Abstract
Objectives
Recent large cohort studies suggest an association between high plasma prolactin and cardiovascular mortality. The objective of this systematic review was to systematically assess the effect of reducing prolactin with dopamine agonist on established cardiovascular risk factors in patients with prolactinomas.
Design
Bibliographical search was done until February 2019 searching the following databases: PubMed, EMBASE, WHO and LILAC. Eligible studies had to include participants with verified prolactinomas where metabolic variables were assessed before and after at least 2 weeks treatment with dopamine agonists.
Methods
Baseline data and outcomes were independently collected by two investigators. The study was registered with PROSPERO (registration number CRD42016046525).
Results
Fourteen observational studies enrolling 387 participants were included. The pooled standardized mean difference of the primary outcome revealed a reduction of BMI and weight of −0.21 (95% CI −0.37 to −0.05; P = 0.01; I2 = 71%), after treatment. Subgroup analysis suggested that the reduction of weight was primarily driven by studies with high prolactin levels at baseline (P = 0.04). Secondary outcomes suggested a small decrease in waist circumference, a small-to-moderate decrease in triglycerides, fasting glucose levels, HOMA-IR, HbA1c and hsCRP, and a moderate decrease in LDL, total cholesterol and insulin.
Conclusion
This systematic review suggests a reduction of weight as well as an improved lipid profile and glucose tolerance after treatment with dopamine agonist in patients with prolactinomas. These data are based on low-quality evidence.
Keywords: prolactinoma, dopamine agonist, weight, BMI, metabolic effect
Introduction
In recent large cohort studies, high prolactin levels within and above the normal physiological range have been associated with increased risk of cardiovascular mortality (1, 2, 3). It is speculated whether this association is mediated through prolactin-induced increase in cardiovascular risk factors including obesity, dyslipidemia and insulin resistance (4, 5, 6, 7, 8, 9, 10). In a German population-based cohort study, prolactin levels within the normal range showed a positive correlation to all-cause as well as cardiovascular mortality (1). Furthermore, prolactin levels prospectively correlated with higher levels of low-density lipoprotein (LDL) in women, and with hypertension and incidence of diabetes in men (11). In two large cohort studies hyperprolactinemia was associated with increased incidence of cardiovascular disease and cardiovascular mortality in men only (2, 3). However, the association between prolactin and excess disease burden is not consistent: In a case–control study with 1204 cases of hyperprolactinemia, no increased morbidity or mortality was found (12), and in a large cohort, levels of prolactin did not differ between those participants who suffered from fatal or nonfatal coronary artery disease and those who did not (13).
Prolactin receptors are widely distributed in the liver (14), endocrine pancreas (15) and in adipose tissue (16) pointing to a possible direct metabolic effect of prolactin. A number of small studies have found that prolactin levels are associated with inflammation, endothelial dysfunction, insulin resistance and dyslipidemia that could contribute to cardiovascular complications (7, 17, 18, 19, 20). Furthermore, prolactin levels correlated with insulin resistance in patients with polycystic ovary syndrome (21).
Dopamine is the natural negative regulator of prolactin release from the anterior pituitary gland, and dopamine D2 receptor agonists are the first line of treatment for most patients with prolactinoma.
A link between high prolactin levels and cardiovascular mortality would have important clinical implications. According to current guidelines (22), asymptomatic patients with hyperprolactinemia are not necessarily offered treatment; the same is true for secondary hyperprolactinemia as a side effect to widely used medications, such as antipsychotic drugs or in patients with kidney failure.
The purpose of this systematic review was to systematically assess the metabolic effects of dopamine agonist treatment in patients with prolactinomas.
Methods
Study design
A systematic review and meta-analysis. The study was registered with PROSPERO (registration number CRD42016046525).
Study selection
Eligible studies were observational or randomized clinical trials assessing the effect of dopamine agonist treatment in patients with prolactinomas verified by MRI or CT scan. For inclusion, the studies should report on metabolic variables before and after dopamine-agonist treatment. Patients should have been treated with dopamine agonist for more than 2 weeks. Studies that included patients with other treatments for prolactinomas, for example, surgical resection or radiotherapy of the pituitary adenoma were not included. There was no age or language restriction.
Search strategy
The following bibliographical databases were searched until February 2019: PubMed, EMBASE, WHO and LILAC using the text words terms: (prolactin OR hyperprolactinemia OR prolactinoma) AND (dopamine agonist OR dostinex OR cabergoline OR bromocriptine). The search was restricted to titles.
One investigator (SB) conducted the main search. Based on title and abstract, obviously irrelevant titles were removed and the remaining studies were considered for inclusion after thorough review of the full manuscript.
Data extraction
Demographical data, baseline data of participants, study outcomes, diagnostic procedures, patient co-morbidity, hormone replacement among participants, type and duration of treatment, and results were independently collected by two investigators (SB, JF). In studies where more than one follow-up was reported, data from the latest follow-up, where the number and gender distributions of participants were reported, were included in the analysis.
Authors were contacted by mail in case of queries regarding reported data. Two requests were sent with 14-days interval if no response was received after the first request.
Outcomes
Primary outcome
The primary outcome was change in weight or body mass index (BMI) from baseline to the end of the observation.
