Abstract
Background
Occurrence of cough during swallowing is common among asthma patients, but has not been investigated in detail.
Objective
We conducted an observational study to determine the prevalence of swallowing-related cough (SRC) and its characteristics in asthma patients.
Methods
Asthma patients attending our outpatient department between May 2005 and April 2007 were interviewed to investigate if they had ever experienced SRC, as well as postnasal drip or heartburn and cough related to these conditions.
Results
Among 417 patients who completed the questionnaire, 121 patients (29.0%) had experienced SRC. Spicy and sour foods were the most frequent tussigenic foods, causing cough in 76.0% and 53.7% of the 121 patients, respectively. In patients without SRC, the prevalence rates of postnasal drip and postnasal drip-induced cough were 35.8% (106 of 296) and 7.8% (23 of 296), respectively. The corresponding prevalence rates in patients with SRC were 50.4% (61 of 121) and 37.2% (45 of 121), which were both significantly higher than in patients without cough (p = 0.006 and p < 0.001 respectively). In patients without SRC, the prevalence rates of heartburn and heartburn-induced cough were 22.2% (66 of 296) and 2.4% (7 of 296), respectively. The corresponding prevalence rates in patients with SRC were 45.5% (55 of 121) and 16.5% (20 of 121), with both being significantly higher than in patients without cough (p = 0.002 and p < 0.001, respectively).
Conclusion
SRC was frequent in asthma patients, and was closely related to postnasal drip and heartburn. Irritable larynx is one of the possible underlying mechanisms of SRC. This study was registered with the University Hospital Medical Information Network clinical trials registry (registration number: UMIN000017426).
Keywords: Asthma, Cough hypersensitivity, Gastroesophageal reflux, Irritable larynx, Postnasal drip
INTRODUCTION
Some asthma patients complain of cough when swallowing, but the mechanisms involved have not been investigated in detail. Exacerbation of asthma symptoms in relation to food intake occurs in patients with aspirin-induced asthma due to salicylates or food preservatives [1], and has also been reported in alcohol-induced asthma [2] and gastroesophageal reflux disease (GERD) [3,4] at some time after food intake. However, the occurrence of cough directly related to and at the time of swallowing has never been investigated. We hypothesized that mechanical or chemical stimulation of the pharyngolaryngeal mucosa, such as by capsaicin in chili peppers [5] or acid in sour foods [6], might be involved in the pathogenesis of swallowing-related cough (SRC).
Postnasal drip (PND) is a major cause of cough related to stimulation of the pharyngeal mucosa by inflammatory materials in the nasal secretions [7,8] and to activation of the pharyngobronchial reflex [9]. We hypothesized that the mechanisms of SRC and PND-induced cough could be similar since both involve pharyngeal stimulation. GERD is another condition in which laryngopharyngeal stimulation is known to cause cough, with regurgitation of acid gastric contents affecting the region from the lower esophagus to the laryngopharynx, or microaspiration into the airways leading to cough [3].
Against this background, we investigated the prevalence of SRC and its characteristics among patients with bronchial asthma or cough-variant asthma, as well as the relationship between SRC and cough induced by PND or heartburn.
MATERIALS AND METHODS
Patients
From May 2005 to April 2007, outpatients with bronchial asthma or cough-variant asthma attending the National Hospital Organization Disaster Medical Center (Tokyo, Japan) were enrolled consecutively in this study. Based on the simplified diagnostic criteria for cough-variant asthma [10], this condition was defined as persistent cough for >3 weeks with no observed symptomatic wheezing or audible wheezing on auscultation that responded to inhalation of β2-stimulants. Bronchial asthma was diagnosed by the attending physicians in patients with a history of wheezing that responded to bronchodilators.
Methods
Patients were interviewed to determine if they had ever experienced SRC, as well as being asked about tussigenic foods, the timing of cough during the swallowing process, and the severity of cough. SRC was defined as cough that was induced during swallowing and by certain foods or beverages, which improved after initiation of medication for asthma, thus differentiating it from aspiration-induced cough. Causative foods were categorized as follows: spicy, sour, salty, and others. To assess the timing of SRC, the swallowing process was categorized as follows: (1) the oral phase during which food or liquid still remains in the oral cavity before being swallowed, (2) the pharyngeal phase during which the bolus passes through the pharyngolaryngeal space and come into contact with the retropharyngeal mucosa, and (3) the esophageal phase in which the bolus traverses the esophagus. The severity of SRC was categorized as mild, moderate, or severe. Mild SRC was defined as transient cough that allowed patients to continue eating soon after its cessation. Moderate SRC was defined as persistent cough that forced the patient to discontinue eating. Severe SRC was defied as cough inducing secondary events such as vomiting or wheezing. In addition, whether the subjects had ever experienced PND and PND-induced cough (excluding simple clearing of the throat), as well as whether they had ever experienced heartburn and heartburn-induced cough, was determined by interview and the prevalence rates of these conditions were compared between SRC-positive and SRC-negative patients.
