs at 535 nm (Perkin-Elmer, D-7770 Uberlingen, Germany). All biochemical measurements were performed in the same laboratory with the use of standard laboratory methods.
Glucose homeostasis
Homeostatic model assessment of insulin resistance (HOMA-IR) and quantitative insulin sensitivity check index (QUICKI) were also calculated based on suggested formulas [22].
Anthropometric and blood pressure measurements
Anthropometric measures were taken in the standing position, with participants wearing light clothing and footwear at baseline and at weeks 4, 8, and 12. Height and weight were measured with an accuracy of 0.1 cm and 0.1 kg, respectively. BMI was calculated by dividing weight in kilogram by the square of height in meters. Waist circumference was measured at the middle point between the lower rib margin and the iliac crest. Blood pressure was measured after 10 minutes of rest at baseline and at weeks 4, 8, and 12. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured with participants in the sitting position with the use of a validated mercury sphygmomanometer.
Dietary intake assessment
A 3-day (1 weekend day and 2 nonconsecutive weekdays) food records were performed at baseline and at weeks 2, 6, and 10. A nutritionist individually taught subjects how to measure their food portions and how to complete the food records. Dietary records were analyzed using a nutrition analysis program (Nutritionist IV, version 3.5.2; First DataBank Division, San Bruno, CA, USA) modified for Iranian foods.
Primary and secondary outcomes
The primary outcome measures were changes in serum lipid profiles, glycemic variables, lipid peroxidation marker, and antioxidant status. Secondary outcome measures were changes in blood pressure and anthropometric variables.
Statistical analyses
To determine the effect of resistant starch on serum lipid profiles, glycemic variables, lipid peroxidation marker, antioxidant status, blood pressure, and anthropometric variables, we applied a general linear model analysis of variance for repeated measurements. Also, we computed the median value of studied variables and divided the subjects into 2 groups as higher than or lower than median groups to show the effect of resistant starch on that variable in subjects with high or low level of variable. Multiple comparisons between the intervention and the placebo were followed by the Bonferroni post hoc test. In this method, in case the Mauchly's test of sphericity assumption was violated, the Epsilon and Huynh-Feldt test results are reported. Data were analyzed with the use of SPSS version 16 (SPSS, Inc., Chicago, IL, USA). For all analyses, p < 0.05 was considered significant and all data are shown as means ± standard deviations if not indicated otherwise.
RESULTS
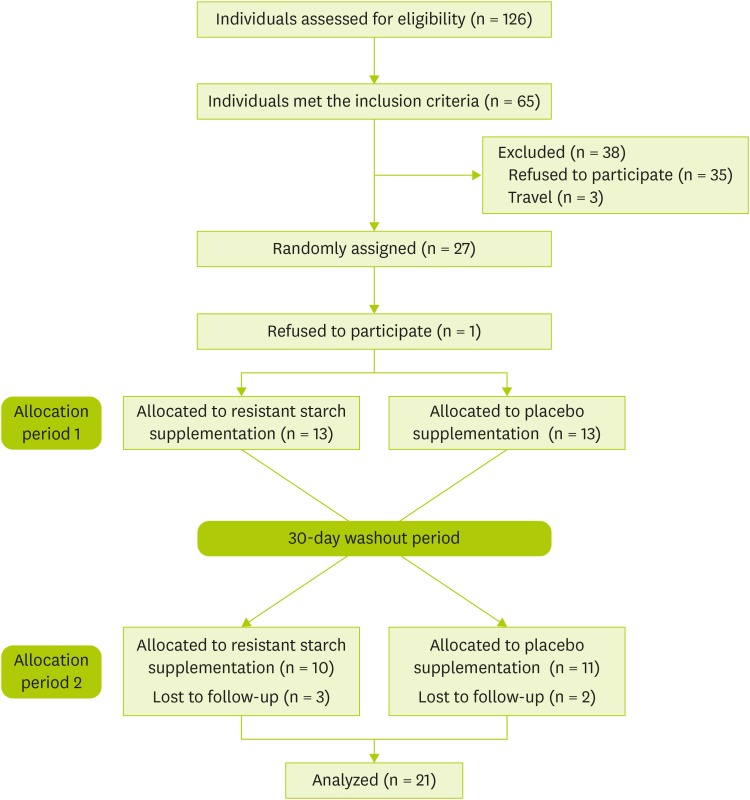
A total of 27 participants were randomly assigned into 2 groups. After randomization, 6 participants were discontinued due to lost to follow-up. In total, 21 participants completed the trial (Figure 1). The participants in this study consisted of 8 males and 13 females. The mean of their ages was 35 ± 7.0 years (Table 1). The results obtained from the participants' three-day records regarding intake of energy, carbohydrates, protein, fat, and fiber at the baseline and weeks 2, 6, and 10 did not show any significant difference (Table 2).