Secondary outcomes
Secondary outcomes were waist circumference (WC), total cholesterol, low-density lipoprotein (LDL), triglycerides (TRG), fasting plasma glucose, HOMA-IR, fasting serum insulin, glycated hemoglobin (HbA1c), plasma testosterone, luteinizing hormone (LH), follicle-stimulating hormone (FSH), high-sensitivity C-reactive protein (hsCRP) and systolic and diastolic blood pressure.
Assessment of study quality
The risk of bias in the included studies was assessed by using the Newcastle–Ottawa Scale (NOS) for cohort studies as suggested by the Cochrane collaboration (23), which assesses selection bias, comparability and outcome modified for the current systematic review. Selection of cases was awarded points if patients were consecutively approached for inclusion, if both genders were included in the study and if hypothyroidism was ruled out. In the outcome domain we assessed if the staff assessed weight or if weight was reported by the participants themselves, if staff were blinded to baseline weight and if attrition were less than 5% at follow-up. For each study, we assessed the above items providing each with assessment of ‘yes’ or ‘no’. In case there was inadequate information to judge either ‘yes’ or ‘no’ we used ‘unclear’. The total risk of bias score was based on one point for each ‘yes’ in the selection bias domain or the outcome domain. We also included whether the study was conducted prospectively or retrospectively. We considered this item as posing a risk of random error opposed to a systematic error, which was assessed in the selection bias domain and the outcome domains and did not include this item in the total score which represents an estimated pooled risk of bias. Studies with a total score of 4 or more were considered with less risk of bias.
Statistical methods
For all outcomes we reported the pooled effect estimates using the standardized mean difference (SMD). SMD is the mean difference in outcome between the post- and pre-intervention assessment divided by the pooled standard deviation (SD). The result is a unit free effect size and by convention, SMD of 0.2, 0.5 and 0.8 are considered small, medium and large effects sizes.
In case mean (x̄) was not reported, we calculated the mean from median (m) and range (a–b) using the formula x̄ ≈ (a + 2m + b) / 4, as proposed by Hozo et al. (24), and SD = (b - a) / 4. In case results were reported separately for men and women, a pooled mean and SD was calculated as recommended in Cochrane Handbook for Systematic Reviews (23).
We expected some degree of heterogeneity due to differences in population, duration of treatment and dose of DA, and we a priori planned to use random-effects analysis to pool estimates from included studies.
P values below 0.05 were considered significant.
For data analyses, we used Comprehensive Meta-Analysis 2.0. The heterogeneity for each outcome was reported as I2.
Subgroup analysis
For the primary outcomes, weight and BMI, we planned to explore the potential effect of confounding variables: duration of treatment (<6 months was short duration of treatment vs duration of treatment of ≥6 months), percentage of included men, prolactin levels at baseline, percentage of macroprolactinoma, the effect of study design as well as the effect of risk of bias. The division of groups for subgroup analysis was based on the median value.
Deviation from the protocol
There have been some changes from the original protocol: the duration of treatment was found to be equivalent to follow-up in the included studies; therefore, no subgroup analysis on follow-up was performed. Comorbidities were an exclusion criterion in seven studies and were not reported in five studies, and as a result of the inconsistency in this data point, a subgroup analysis on comorbidities was not performed. Only three studies reported change of testosterone levels (6, 25, 26) or estradiol levels (25, 27, 28) , which is why this subgroup analysis was abandoned. Mixed treatment modalities were added as exclusion criteria to the original protocol. In the data analysis we conducted subgroup analysis by study design and risk of bias in addition to the original protocol. To improve the clinical applicability of the analysis, the effect sizes are also presented as mean difference.
Results
Study selection and characteristics
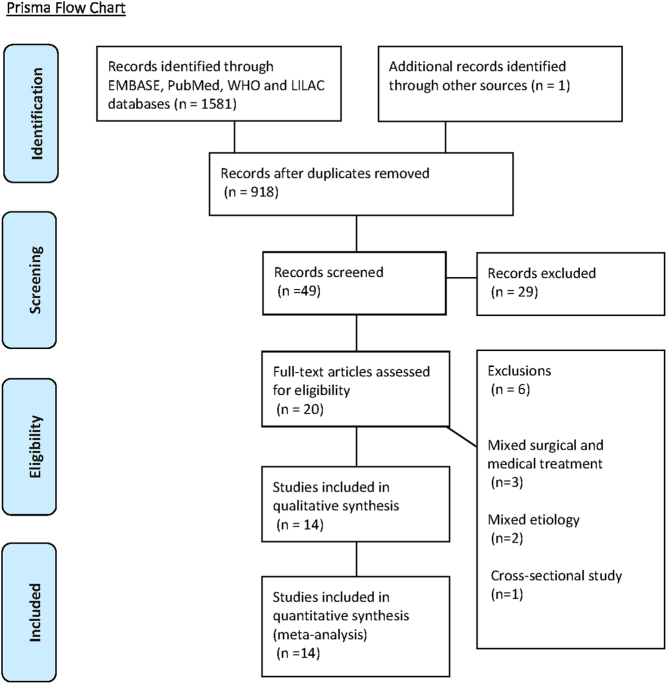
The bibliographical search was conducted until February 2019. As illustrated in Fig. 1, we included 14 observational studies assessing the effect of dopamine agonist therapy on metabolic variables in 387 patients with prolactinomas (4, 5, 6, 7, 8, 25, 26, 27, 28, 29, 30, 31, 32, 33).
Figure 1.
Prisma flow chart.