For statistical analysis, the significance of differences was assessed by using Pearson chi-square test. All p values were 2-tailed and p < 0.05 was considered to indicate statistical significance. Analyses were performed by using JMP ver. 10 (SAS Institute Ltd., Tokyo, Japan).
This study was performed in conformity with the Declaration of Helsinki and was approved by the ethics committee of the National Hospital Organization Disaster Medical Center. Verbal informed consent was obtained from all participants. This study was registered with the University Hospital Medical Information Network (UMIN) clinical trials registry (registration number: UMIN00001726).
RESULTS
Four hundred seventeen patients were enrolled, including 303 with bronchial asthma and 114 with cough-variant asthma. The prevalence of SRC was 29.0% (121 of 417), and it was significantly higher among females (p = 0.020) (Table 1). Age was not correlated with the prevalence of SRC (p = 0.424). The tussigenic foods are listed in Table 2. Spicy and sour foods were most frequently tussigenic, affecting 76.0% and 53.7% of the patients, respectively. Not only the taste of food, but also its texture (such as crispy or powdered food) was related to induction of cough. SRC was generally noted during the pharyngeal phase of swallowing (108 of 121, 89.3%), while it was not commonly associated with the oral phase (oral phase only in 8 [6.6%]; oral and pharyngeal phase in 4 [3.3%]: total 9.9%) or the esophageal phase (esophageal phase only in 5 [4.1%]; esophageal and pharyngeal phase in 8 [6.6%]: total 10.7%) (Table 1). SRC was classified as mild in 46 patients (38.0%), moderate in 53 patients (43.8%), and severe in 22 patients (18.2%), including 16 patients who had wheezing and 6 patients with vomiting. When asthma treatment was stratified according to the steps in the Global Initiative for Asthma guideline, it was not associated with the occurrence of SRC (p = 0.703) or with the severity of SRC in the SRC-positive group (p = 0.322). In 15 patients who had active SRC at the time of interview, inhalation of a β-stimulant 15 minutes before meals was initiated and SRC improved in 11 of these patients. In 4 of these 15 patients, inhalation of salmeterol was shifted from after to before meals and SRC improved in 3 of them. In another 5 patients, salmeterol was newly added before meals and SRC improved in 3 of them. A short acting β-stimulant was added before meals in the remaining 6 patients and SRC improved in 5 of them. The effects of SRC on daily life were as follows: inability to eat favorite spicy foods, difficulty in tasting or seasoning foods for a professional cook, apprehension about social activities such as dinner with friends, patients mistaking SRC for aspiration, actual aspiration during SRC in a patient with Parkinson disease, and inability to eat during exacerbation of asthma.
Table 1. Characteristics of the subjects.
| Characteristic | SRC (+) | SRC (−) | Total | |
|---|---|---|---|---|
| No. of subjects | 121 (29.0) | 296 (71.0) | 417 | |
| Female sex§ | 80 (65.6) | 159 (53.7) | 239 (57.3) | |
| Age (yr) | 59 (17–84) | 56 (16–86) | 57 (16–86) | |
| Step | ||||
| I | 0 | 0 | 0 | |
| II | 23 | 53 | 76 | |
| III | 28 | 64 | 92 | |
| IV | 70 | 176 | 246 | |
| V | 0 | 3 | 3 | |
| Timing of SRC | ||||
| Oral phase | 12 (9.9)† | - | ||
| Pharyngeal phase | 108 (89.3) | - | ||
| Esophageal phase | 13 (10.7)‡ | - | ||
| Severity of SRC* | ||||
| Mild | 46 (38.0) | - | ||
| Moderate | 53 (43.8) | - | ||
| Severe | 22 (18.2) | - | ||
Values are presented as number (%) or median (range).
SRC, swallowing-related cough.