Figure 1. CONSORT diagram showing the flow of participant eligibility, screening, randomization, and follow-up through each stage of the randomized crossover trial.
Table 1. Demographic characteristics of participants (n = 21).
| Characteristics | Values |
|---|---|
| Sex (women/men) | 13/8 |
| Age (yr) | 35 ± 7.0 |
| Weight (kg) | 90.5 ± 9.8 |
| Height (cm) | 167.1 ± 0.07 |
| BMI (kg/m2) | 32.5 ± 3.5 |
Values are means ± standard deviations unless otherwise indicated.
BMI, body mass index.
Table 2. Dietary nutrient intakes in a 12-week crossover trial in overweight or obese subjects (n = 21) by intervention period with resistant starch and placebo.
| Dietary nutrient | Intervention period | Placebo period | p value* | |
|---|---|---|---|---|
| Energy intake (kcal/day) | 0.58 | |||
| Baseline | 2,488 ± 719 | 2,399 ± 212 | ||
| Week 2 | 2,693 ± 728 | 2,234 ± 464 | ||
| Week 6 | 2,155 ± 392 | 2,548 ± 695 | ||
| Week 10 | 2,228 ± 476 | 2,448 ± 558 | ||
| Carbohydrates (grams per day) | 0.60 | |||
| Baseline | 365.5 ± 136.7 | 406.5 ± 32.1 | ||
| Week 2 | 367.4 ± 148.5 | 369.3 ± 87.2 | ||
| Week 6 | 340.4 ± 53.0 | 368.2 ± 153.7 | ||
| Week 10 | 356.9 ± 86.2 | 383.9 ± 127.2 | ||
| Protein (grams per day) | 0.64 | |||
| Baseline | 84.8 ± 21.0 | 77.6 ± 22.5 | ||
| Week 2 | 80.8 ± 20.6 | 67.7 ± 13.8 | ||
| Week 6 | 66.5 ± 25.9 | 70.6 ± 19.2 | ||
| Week 10 | 68.2 ± 5.7 | 66.2 ± 14.0 | ||
| Fat (grams per day) | 0.76 | |||
| Baseline | 81.5 ± 10.6 | 74.5 ± 10.7 | ||
| Week 2 | 80.1 ± 14.9 | 69.8 ± 15.9 | ||
| Week 6 | 72.9 ± 16.4 | 83.1 ± 13.2 | ||
| Week 10 | 71.7 ± 12.1 | 81.3 ± 21.3 | ||
| Fiber (grams per day) | 0.46 | |||
| Baseline | 19.7 ± 2.8 | 16.1 ± 4.7 | ||
| Week 2 | 16.6 ± 2.5 | 18.6 ± 4.1 | ||
| Week 6 | 15.3 ± 4.0 | 17.4 ± 5.2 | ||
| Week 10 | 16.0 ± 1.7 | 16.9 ± 1.4 | ||
Values are means ± standard deviations.
*The p values were computed by using general linear model analysis of variance for repeated measurements.