As shown in Table 1 nine of the included studies were prospective (5, 7, 8, 26, 27, 28, 29, 30, 32), three studies (4, 25, 33) were retrospective and in two studies the design was not clearly stated (6, 31). As shown in Table 2 study participants were treated with cabergoline (CAB) in eight studies (4, 5, 7, 25, 26, 27, 30, 33), bromocriptine (BRC) in two studies (28, 31) and in four studies participants received either CAB or BRC (6, 8, 29, 32).
Table 1.
Risk of bias in included studies for the primary outcome – ‘change in weight’.
| Random error | Selection bias | Outcome | Total points | Notes | |||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Prospective | Consecutive enrollment | Both genders | Hypothyroidism ruled out | Weight assessment by staff | Blinded to baseline weight | <5% missing data | ||
| Auriemma 2014 (5) | Yes | Yes | Yes | Yes | Yes | Unclear | No | 4 | Assessment from 3 to 6 months interval from baseline not included in report. 25/95 patients excluded due to treatment duration <12 months |
| Auriemma 2015 (26) | Yes | Yes | No | Yes | Yes | Unclear | No | 3 | Assessment from 3 to 6 months interval not included in report. 7/45 patients not included due to treatment duration <12 months |
| Barbosa 2014 (33) | Yes | Unclear | Yes | Yes | Yes | Unclear | No | 3 | 10/35 patients not included due to discontinuation of dopamine agonist therapy due to irregular supply. Exclusion criteria: chronic disease |
| Berinder 2011 (6) | Unclear | Unclear | Yes | No | Yes | Unclear | Yes | 2 | Exclusion criteria: diabetes and dyslipidemia |
| Ciresi 2013 (4) | No | Yes | Yes | Unclear | Yes | Unclear | No | 3 | Patients receiving hypoglycemic agents at baseline were excluded. One patient had diabetes, 13 patients with metabolic syndrome |
| Docknic 2002 (30) | Unclear | Unclear | Yes | Yes | Yes | Unclear | Yes | 4 | 52% of included patients were men |
| Inancli 2013 (29) | Yes | Unclear | No | Yes | Yes | Unclear | Yes | 3 | Exclusion criteria: CVD, diabetes and hypertension |
| Medic-Stojanovska 2015 (31) | Yes | Unclear | No | Yes | Yes | Unclear | Yes | 3 | Exclusion criteria: CVD, diabetes mellitus |
| Pala 2015 (27) | Yes | Yes | Yes | Unclear | Yes | Unclear | Yes | 5 | Only 1 male patient. Exclusion criteria: Diabetes, dyslipidemia |
| Silva 2011 (8) | Yes | Unclear | Yes | Yes | Yes | Unclear | Yes | 4 | 10/35 patients excluded due to discontinuation of DA therapy. 2 diabetes, Exclusion criteria: Acute or chronic diseases |
| Schwetz 2017 (25) | No | No | Yes | Unclear | Yes | Unclear | Yes | 3 | Patients failing to demonstrate normoprolactenemia at follow-up were excluded from analysis |
| Serri 2006 (7) | Yes | No | Yes | Unclear | Yes | Unclear | Yes | 4 | 7/15 patients were male. Exclusion criteria: CVD, HT, diabetes |
| Yavuz 2003 (28) | Yes | Unclear | No | Unclear | Yes | Unclear | Yes | 2 | No comorbidities |
| Iglesiasa 2016 (32) | No | Unclear | No | Unclear | N/A | N/A | No | 0 | |
aIglesias et al. did not report weight changes in response to dopamine receptor antagonist treatment.
Table 2.
Background characteristics and study data.
| Study | n | Country | Follow-up, months | Drop out, % | Age, mean ± s.d. | Male, % | Macroprolactinoma, % | Treatmenta | Overweight or obese at baseline, % | Baseline prolactin, mU/L mean ± s.d. | Baseline BMI, mean ± s.d. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Schwetz 2017 (25) | 53 | Austria | 9 | 0 | 39 ± 17 | 58 | 59 | CAB | – | 7607 ± 4414 | 27.9 ± 5.9 |
| Iglesias 2016 (32) | 27 | Spain | 57 | 0 | 39 ± 13 | 100 | 74 | CAB | – | 43693 ± 27401 | 31.3 ± 5.1 |
| Auriemma 2015 (26) | 32 | Italy | 24 | 0 | 42 ± 5 | 100 | 78 | CAB | 97 | 42996 ± 93443 | 31.7 ± 3.9 |
| Auriemma 2014 (5) | 61 | Italy | 60 | 0 | 34 ± 10 | 21 | 33 | CAB | 64 | 16733 ± 5073 | 27.6 ± 5.3 |
| Barbosa 2014 (33) | 21 | Brazil | 6 | 40 | – | – | 23 | CAB, BRC | 69 | 9080 ± 7034 | 29.3 ± 15.4 |
| Ciresi 2013 (4) | 43 | Italy | 12 | 0 | 34 ± 11 | 19 | – | CAB | – | 3715 ± 5718 | 25.57 ± 5.18 |
| Inancli 2013 (29) | 21 | Turkey | 6 | 0 | 30 ± 10 | 0 | 14 | CAB | 19b | 3201 ± 1230 | 27.1 ± 5.9 |
| Berinder 2011 (6) | 14 | Sweden | 6 | 7 | 40 ± 14 | 43 | 43 | CAB, BRC | 21b | M:26809(2617–204,255) /F: 1511(1043–2787) | 25.8 ± 7.8 |
| Silva 2011 (8) | 22 | Brazil | 6 | 37 | 42 ± 35 | 23 | 18 | CAB, BRC | 62 | 5720 ± 3621 | 29.2 ± 15.3 |
| Serri 2006 (7) | 15 | Canada | 3 | – | 39 ± 13 | 47 | 13 | CAB | 47b | 20,160 ± 18,780 | 29 ± 6 |
| Doknic 2002 (30) | 23 | Serbia | 6 | 0 | 37 ± 3 | 52 | 65 | BRC | 39c | 42,682 ± 37,429 | 27.5 ± 3.4 |
| Medic 2015 (31) | 20 | Serbia | 4 | 0 | 30 ± 7 | 0 | 30 | CAB, BRC | – | 2919 ± 1102 | 24 ± 6.4 |
| Pala 2015 (27) | 19 | India | 6 | 32 | 27 ± 6 | 5 | 21 | CAB | 37 | 2514 ± 2232 | 24.2 ± 4.0 |
| Yavuz 2003 (28) | 16 | Turkey | 6 | 0 | 31 ± 10 | 0 | – | BRC | – | 3318 ± 1637 | 26.3 ± 5.3 |
aCabergoline (CAB); Bromocriptine (BRC)
bBMI > 30.