*Mild SRC was defined as transient cough that allowed patients to continue eating soon after its cessation. Moderate SRC was defined as persistent cough that forced the patient to discontinue eating. Severe SRC was defied as cough inducing secondary events such as vomiting or wheezing. †Oral phase only in 8 (6.6%), oral and pharyngeal phase in 4 (3.3). ‡Esophageal phase only in 5 (4.1), esophageal and pharyngeal phase in 8 (6.6%). §p = 0.020.
Table 2. Tussigenic foods and beverages (n = 121).
| Tussigenic foods and beverages | No. (%) | ||
|---|---|---|---|
| Spicy | 92 (76.0) | ||
| Sour | 65 (53.7) | ||
| Salty | 12 (9.9) | ||
| Others | 38 (31.4) | ||
| Cold foods and beverages | 8 (6.6) | ||
| Crispy foods | 8 (6.6) | ||
| Powdered foods | 8 (6.6) | ||
| Carbonated drinks | 6 (5.0) | ||
| Hot foods | 3 (2.5) | ||
| Alcohol | 2 (1.7) | ||
| Dehydrated foods | 1 (0.8) | ||
| Strong taste | 1 (0.8) | ||
| Anything | 4 (3.3) | ||
| Specific foods* | 8 (6.6) | ||
| Relation to swallowing | 3 (2.5) | ||
| Swallowing a large amount | 2 (1.7) | ||
| First swallow of each meal | 1 (0.8) | ||
*Japanese miso soup in 2, grapes and pears in 1, almond chocolate, shrimp, beer, melon, and peanuts in 1 each.
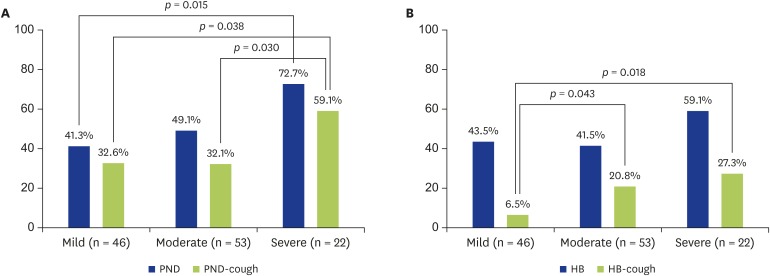
Among the 121 SRC-positive patients, 61 patients (50.4%) had a history of PND and 45 patients (37.2%) had a history of PND-induced cough. Among the 296 SRC-negative patients, 106 patients (35.8%) had a history of PND and 23 patients (7.8%) had a history of PND-induced cough. The frequency of PND and PND-induced cough were both significantly higher in the SRC-positive group (p = 0.006 and p < 0.001, respectively) (Table 3). Both PND and PND-induced cough increased in frequency according to the severity of SRC, but this trend did not reach statistical significance (p = 0.051 and p = 0.063, respectively) (Fig. 1A). PND showed a significantly higher frequency among patients with severe SRC (16 of 22, 72.7%) compared to those with mild SRC (19 of 46, 41.3%) (p = 0.015). The frequency of PND-induced cough was also significantly higher among patients with severe SRC (13 of 22, 59.1%) compared to those with mild SRC (15 of 46, 32.6%) (p = 0.038) or moderate SRC (17 of 53, 32.1%) (p = 0.030).
Table 3. Relationship between SRC and PND or heartburn.
| Variable | Total (n = 417) | SRC (+) (n = 121) | SRC (−) (n = 296) | p value |
|---|---|---|---|---|
| PND | 167 (40.0) | 61 (50.4) | 106 (35.8) | 0.006 |
| PND-cough | 68 (16.3) | 45 (37.2) | 23 (7.8) | < 0.001 |
| PND-cough/PND | 40.7% | 73.8% | 21.7% | < 0.001 |
| HB | 121 (29.0) | 55 (45.5) | 66 (22.2) | 0.002 |
| HB-cough | 27 (6.5) | 20 (16.5) | 7 (2.4) | < 0.001 |
| HB-cough/HB | 22.3% | 36.4% | 10.6% | 0.001 |
Values are presented as number (%) unless otherwise indicated.
SRC, swallowing-related cough; PND, postnasal drip; PND-cough, postnasal drip-induced cough; HB, heartburn; HB-cough, heartburn-induced cough.