Primary outcomes
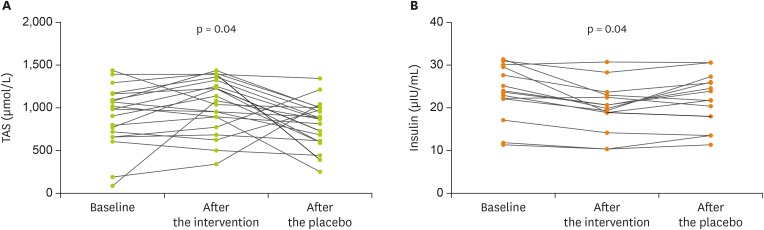
As shown in Table 3, we found no significant differences in serum concentrations of total cholesterol, TGs, HDL-C, LDL-C, FBS, insulin, HOMA-IR, QUICKI, SOD activity, and MDA in the 3 phases of baseline, after intervention with resistant starch and after placebo. However, changes in TAS (p = 0.04) and serum concentrations of insulin in 52.4% participants with insulin levels above 16 µIU/mL at the baseline (p = 0.04) were significantly different in the three phases (Figure 2). In addition, the mean of serum HDL-C after the intervention was significantly higher than after baseline value (p = 0.04) (Table 3).
Table 3. Comparison of primary and secondary outcome variables in overweight or obese subjects (n = 21) in the three phases of baseline, after intervention with resistant starch and after placebo for 12 weeks.
| Outcomes | Baseline | After the intervention | After the placebo | p value* | ||
|---|---|---|---|---|---|---|
| Serum lipids (mg/dL) | ||||||
| Total cholesterol | 183.2 ± 35.4 | 184.4 ± 36.4 | 184.0 ± 36.7 | 0.95 | ||
| TGs | 140.3 ± 59.9 | 150.4 ± 39.8 | 153.2 ± 48.7 | 0.45 | ||
| HDL-C | 40.3 ± 5.6a | 41.8 ± 6.0b | 41.7 ± 5.9 | 0.23 | ||
| LDL-C | 89.1 ± 16.8 | 90.5 ± 20.4 | 89.6 ± 18.1 | 0.86 | ||
| Glycemic variables | ||||||
| FBS (mg/dL) | 103.1 ± 7.1 | 105.3 ± 7.1 | 106.1 ± 9.7 | 0.25 | ||
| Serum insulin (µIU/mL) | 20.2 ± 7.4 | 18.2 ± 5.2 | 19.6 ± 6.2 | 0.15 | ||
| Insulin ≥ 16 | 24.9 ± 4.2 | 20.8 ± 4.6 | 22.7 ± 5.3 | 0.04 | ||
| Insulin < 16 | 11.9 ± 1.3 | 13.6 ± 1.3 | 13.4 ± 1.6 | 0.25 | ||
| HOMA-IR | 5.1 ± 2.0 | 4.7 ± 1.5 | 5.0 ± 1.7 | 0.45 | ||
| QUICKI | 0.306 ± 0.01 | 0.303 ± 0.01 | 0.305 ± 0.01 | 0.64 | ||
| Antioxidant status | ||||||
| Serum TAS (µmol/L) | 968.9 ± 30.7 | 1,047.1 ± 32.9 | 819.6 ± 27.6 | 0.04 | ||
| Serum SOD activity (U/g Hb) | 1,507.4 ± 590.1 | 1,386.2 ± 499.1 | 1,326.8 ± 621.5 | 0.55 | ||
| Serum MDA (µIU/mL) | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.2 ± 0.2 | 0.30 | ||
| Blood pressure (mm Hg) | ||||||
| SBP | 115.0 ± 11.1 | 115.0 ± 10.9 | 115.7 ± 12.1 | 0.91 | ||
| DBP | 80.0 ± 10.0 | 76.7 ± 11.3 | 78.8 ± 7.7 | 0.56 | ||
| Metabolic characteristics | ||||||
| Weight (kg) | 90.5 ± 9.8 | 90.9 ± 9.4 | 91.3 ± 8.9 | 0.16 | ||
| BMI (kg/m2) | 32.5 ± 3.5 | 32.6 ± 3.4 | 32.7 ± 3.2 | 0.16 | ||
| Waist circumference (cm) | 106.4 ± 7.2 | 105.3 ± 6.1 | 106.5 ± 5.1 | 0.21 | ||
Values are means ± standard deviations.