cBMI > 27.
F, female; M, male.
The median percentage of male participants was 23% (range, 0–100%). The median of mean age was 37 years (range, 27–42 years). The median percentages of study participants with macroprolactinoma were 31.5% (range, 13–78%). The range of mean plasma prolactin was 2514–43,693 mU/L and the median follow-up time was 6 months (range, 3–60 months). The median number of participants that reached normoprolactinemia at follow-up was 97% (range, 56–100%) (4, 5, 6, 7, 8, 26, 27, 28, 29, 30, 32).
Because of heterogeneity in reported data, it has not been possible to compare the data regarding treatment dosage: In two studies the dosage was not reported (8, 29); two studies reported the initial dosage (27, 30), two studies reported a range of doses administered, without reporting a median dose (28, 31); one study reported the accumulated dose of 108 mg CAB administered over a mean period of 56.9 ± 46 months (33) and four studies reported doses administered in different periods of the studies (4, 5, 7, 26).
Raw data are presented in Supplementary Tables 3, 4, 5, 6, and 7 (see section on supplementary data given at the end of this article.
In the nine studies reporting obesity at baseline, the median percentage of participants with overweight at baseline was 47% (range, 19–97%) (5, 6, 7, 8, 26, 27, 29, 30, 31). The median fasting glucose levels at baseline were 5.2 mmol/L (range, 4.4–6.5), and median HbA1c was 35.5 mmol/mol (34.4–36.8). The median mean value of LDL at baseline was 3.3 mmol/L (range, 2.8–3.7). Only 3 of 14 studies reported on baseline levels of sex hormones. The prevalence of comorbidities was not reported, or participants with comorbidities were excluded, in 12 studies.
Assessment of risk of bias in included studies
None of the included studies had a control group and the comparability item was left out. No participants were lost to follow-up in 9 of 14 studies (4, 5, 25, 26, 28, 30, 31, 32, 33), in four studies 7–40% of participants were not included in final analysis (6, 8, 27, 29), and in one study this item was unclear (7). Based on our modified NOS bias assessment, we found that eight studies had a high risk of bias, and five studies had a lesser risk of bias in the 13 studies that reported on the primary outcome. Risk of bias for the primary outcome is presented in Table 1.
Primary outcome: the effect of dopamine agonist therapy on weight or body mass index
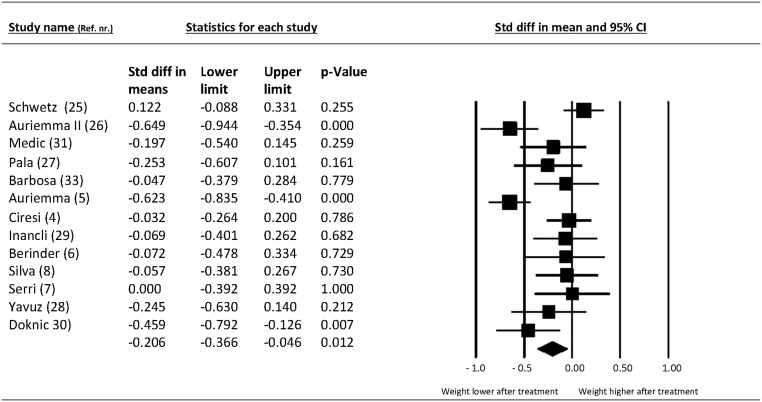
At follow-up, 12 studies reported change in BMI, one study reported change in weight exclusively and, finally, one study reported neither change in weight nor BMI. Therefore, 13 studies with 360 participants were available for analysis. The standardized mean change in BMI and weight was −0.21 SMD (95% CI −0.37 to −0.05; P = 0.01) at follow-up compared to baseline values. For the 12 studies that only reported on BMI, the mean reduction in BMI after treatment was −1.17 BMI points (95% CI −1.99 to −0.38). The forest plot is presented in Fig. 2 and inspection of funnel plot (not shown) for primary outcome did not suggest publication bias.
Figure 2.
Change in weight/BMI after dopamine receptor agonist treatment in patients with prolactinomas.