Fig. 1. Relationship between the severity of SRC and PND or heartburn (HB). (A) The frequency of PND and PND-induced cough increased along with the severity of SRC (p = 0.051, p = 0.063 respectively). PND was significantly more frequent in patients with severe SRC than in those with mild SRC (16/22 vs. 19/46, p = 0.015). Also, PND-induced cough was significantly more frequent in patients with severe SRC compared to patients with mild SRC (13/22 vs. 15/46, p = 0.038) or moderate SRC (17/53, p = 0.030). (B) The frequency of HB was not associated with the severity of SRC (p = 0.358). However, the frequency of HB-induced cough increased along with the severity of SRC (p = 0.053). Compared to patients with mild SRC (3 of 46), HB-induced cough was significantly more frequent in patients with moderate SRC (11/53, p = 0.043) or severe SRC (6/22, p = 0.018). SRC, Swallowing-related cough; PND, Postnasal drip; HB, Heartburn.
In the SRC-positive group, 55 patients (45.5%) had a history of heartburn and 20 patients (16.5%) had a history of heartburn-induced cough. In the SRC-negative group, 66 patients (22.2%) had a history of heartburn and 7 patients (2.4%) had a history of heartburn-induced cough. The frequency of heartburn was significantly higher in the SRC-positive group, as was the frequency of heartburn-induced cough (p = 0.002 and p < 0.001, respectively) (Table 3). While the frequency of heartburn was not associated with the severity of SRC (p = 0.358), heartburn-induced cough tended to increase along with the severity of SRC (p = 0.053). Compared to the frequency of heartburn-induced cough in patients with mild SRC (3 of 46, 6.5%), the frequency was significantly higher in patients with moderate SRC (11 of 53, 20.8%) (p = 0.043) and patients with severe SRC (6 of 22, 27.3%) (p = 0.018) (Fig. 1B).
In the SRC-positive group, the frequency of PND and heartburn did not show a significant difference and there was also no difference in the frequency of PND-induced cough and heartburn-induced cough (p = 0.642 and p = 0.429, respectively).
DISCUSSION
In the present study, 29% of asthma patients attending a hospital outpatient clinic had symptoms consistent with SRC. This condition showed female predominance and cough usually occurred during the pharyngeal phase of swallowing. In addition, SRC was closely related to a history of PND and/or heartburn along with a history of PND/heartburn-induced cough. Aspiration-induced cough also occurs during swallowing, but SRC could be differentiated from such cough by 3 factors: (1) alleviation through better control of asthma, (2) induction by specific foods, and (3) prevention by administration of a β2-stimulant before meals.
Cough receptors are widely distributed throughout the airways from the pharynx to the bronchi [11,12]. There are 2 distinct types of cough receptors, which are rapidly adapting mechanoreceptors and C-fiber receptors [11,12]. Rapidly adapting mechanoreceptors are activated by mechanical stimuli, such as inflation and deflation of the lungs or acute mechanical distortion of the airway walls. In contrast, C-fiber receptors are activated by a variety of chemical stimuli, including cigarette smoke, bradykinin, citric acid, and vanilloid capsaicin, and these fibers are much less sensitive to mechanical stimulation [12]. Immediate onset of SRC during the pharyngeal phase of swallowing when the food bolus comes into contact with the pharyngolaryngeal mucosa suggests that these cough receptors are stimulated by tussigenic foods. Thus, our subjects with SRC during the pharyngeal phase might have developed cough due to pharyngeal hypersensitivity related to laryngeal hypersensitivity syndrome or irritable larynx [7,13] and mediated via the superior or recurrent laryngeal nerve [7,13]. In asthma patients, upper airway cough receptors may be involved in its pathogenesis in addition to intrathoracic airway cough receptors [7], and it has been reported that asthma patients with chronic cough show increased laryngeal hypersensitivity following inhalation of histamine, a finding consistent with the existence of irritable larynx [7]. The high prevalence rate of SRC among asthma patients revealed by our study suggests that these patients also frequently have laryngopharyngeal hypersensitivity. This study also identified 2 other types of SRC, which occurred during the oral or esophageal phases of swallowing, but these only accounted for 9.9% and 4.1% of all SRC, respectively. SRC during the oral phase of swallowing might be caused by influx of saliva containing tussigenic food particles into the pharyngeal space or by inhalation of tussigenic air into the airways [14]. On the other hand, SRC during the esophageal phase could be due to stimulation of the esophageal mucosa, as occurs in GERD [3], or may represent a delayed response to pharyngeal stimulation.