TG, triacylglycerol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; FBS, fasting blood sugar; HOMA-IR, homeostatic model assessment of insulin resistance; QUICKI, quantitative insulin sensitivity check index; TAS, total antioxidant status; SOD, superoxide dismutase; MDA, malondialdehyde; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index .
a,bDifferent alphabet indicates significant difference (p < 0.05) in the same raw by paired t-test. Pairwise comparisons between periods were performed with the use of Bonferroni adjustment to account for multiple comparisons. *The p values were computed by using general linear model analysis of variance for repeated measurements.
Figure 2. Changes in TAS (A) and serum concentration of insulin in 52.4% participants with insulin levels above median (16 µIU/mL) at the baseline (B) in overweight or obesity subjects in the three phases of baseline, after intervention with resistant starch and after placebo for 12 weeks. Values are means ± standard deviations. On the basis of general linear model analysis of variance for repeated measurements there were significant differences in the three phases (p = 0.04).
TAS, total antioxidant status.
Secondary outcomes
There were no significant differences in SBP and DBP, weight, BMI, or waist circumference in the three phases of baseline, after intervention with resistant starch, and after placebo (Table 3).
DISCUSSION
In the present study, we showed that the consumption of resistant starch for 12 weeks led to statistically and clinically significant improvements in TAS, serum concentrations of insulin and HDL-C whereas serum lipid profiles, glycemic variables, and antioxidant and lipid peroxidation markers remained unchanged.
Nowadays, resistant starch has drawn the interest of investigators due to its health benefits and nutritional characteristic [23]. According to data from the National Health and Nutrition Examination Survey III, the findings on overweight and obese adults indicated a strong correlation between BMI and abnormal lipid profiles [24]. In this study, we found no effect of resistant starch on total cholesterol, TGs, and LDL-C. A meta-analysis of 14 clinical studies found that resistant starch is potentially useful for improving lipid profiles, particularly when administered for duration longer than 4 weeks [25]. Consistent with our results, Kwak et al. [12] did not observed significant improvement in lipid and lipoprotein profile after the intervention with 6.5 g/day resistant starch. However, we found significant increase in HDL-C after the intervention with 13.5 g/day resistant starch. This lack of effect in study by Kwak et al. [12] might be explained by the fact that the lipid-lowering effects of resistant starch may be dependent upon high levels of intake [26]. In our study the beneficial effect of resistant starch on HDL-C could be through decreasing the dietary cholesterol absorption in the intestine, increasing of faecal cholesterol excretion, and increasing the production of SCFA via gut microbiota fermentation, in particular, propionate and butyrate, which may inhibit of hepatic cholesterol synthesis [7].
Findings from the current study showed that the administration of resistant starch supplements affect serum concentrations of insulin in 52.4% participants with insulin levels above 16 µIU/mL. However, we found no significant differences in this parameter in subjects with insulin levels below 16 µIU/mL. Therefore, it is possible that normal insulin levels are not affected by resistant starch; although, this warrants further investigation. In our study, a best explanation for reducing insulin responses is increasing the production of SCFAs [7]. SCFAs have been shown to have insulin-like effects; reducing gluconeogenesis, activating glycogen synthase in hepatocytes and stimulating glycolysis, all functions to lower circulating glucose [27]. Ble-Castillo et al. [28] reported that consumption of 24 g/day resistant starch supplementation for 4 weeks did not affect FBS and insulin resistance in obese type 2 diabetes mellitus (T2DM) patients. Lobley et al. [29] reported that intake of 25 g/day resistant starch for 3 weeks improved plasma insulin, insulin sensitivity and HOMA-IR in obese subjects, but fasting glycemia remained unchanged. Maki et al. [9] also observed a significant increase in the insulin sensitivity index, but no significant change in FBS after consumption of 15 and 30 g/day resistant starch for 4 weeks. This discrepancy in results may be due to differences in source of resistant starch, dose and type of resistant starch supplementation, and the pathologic state of the subjects. It seems that a high daily intake of resistant starch has a positive effect on improving glucose metabolism and could decrease the development of T2DM [11]. Further dose-response investigation would therefore be required for a recommended daily intake for specific groups and general public.