Heterogeneity and subgroup analysis of primary outcome
The primary outcome was associated with an I2 of 71% suggesting substantial heterogeneity. Subgroup analysis suggested that the reduction of weight was −0.09 SMD (95% CI −0.16 to 0.06; P = 0.35; I2 = 0%) in studies with low prolactin levels at baseline compared to −0.33 SMD (95% CI −0.58 to −0.09; P = 0.008; I2 = 72%) in studies with high prolactin levels at baseline. As shown in Supplementary Table 1, no other subgroup analyses explained the observed heterogeneity.
Secondary outcomes
After treatment with DA, the pooled SMD suggested a small decrease in WC, a small-to-moderate decrease in TRG, fasting glucose levels, HOMA-IR, HbA1c and hsCRP, and a moderate decrease in LDL, total cholesterol and insulin (Supplementary Table 2). There was no change in blood pressure, a small increase in LF and FSH, no increase in estrogen and estradiol and a large increase in testosterone after treatment.
Adverse events from treatment
Adverse events were reported in 4 of 14 studies. In one study, 24% of the patients had a transient gastrointestinal intolerance to DA treatment (29). In another study non-specified transient side effects occurred in 21% (8). One study reported that no participants experienced adverse events (7) and in one study 87.5% of participants experienced nausea, dizziness and sleep disturbances during treatment (28). None of the studies reported serious adverse events.
Discussion
In this systematic review, we included 14 observational studies assessing the effect of DA treatment on metabolic variables in 387 patients with prolactinoma. For the primary outcome, weight and BMI, DA treatment significantly reduced body weight, with a weight reduction of 0.2 SMD. In addition, DA treatment was associated with small-to-moderate effects on all secondary endpoints except blood pressure, estrogen and estradiol. Too few studies reported on harmful events to provide any firm conclusions regarding this aspect of DA treatment in patients with prolactinomas.
A reduction in body weight was observed in 11 of 13 included studies; however, the effect was most prevalent in studies with high prolactin levels at baseline. In concurrence with previous reports (17, 34, 35) the included studies had a high prevalence of obese participants at baseline which suggest an association between prolactin and obesity. There are several hypotheses, which may explain the observed reduction of weight after DA treatment in patients with hyperprolactinemia. One possible mechanism could be a direct effect of DA on metabolism. Randomized clinical trials of patients with type 2 diabetes and obesity suggest that quick-release BRC lowers HbA1c and fasting plasma glucose; however, in these studies a neutral effect on weight and lipid profile were observed (36, 37, 38).
Hyperprolactinemia causes hypogonadotropic hypogonadism and the observed effect on weight may be a result of restoration of eugonadism. It is well established that obesity can cause male hypogonadotropic hypogonadism. By contrast, it is debated whether hypogonadism induces obesity. Some studies have shown that low testosterone levels reduce the fat-to-muscle ratio, but do not alter body weight (39, 40, 41, 42). In opposition to these results a RCT (43) and two observational studies examining changes in body weight in male patients with hypogonadism before and after initiation of testosterone treatment found a mean decrease in BMI of approximately four BMI points (44, 45, 46). For women obesity has been associated with higher levels of estrogens (41, 47) but to our knowledge no studies looking at weight change related to treatment of secondary hypogonadism in women has been published.
A direct effect of prolactin on adipocytes and lipid metabolism cannot be excluded, since prolactin receptors have been identified on adipose tissue (48). However, one in vitro study on human adipocytes from women in the fertile age found that prolactin inhibited lipid storage outside breast tissue (16), thereby not supporting a link between obesity and prolactin. It should be emphasized that the design of the included studies in the present meta-analysis does not allow any conclusions on possible causal mechanisms between weight reduction and DA treatment.
For the secondary outcomes, we found improvement in all cardiovascular risk factors except blood pressure, estrogen and estradiol. We found a small decrease in WC, a small-to-moderate decrease in TRG, fasting glucose levels, HOMA-IR, HbA1c and hsCRP, and a moderate decrease in LDL, total cholesterol and fasting insulin levels.
DA treatment has a neutral effect on lipid profile (37). However, the favorable effect of quick-release BRC on glucose metabolism has led to the approval of BRC for treatment of type 2 diabetes by the FDA (49), and studies have found that BRC as add-on therapy in patients with type 2 diabetes lowers HbA1c (weighted mean difference, −6.52 mmol/mol; (95% CI −8.07 to −4.97) (37, 50, 51) which likely explains the observed effect on the glucose tolerance.
Reversal of hypogonadism might be contributing to the improvement in both lipid profile and glucose tolerance, but study results are conflicting: in a randomized double-blind trial allocating men with hypogonadism (n = 220) and type 2 diabetes or metabolic syndrome to testosterone supplementation or placebo; no consistent improvement was found in lipid profile or WC, but there was significant reduction in HOMA-IR (43). In two registry-based studies (n = 255 and n = 561) investigating males with hypogonadism, a significant reduction in total cholesterol, LDL, TRG, HbA1c, fasting blood glucose and CRP after testosterone treatment (44, 52).
There are several important limitations to the current review. No randomized trials were included, and all the reported outcomes were associated with moderate-to-large heterogeneity. The strength of this review is that more than half of the included studies were prospective and the thorough and systematic approach regarding search strategy as well as data extraction and data synthesis. Furthermore, the protocol was made available in a publicly accessible database (Prospero) prior to data collection and analysis.