The mechanism of PND-induced cough has been reported to involve both mechanical stimulation by nasal secretions and chemical stimulation by inflammatory cytokines contained in the secretions [7,8,15], or may be related to activation of the pharyngobronchial reflex [9], and PND-induced cough is closely associated with irritable larynx [7]. According to previous studies, PND-induced cough is not so frequent among PND patients [16,17], with a rate of 21% being reported by O'Hara and Jones [16] that decreased to only 8% when patients with respiratory diseases were excluded [16]. In our study, the frequency of PND-induced cough was 40.7% among PND patients with asthma and 73.8% among SRC-positive asthma patients. These results suggest that the prevalence of PND-induced cough among PND patients might depend on the study population, especially whether or not the patients have asthma, since laryngeal hypersensitivity may be more frequent among asthma patients with chronic cough [7]. The higher prevalence of PND-induced cough among our SRC-positive patients also suggested that SRC and PND-induced cough might have the same underlying mechanism related to irritable larynx or laryngeal hypersensitivity syndrome. In this study, we found that heartburn and heartburn-induced cough were also significantly associated with SRC. Cough due to GERD depends on chemical stimulation of the mucosa extending from the esophagus to the pharyngolarynx by acidic gastric contents [3] and it is thought to be more frequent in persons with laryngeal hypersensitivity.
This study had several limitations as follows. Reporting of events (SRC, PND, heartburn, and PND/heartburn-induced cough) was dependent on each patient's memory and thus was subjective and lacked detailed information about the symptoms. In addition, the interview was performed and decisions were made by a single doctor. Furthermore, there were no objective assessments such as measurement of the peak flow rate, spirometry, or otolaryngological evaluation (including assessment of background rhinitis underlying PND). However, diagnosis of PND and PND-induced cough is essentially subjective, since there is no objective test for PND and no way to quantify the amount of nasal discharge or directly prove that it causes cough [15]. In addition, not all GERD patients experience heartburn, including up to 75% of patients with GERD-related cough [18], so our patients with heartburn and heartburn-induced cough represent a subset of GERD and its overall relationship to SRC remains unknown. The relationship of SRC to other common triggers of cough, such as cold air, dry air, or speaking should also have been examined for further evaluation of underlying cough hypersensitivity syndrome. Premedication with a β-stimulant before meals prevented emergence of SRC in our experience and seemed to be promising, but the intervention was not controlled and a placebo effect was not excluded. Furthermore, we did not evaluate the prevalence of SRC in nonasthma patients or the general population, but improvement of SRC by premedication with a β-stimulant before meals suggests its prevalence might be lower in other populations. Moreover, the effects of food on the pharyngolaryngeal mucosa might differ according to diet and culture, e.g., our results might have been different if this study had been performed in a population accustomed to eating spicy foods. Finally, we investigated a history of SRC rather than current SRC, which means that our results do not necessarily reflect the relationship between SRC and the status of asthma or its treatment.
Our results suggested that SRC is closely related to cough induced by PND or heartburn (GERD). In addition, a subgroup of asthma patients may have hypersensitivity of the mucosa or sensory neurons in the region extending from the pharynx to the esophagus. SRC could be a surrogate marker for irritable larynx or laryngeal hypersensitivity syndrome, which means that evaluation of these conditions might be possible through development of challenge tests such as the ‘capsaicin swallowing test.’
We found that patients were affected by SRC in specific ways related to their eating habits, but impairment of quality of life due to SRC was not assessed fully and should be investigated in the future. Inhalation of a β-stimulant before meals may control SRC. Accordingly, the timing of routine use of inhaled corticosteroids/long-acting β-agonists should be switched to before meals rather than increasing the dose or adding other medications when patients have SRC.
In conclusions, SRC was frequent among asthma patients, and it was significantly associated with PND-induced cough or heartburn-induced cough. Accordingly, irritable larynx may be a common underlying mechanism of these conditions. Further investigation should be performed to evaluate the clinical features of SRC as one of the symptoms of asthma.
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest.
- Conceptualization: Mitsuhiro Kamimura.
- Data curation: Mitsuhiro Kamimura, Atsuto Mouri, Kazuo Takayama, Tomonori Mizutani, Yoichiro Hamamoto, Motoyasu Iikura, Kaneyuki Furihata.
- Formal analysis: Mitsuhiro Kamimura.
- Investigation: Mitsuhiro Kamimura.
- Supervision: Mitsuhiro Kamimura.
- Validation: Mitsuhiro Kamimura.
- Writing - original draft: Mitsuhiro Kamimura.
- Writing - review & editing: Mitsuhiro Kamimura, Motoyasu Iikura, Kaneyuki Furihata.