Decreases in antioxidant enzymes may be ascribed to rapid utilization and exhaustion of their storage in the body when encountering free radicals generated during development of obesity. Previous studies have shown that transfer of pathogenic bacteria products from the intestinal lumen into the circulation increases oxidative stress [30]. In current study, increased TAS by resistant starch consumption may be ascribed to inhibition of this procedure by reducing of inflammation and endotoxin and scavenging of reactive oxygen species [31]. Kwak et al. [12] reported that rice including 6.5 g/day resistant starch decreased oxidative stress after 4 weeks in patients with prediabetes. Similar findings have been reported after intake of 10 g/day resistant starch in patients with T2DM [32]. In contrary to our study, Thampi et al. [33] found decreased MDA concentrations as well as increased SOD and catalase after fiber supplementation compared with a control diet in rats. Possibly, dietary fiber influenced the amount of lipid peroxidation by enhancing the activity of antioxidant enzymes; this hypothesis needs further evaluation.
Our limitations in this study are low sample size and short duration of intervention. Another limitation of this study was that we did not evaluate the gut microbiome. Although we considered no inclusion criteria regarding the variables level, we noticed that the effect of resistant starch on insulin is different in subjects with different baseline levels of this variable; so, we conducted a secondary analysis to show this important finding and we suggest that future studies focus on the effect of resistant starch in hyperinsulinemic patients. Crossover design is the most important advantage of the present study. In a crossover design study, variability is reduced because each participant serves as own control.
CONCLUSION
In conclusion, this randomized, double-blind, placebo-controlled, crossover trial provided some evidence that consumption of resistant starch for 12 weeks led to significant improvements in TAS, serum concentrations of insulin and HDL-C whereas lipid profiles, glycemic variables, and antioxidant and lipid peroxidation markers remained unchanged. Further clinical studies are necessary to confirm the positive effects of resistant starch in subjects with overweight or obesity.
ACKNOWLEDGEMENTS
We are thankful to all participants and staff of the present study.
Footnotes
Funding: Conduction of the study was financially supported by Urmia University of Medical Sciences, Urmia, Iran.
Conflict of Interest: The authors declare that they have no competing interests.
References
- 1.Rahmani A, Sayehmiri K, Asadollahi K, Sarokhani D, Islami F, Sarokhani M. Investigation of the prevalence of obesity in Iran: a systematic review and meta-analysis study. Acta Med Iran. 2015;53:596–607. [PubMed] [Google Scholar]
- 2.Esteghamati A, Meysamie A, Khalilzadeh O, Rashidi A, Haghazali M, Asgari F, Kamgar M, Gouya MM, Abbasi M. Third national Surveillance of Risk Factors of Non-Communicable Diseases (SuRFNCD-2007) in Iran: methods and results on prevalence of diabetes, hypertension, obesity, central obesity, and dyslipidemia. BMC Public Health. 2009;9:167. doi: 10.1186/1471-2458-9-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frayn KN, Coppack SW. Insulin resistance, adipose tissue and coronary heart disease. Clin Sci (Lond) 1992;82:1–8. doi: 10.1042/cs0820001. [DOI] [PubMed] [Google Scholar]
- 4.Valdecantos MP, Pérez-Matute P, Martínez JA. Obesity and oxidative stress: role of antioxidant supplementation. Rev Invest Clin. 2009;61:127–139. [PubMed] [Google Scholar]