Today, large patient populations such as patients with renal failure and those receiving antipsychotic medication are not offered treatment for hyperprolactinemia. In case the findings from this systematic review could be replicated in studies of a higher evidence level, the clinical implications are potentially large.
Conclusion
This systematic review suggests a reduction of weight as well as an improved lipid profile and glucose tolerance after treatment with dopamine agonist in patients with prolactinomas. These data are based on low quality evidence.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
Prospero number: CRD42016046525.
References
- 1.Haring R, Friedrich N, Völzke H, Vasan RS, Felix SB, Dörr M, Meyer Zu Schwabedissen HE, Nauck M, Wallaschofski H. Positive association of serum prolactin concentrations with all-cause and cardiovascular mortality. European Heart Journal 2014. 1215–1221. ( 10.1093/eurheartj/ehs233) [DOI] [PubMed] [Google Scholar]
- 2.Krogh J, Selmer C, Torp-Pedersen C, Gislason GH, Kistorp C. Hyperprolactinemia and the association with all-cause mortality and cardiovascular mortality. Hormone and Metabolic Research 2017. 411–417. ( 10.1055/s-0043-107243) [DOI] [PubMed] [Google Scholar]
- 3.Toulis KA, Robbins T, Reddy N, Kumarendran B, Gokhale K, Wijesinghe H, Cheng KK, Karavitaki N, Wass J, Nirantharakumar K. Males with prolactinoma are at increased risk of incident cardiovascular disease. Clinical Endocrinology 2017. 71–76. ( 10.1111/cen.13498) [DOI] [PubMed] [Google Scholar]
- 4.Ciresi A, Amato MC, Guarnotta V, Lo Castro F, Giordano C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clinical Endocrinology 2013. 845–852. ( 10.1111/cen.12204) [DOI] [PubMed] [Google Scholar]
- 5.Auriemma RS, Granieri L, Galdiero M, Simeoli C, Perone Y, Vitale P, Pivonello C, Negri M, Mannarino T, Giordano C, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology 2013. 299–310. ( 10.1159/000357810) [DOI] [PubMed] [Google Scholar]
- 6.Berinder K, Nyström T, Höybye C, Hall K, Hulting AL. Insulin sensitivity and lipid profile in prolactinoma patients before and after normalization of prolactin by dopamine agonist therapy. Pituitary 2011. 199–207. ( 10.1007/s11102-010-0277-9) [DOI] [PubMed] [Google Scholar]
- 7.Serri O, Li L, Mamputu JC, Beauchamp MC, Maingrette F, Renier G. The influences of hyperprolactinemia and obesity on cardiovascular risk markers: effects of cabergoline therapy. Clinical Endocrinology 2006. 366–370. ( 10.1111/j.1365-2265.2006.02469.x) [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos Silva CM, Barbosa FRP, Lima GAB, Warszawski L, Fontes R, Domingues RC, Gadelha MR. BMI and metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity 2011. 800–805. ( 10.1038/oby.2010.150) [DOI] [PubMed] [Google Scholar]
- 9.Carrero JJ, Kyriazis J, Sonmez A, Tzanakis I, Qureshi AR, Stenvinkel P, Saglam M, Stylianou K, Yaman H, Taslipinar A, et al. Prolactin levels, endothelial dysfunction, and the risk of cardiovascular events and mortality in patients with CKD. Clinical Journal of the American Society of Nephrology 2012. 207–215. ( 10.2215/CJN.06840711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Curhan GC, Forman JP. Plasma prolactin level and risk of incident hypertension in postmenopausal women. Journal of Hypertension 2010. 1400–1405. ( 10.1097/HJH.0b013e328339f254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Therkelsen KE, Abraham TM, Pedley A, Massaro JM, Sutherland P, Hoffmann U, Fox CS. Association between prolactin and incidence of cardiovascular risk factors in the Framingham heart study. Journal of the American Heart Association 2016. e002640 ( 10.1161/JAHA.115.002640) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto-Pedre E, Newey PJ, Bevan JS, Leese GP. Morbidity and mortality in patients with hyperprolactinaemia: the PROLEARS study. Endocrine Connections 2017. 580–588. ( 10.1530/EC-17-0171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuwer AQ, Twickler MT, Hutten BA, Molema FW, Wareham NJ, Dallinga-Thie GM, Bogorad RL, Goffin V, Smink-Bol M, Kastelein JJP, et al. Prolactin levels and the risk of future coronary artery disease in apparently healthy men and women. Circulation. Cardiovascular Genetics 2009. 389–395. ( 10.1161/CIRCGENETICS.109.853572) [DOI] [PubMed] [Google Scholar]
- 14.Simon-Holtorf J, Mönig H, Klomp HJ, Reinecke-Lüthge A, Fölsch UR, Kloehn S. Expression and distribution of prolactin receptor in normal, fibrotic, and cirrhotic human liver. Experimental and Clinical Endocrinology & Diabetes 2006. 584–589. ( 10.1055/s-2006-948310) [DOI] [PubMed] [Google Scholar]
- 15.García-Caballero T, Morel G, Gallego R, Fraga M, Pintos E, Gago D, Vonderhaar BK, Beiras A. Cellular distribution of prolactin receptors in human digestive tissues. Journal of Clinical Endocrinology & Metabolism 1996. 1861–1866. ( 10.1210/jcem.81.5.8626848) [DOI] [PubMed] [Google Scholar]