References
- 1.Sommer DD, Rotenberg BW, Sowerby LJ, Lee JM, Janjua A, Witterick IJ, Monteiro E, Gupta MK, Au M, Nayan S. A novel treatment adjunct for aspirin exacerbated respiratory disease: the low-salicylate diet: a multicenter randomized control crossover trial. Int Forum Allergy Rhinol. 2016;6:385–391. doi: 10.1002/alr.21678. [DOI] [PubMed] [Google Scholar]
- 2.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saritas Yuksel E, Vaezi MF. New developments in extraesophageal reflux disease. Gastroenterol Hepatol (N Y) 2012;8:590–599. [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi S, Richter JE. Diet and gastroesophageal reflux disease: role in pathogenesis and management. Curr Opin Gastroenterol. 2017;33:107–111. doi: 10.1097/MOG.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 5.Lankatilake KN, Uragoda CG. Respiratory function in chilli grinders. Occup Med (Lond) 1993;43:139–142. doi: 10.1093/occmed/43.3.139. [DOI] [PubMed] [Google Scholar]
- 6.Wong CH, Matai R, Morice AH. Cough induced by low pH. Respir Med. 1999;93:58–61. doi: 10.1016/s0954-6111(99)90078-1. [DOI] [PubMed] [Google Scholar]
- 7.Bucca CB, Bugiani M, Culla B, Guida G, Heffler E, Mietta S, Moretto A, Rolla G, Brussino L. Chronic cough and irritable larynx. J Allergy Clin Immunol. 2011;127:412–419. doi: 10.1016/j.jaci.2010.10.038. [DOI] [PubMed] [Google Scholar]
- 8.Riccio AM, Tosca MA, Cosentino C, Pallestrini E, Ameli F, Canonica GW, Ciprandi G. Cytokine pattern in allergic and non-allergic chronic rhinosinusitis in asthmatic children. Clin Exp Allergy. 2002;32:422–426. doi: 10.1046/j.1365-2222.2002.01315.x. [DOI] [PubMed] [Google Scholar]
- 9.Rolla G, Colagrande P, Scappaticci E, Bottomicca F, Magnano M, Brussino L, Dutto L, Bucca C. Damage of the pharyngeal mucosa and hyperresponsiveness of airway in sinusitis. J Allergy Clin Immunol. 1997;100:52–57. doi: 10.1016/s0091-6749(97)70194-5. [DOI] [PubMed] [Google Scholar]
- 10.Committee for the Japanese Respiratory Society Guidelines for Management of Cough. Kohno S, Ishida T, Uchida Y, Kishimoto H, Sasaki H, Shioya T, Tokuyama K, Niimi A, Nishi K, Fujimura M, Matsuse H, Suzaki H. The Japanese Respiratory Society guidelines for management of cough. Respirology. 2006;11(Suppl 4):S135–S186. doi: 10.1111/j.1440-1843.2006.00920_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Widdicombe J. Airway receptors. Respir Physiol. 2001;125:3–15. doi: 10.1016/s0034-5687(00)00201-2. [DOI] [PubMed] [Google Scholar]
- 12.Mazzone SB, Canning BJ. Plasticity of the cough reflex. Eur Respir Rev. 2002;12:236–242. [Google Scholar]
- 13.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology. 2013;18:948–956. doi: 10.1111/resp.12103. [DOI] [PubMed] [Google Scholar]
- 14.Millqvist E. The airway sensory hyperreactivity syndrome. Pulm Pharmacol Ther. 2011;24:263–266. doi: 10.1016/j.pupt.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Pratter MR. Chronic upper airway cough syndrome secondary to rhinosinus diseases (previously referred to as postnasal drip syndrome): ACCP evidence-based clinical practice guidelines. Chest. 2006;129(1 Suppl):63S–71S. doi: 10.1378/chest.129.1_suppl.63S. [DOI] [PubMed] [Google Scholar]
- 16.O'Hara J, Jones NS. “Post-nasal drip syndrome”: most patients with purulent nasal secretions do not complain of chronic cough. Rhinology. 2006;44:270–273. [PubMed] [Google Scholar]
- 17.Sandhu GS, Kuchai R. The larynx in cough. Cough. 2013;9:16. doi: 10.1186/1745-9974-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahrilas PJ, Altman KW, Chang AB, Field SK, Harding SM, Lane AP, Lim K, McGarvey L, Smith J, Irwin RS CHEST Expert Cough Panel. Chronic cough due to gastroesophageal reflux in adults: CHEST guideline and expert panel report. Chest. 2016;150:1341–1360. doi: 10.1016/j.chest.2016.08.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]