- 5.Zhang L, Li HT, Shen L, Fang QC, Qian LL, Jia WP. Effect of dietary resistant starch on prevention and treatment of obesity-related diseases and its possible mechanisms. Biomed Environ Sci. 2015;28:291–297. doi: 10.3967/bes2015.040. [DOI] [PubMed] [Google Scholar]
- 6.Belobrajdic DP, King RA, Christophersen CT, Bird AR. Dietary resistant starch dose-dependently reduces adiposity in obesity-prone and obesity-resistant male rats. Nutr Metab (Lond) 2012;9:93. doi: 10.1186/1743-7075-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Wang F, Ren X, Wang Y, Blanchard C. Resistant starch manipulated hyperglycemia/hyperlipidemia and related genes expression in diabetic rats. Int J Biol Macromol. 2015;75:316–321. doi: 10.1016/j.ijbiomac.2015.01.052. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen TS, Theil PK, Purup S, Nørskov NP, Bach Knudsen KE. Effects of resistant starch and arabinoxylan on parameters related to large intestinal and metabolic health in pigs fed fat-rich diets. J Agric Food Chem. 2015;63:10418–10430. doi: 10.1021/acs.jafc.5b03372. [DOI] [PubMed] [Google Scholar]
- 9.Maki KC, Pelkman CL, Finocchiaro ET, Kelley KM, Lawless AL, Schild AL, Rains TM. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J Nutr. 2012;142:717–723. doi: 10.3945/jn.111.152975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park OJ, Kang NE, Chang MJ, Kim WK. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J Nutr Sci Vitaminol (Tokyo) 2004;50:93–99. [PubMed] [Google Scholar]
- 11.Johnston KL, Thomas EL, Bell JD, Frost GS, Robertson MD. Resistant starch improves insulin sensitivity in metabolic syndrome. Diabet Med. 2010;27:391–397. doi: 10.1111/j.1464-5491.2010.02923.x. [DOI] [PubMed] [Google Scholar]
- 12.Kwak JH, Paik JK, Kim HI, Kim OY, Shin DY, Kim HJ, Lee JH, Lee JH. Dietary treatment with rice containing resistant starch improves markers of endothelial function with reduction of postprandial blood glucose and oxidative stress in patients with prediabetes or newly diagnosed type 2 diabetes. Atherosclerosis. 2012;224:457–464. doi: 10.1016/j.atherosclerosis.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Robertson MD, Wright JW, Loizon E, Debard C, Vidal H, Shojaee-Moradie F, Russell-Jones D, Umpleby AM. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J Clin Endocrinol Metab. 2012;97:3326–3332. doi: 10.1210/jc.2012-1513. [DOI] [PubMed] [Google Scholar]
- 14.Keenan MJ, Zhou J, Hegsted M, Pelkman C, Durham HA, Coulon DB, Martin RJ. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv Nutr. 2015;6:198–205. doi: 10.3945/an.114.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aigster A. Physicochemical and Sensory Properties of Resistant Starch-Based Cereal Products and Effects on Glycemic and Oxidative Stress Responses in Hispanic Women [dissertation] Blacksburg, VA: Virginia Polytechnic Institute and State University; 2009. [Google Scholar]
- 16.Mutlu-Türkoğlu U, Öztezcan S, Telci A, Orhan Y, Aykaç-Toker G, Sivas A, Uysal M. An increase in lipoprotein oxidation and endogenous lipid peroxides in serum of obese women. Clin Exp Med. 2003;2:171–174. doi: 10.1007/s102380300002. [DOI] [PubMed] [Google Scholar]
- 17.Grabitske HA, Slavin JL. Gastrointestinal effects of low-digestible carbohydrates. Crit Rev Food Sci Nutr. 2009;49:327–360. doi: 10.1080/10408390802067126. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Chen J, Song YH, Zhao R, Xia L, Chen Y, Cui YP, Rao ZY, Zhou Y, Zhuang W, Wu XT. Effects of the resistant starch on glucose, insulin, insulin resistance, and lipid parameters in overweight or obese adults: a systematic review and meta-analysis. Nutr Diabetes. 2019;9:19. doi: 10.1038/s41387-019-0086-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woolliams JA, Wiener G, Anderson PH, McMurray CH. Variation in the activities of glutathione peroxidase and superoxide dismutase and in the concentration of copper in the blood in various breed crosses of sheep. Res Vet Sci. 1983;34:253–256. [PubMed] [Google Scholar]