- 16.Ling C, Svensson L, Odén B, Weijdegård B, Edén B, Edén S, Billig H. Identification of functional prolactin (PRL) receptor gene expression: PRL inhibits lipoprotein lipase activity in human white adipose tissue. Journal of Clinical Endocrinology & Metabolism 2003. 1804–1808. ( 10.1210/jc.2002-021137) [DOI] [PubMed] [Google Scholar]
- 17.Perić B, Kruljac I, Šundalić S, Pećina HI, Jović A, Štefanović M, Butorac D, Vrkljan M. Obesity and hypercholesterolemia in patients with prolactinomas: could DHEA-S and growth hormone be the missing link? Endocrine Research 2016. 200–206. ( 10.3109/07435800.2015.1135444) [DOI] [PubMed] [Google Scholar]
- 18.Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, Creatsa M, Christodoulakos G, Alevizaki M, Sfikakis PP, et al. Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension 2009. 54 98–105. ( 10.1161/HYPERTENSIONAHA.109.132100) [DOI] [PubMed] [Google Scholar]
- 19.Reuwer AQ, Van Eijk M, Houttuijn-Bloemendaal FM, Van Der Loos CM, Claessen N, Teeling P, Kastelein JJP, Hamann J, Goffin V, Von Der Thüsen JH, et al. The prolactin receptor is expressed in macrophages within human carotid atherosclerotic plaques: A role for prolactin in atherogenesis? Journal of Endocrinology 2011. 107–117. ( 10.1677/JOE-10-0076) [DOI] [PubMed] [Google Scholar]
- 20.Landgraf R, Landgraf-Leurs MMC, Weissmann A, Hörl R, von Werder K, Scriba PC. Prolactin: a diabetogenic hormone. Diabetologia 1977. 99–104. ( 10.1007/BF00745135) [DOI] [PubMed] [Google Scholar]
- 21.Bahceci M, Tuzcu A, Bahceci S, Tuzcu S. Is hyperprolactinemia associated with insulin resistance in non-obese patients with polycystic ovary syndrome? Journal of Endocrinological Investigation 2003. 655–659. ( 10.1007/BF03347025) [DOI] [PubMed] [Google Scholar]
- 22.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JAH. & Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology & Metabolism 2011. 273–288. ( 10.1210/jc.2010-1692) [DOI] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). London, UK: Cochrane, 2017. (available at: (http://training.cochrane.org/handbook) [Google Scholar]
- 24.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005. 13 ( 10.1186/1471-2288-5-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwetz V, Librizzi R, Trummer C, Theiler G, Stiegler C, Pieber TR, Obermayer-Pietsch B, Pilz S. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metabolic Brain Disease 2017. 155–161. ( 10.1007/s11011-016-9882-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Auriemma RS, Galdiero M, Vitale P, Granieri L, Lo Calzo F, Salzano C, Ferreri L, Pivonello C, Cariati F, Coppola G, et al. Effect of chronic cabergoline treatment and testosterone replacement on metabolism in male patients with prolactinomas. Neuroendocrinology 2015. 66–81. ( 10.1159/000371851) [DOI] [PubMed] [Google Scholar]
- 27.Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetology & Metabolic Syndrome 2015. 99 ( 10.1186/s13098-015-0094-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yavuz D, Deyneli O, Akpinar I, Yildiz E, Gözü H, Sezgin O, Haklar G, Akalin S. Endothelial function, insulin sensitivity and inflammatory markers in hyperprolactinemic pre-menopausal women. European Journal of Endocrinology 2003. 187–193. ( 10.1530/eje.0.1490187) [DOI] [PubMed] [Google Scholar]
- 29.Barbosa FR, Silva CM, Lima GA, Warszawski L, Domingues RC, Dominic M, Fontes R, Vieira Neto L, Gadelha MR. Prevalence of obstructive sleep apnea in patients with prolactinoma before and after treatment with dopamine agonists. Pituitary 2014. 441–449. ( 10.1007/s11102-013-0524-y) [DOI] [PubMed] [Google Scholar]
- 30.Inancli SS, Usluogullari A, Ustu Y, Caner S, Tam AA, Ersoy R, Cakir B. Effect of cabergoline on insulin sensitivity, inflammation, and carotid intima media thickness in patients with prolactinoma. Endocrine 2013. 193–199. ( 10.1007/s12020-012-9857-y) [DOI] [PubMed] [Google Scholar]
- 31.Doknic M, Pekic S, Zarkovic M, Medic-Stojanoska M, Dieguez C, Casanueva F, Popovic V. Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. European Journal of Endocrinology 2002. 77–84. ( 10.1530/eje.0.1470077) [DOI] [PubMed] [Google Scholar]
- 32.Medic-Stojanoska M, Icin T, Pletikosic I, Bajkin I, Novakovic-Paro J, Stokic E, Spasic DT, Kovacev-Zavisic B, Abenavoli L. Risk factors for accelerated atherosclerosis in young women with hyperprolactinemia. Medical Hypotheses 2015. 321–326. ( 10.1016/j.mehy.2015.01.024) [DOI] [PubMed] [Google Scholar]
- 33.Iglesias P, Villabona C, Díez JJ. Concentraciones séricas de glucosa, colesterol y triglicéridos en varones con prolactinoma tratados con cabergolina. Medicina Clínica 2016. 466–467. ( 10.1016/j.medcli.2016.05.025) [DOI] [PubMed] [Google Scholar]
- 34.Greenman Y, Tordjman K, Stern N. Increased body weight associated with prolactin secreting pituitary adenomas: weight loss with normalization of prolactin levels. Clinical Endocrinology 1998. 48 547–553. ( 10.1046/j.1365-2265.1998.00403.x) [DOI] [PubMed] [Google Scholar]
- 35.Korner J, Lo J, Freda PU, Wardlaw SL. Treatment with cabergoline is associated with weight loss in patients with hyperprolactinemia. Obesity Research 2003. 11 311–312. ( 10.1038/oby.2003.46) [DOI] [PubMed] [Google Scholar]
- 36.Ramteke KB, Ramanand SJ, Ramanand JB, Jain SS, Raparti GT, Patwardhan MH, Murthy M, Ghanghas RG. Evaluation of the efficacy and safety of bromocriptine QR in type 2 diabetes. Indian Journal of Endocrinology & Metabolism 2011. 15 S33–S39. ( 10.4103/2230-8210.83062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang W, Gao L, Li N, Wang B, Wang L, Wang Y, Yang H, You L, Hou J, Chen S, et al. Efficacy and safety of bromocriptine-QR in Type 2 diabetes: a systematic review and meta-analysis. Hormone and Metabolic Research 2015. 47 805–812. ( 10.1055/s-0035-1559684) [DOI] [PubMed] [Google Scholar]
- 38.Manning PJ, Grattan D, Merriman T, Manning T, Williams S, Sutherland W. Pharmaceutical interventions for weight-loss maintenance: no effect from cabergoline. International Journal of Obesity 2018. 42 1871–1879. ( 10.1038/s41366-018-0165-3) [DOI] [PubMed] [Google Scholar]
- 39.Grossmann M. Hypogonadism and male obesity: focus on unresolved questions. Clinical Endocrinology 2018. 89 11–21. ( 10.1111/cen.13723) [DOI] [PubMed] [Google Scholar]
- 40.Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Tajar A, Bartfai G, Boonen S, Casanueva FF, et al. Age-associated changes in hypothalamic-pituitary-testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. European Journal of Endocrinology 2013. 168 445–455. ( 10.1530/EJE-12-0890) [DOI] [PubMed] [Google Scholar]
- 41.Escobar-Morreale HF, Santacruz E, Luque-Ramírez M, Carretero JIB. Prevalence of “obesity-associated gonadal dysfunction” in severely obese men and women and its resolution after bariatric surgery: a systematic review and meta-analysis. Human Reproduction Update 2017. 23 390–408. ( 10.1093/humupd/dmx012) [DOI] [PubMed] [Google Scholar]
- 42.Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clinical Endocrinology 2005. 63 280–293. ( 10.1111/j.1365-2265.2005.02339.x) [DOI] [PubMed] [Google Scholar]
- 43.Jones TH, Arver S, Behre HM, Buvat J, Meuleman E, Moncada I, Morales AM, Volterrani M, Yellowlees A, Howell JD, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study). Diabetes Care 2011. 828–837. ( 10.2337/dc10-1233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saad F, Yassin A, Haider A, Doros G, Gooren L. Elderly men over 65 years of age with late-onset hypogonadism benefit as much from testosterone treatment as do younger men. Korean Journal of Urology 2015. 56 310–317. ( 10.4111/kju.2015.56.4.310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yassin A, Almehmadi Y, Saad F, Doros G, Gooren L. Effects of intermission and resumption of long-term testosterone replacement therapy on body weight and metabolic parameters in hypogonadal in middle-aged and elderly men. Clinical Endocrinology 2016. 84 107–114. ( 10.1111/cen.12936) [DOI] [PubMed] [Google Scholar]
- 46.Saad F, Haider A, Doros G, Traish A. Long-term treatment of hypogonadal men with testosterone produces substantial and sustained weight loss. Obesity 2013. 21 1975–1981. ( 10.1002/oby.20407) [DOI] [PubMed] [Google Scholar]
- 47.Seyfart T, Friedrich N, Kische H, Bülow R, Wallaschofski H, Völzke H, Nauck M, Keevil BG, Haring R. Association of sex hormones with physical, laboratory, and imaging markers of anthropometry in men and women from the general population. PLOS ONE 2018. 13 e0189042 ( 10.1371/journal.pone.0189042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carré N, Binart N. Prolactin and adipose tissue. Biochimie 2014. 97 16–21. ( 10.1016/j.biochi.2013.09.023) [DOI] [PubMed] [Google Scholar]
- 49.U.S. Food and Drug Administration. Cycloset prescribing information. White Oak, MD, USA: FDA, 2009. (available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020866lbl.pdf) [Google Scholar]
- 50.Lopez Vicchi F, Luque GM, Brie B, Nogueira JP, Garcia Tornadu I, Becu-Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacological Research 2016. 74–80. ( 10.1016/j.phrs.2015.12.029) [DOI] [PubMed] [Google Scholar]
- 51.Bahar A, Kashi Z, Daneshpour E, Akha O, Ala S. Effects of cabergoline on blood glucose levels in type 2 diabetic patients: a double-blind controlled clinical trial. Medicine 2016. e4818 ( 10.1097/MD.0000000000004818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Traish AM, Haider A, Doros G, Saad F. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observational, long-term registry study. International Journal of Clinical Practice 2014. 68 314–329. ( 10.1111/ijcp.12319) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a