- 20.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 21.Kamal AA, Gomaa A, el Khafif M, Hammad AS. Plasma lipid peroxides among workers exposed to silica or asbestos dusts. Environ Res. 1989;49:173–180. doi: 10.1016/s0013-9351(89)80062-3. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Fleming SE. Use of HbA1C testing to diagnose pre-diabetes in high risk African American children: a comparison with fasting glucose and HOMA-IR. Diabetes Metab Syndr. 2012;6:157–162. doi: 10.1016/j.dsx.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Meenu M, Xu B. A critical review on anti-diabetic and anti-obesity effects of dietary resistant starch. Crit Rev Food Sci Nutr. 2019;59:3019–3031. doi: 10.1080/10408398.2018.1481360. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J Am Coll Surg. 2008;207:928–934. doi: 10.1016/j.jamcollsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 25.Behall KM, Howe JC. Effect of long-term consumption of amylose vs amylopectin starch on metabolic variables in human subjects. Am J Clin Nutr. 1995;61:334–340. doi: 10.1093/ajcn/61.2.334. [DOI] [PubMed] [Google Scholar]
- 26.Yuan HC, Meng Y, Bai H, Shen DQ, Wan BC, Chen LY. Meta-analysis indicates that resistant starch lowers serum total cholesterol and low-density cholesterol. Nutr Res. 2018;54:1–11. doi: 10.1016/j.nutres.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Anderson JW, Bridges SR. Short-chain fatty acid fermentation products of plant fiber affect glucose metabolism of isolated rat hepatocytes. Proc Soc Exp Biol Med. 1984;177:372–376. doi: 10.3181/00379727-177-41958. [DOI] [PubMed] [Google Scholar]
- 28.Ble-Castillo JL, Aparicio-Trápala MA, Francisco-Luria MU, Córdova-Uscanga R, Rodríguez-Hernández A, Méndez JD, Díaz-Zagoya JC. Effects of native banana starch supplementation on body weight and insulin sensitivity in obese type 2 diabetics. Int J Environ Res Public Health. 2010;7:1953–1962. doi: 10.3390/ijerph7051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobley GE, Holtrop G, Bremner DM, Calder AG, Milne E, Johnstone AM. Impact of short term consumption of diets high in either non-starch polysaccharides or resistant starch in comparison with moderate weight loss on indices of insulin sensitivity in subjects with metabolic syndrome. Nutrients. 2013;5:2144–2172. doi: 10.3390/nu5062144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, Mascianà R, Forgione A, Gabrieli ML, Perotti G, Vecchio FM, Rapaccini G, Gasbarrini G, Day CP, Grieco A. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–1887. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 31.Russo I, Luciani A, De Cicco P, Troncone E, Ciacci C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn's mucosa through modulation of antioxidant defense machinery. PLoS One. 2012;7:e32841. doi: 10.1371/journal.pone.0032841. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Karimi P, Farhangi MA, Sarmadi B, Gargari BP, Zare Javid A, Pouraghaei M, Dehghan P. The therapeutic potential of resistant starch in modulation of insulin resistance, endotoxemia, oxidative stress and antioxidant biomarkers in women with type 2 diabetes: a randomized controlled clinical trial. Ann Nutr Metab. 2016;68:85–93. doi: 10.1159/000441683. [DOI] [PubMed] [Google Scholar]
- 33.Thampi BS, Manoj G, Leelamma S, Menon VP. Dietary fiber and lipid peroxidation: effect of dietary fiber on levels of lipids and lipid peroxides in high fat diet. Indian J Exp Biol. 1991;29:563–567. [PubMed] [Google Scholar]