Abstract
Purpose
Managing radiotherapy (RT)-induced pain is essential for reducing the likelihood of treatment interruption and improving the chance of tumor control. The current study aimed to examine the role of radiation therapist (RTTs) interaction and effective information communication in modulating patients’ experiences of pain and discomfort during RT.
Methods
Participants were 91 cancer patients undergoing RT for the first time referred to the Radiotherapy Unit of Sant’Orsola-Malpighi Hospital in Bologna, Italy. Patient-reported outcome measures included patient satisfaction with the quality of the relationship and the information received by the RTTs, assessed by the Communication with RTTs — Shortened, scale, and pain and discomfort experienced during RT, assessed through two VAS scales. Attitudes toward RT were also assessed. All measures were collected as patients were approximately halfway through the overall RT duration (on average at the end of the 12th session).
Results
Patient satisfaction with RTT relationships and treatment-information communication was significantly related to RT-induced pain intensity and patient attitudes toward RT. The more satisfied patients were with RTT interactions and communication, the more positive their attitudes were toward RT and the lower the pain intensity experienced during treatment.
Conclusion
Clinical implications can be drawn in terms of highlighting the need for RTTs to be mindful of their technical and supportive role in delivery of patient care and in structuring treatment information content in a way that contrasts potential nocebo effects related to patients' negative expectations about RT. The findings support the idea that RTTs may benefit from training interventions and structured education sessions with a focus on interpersonal skills and patient-centered communication.
Keywords: radiotherapy, experiences of pain, information, communication
Introduction
Radiotherapy (RT), which is widely considered as an effective treatment in more than half of cancer patients worldwide, is also recognized as a potential cause of pain, mainly due to RT-related adverse effects (ie, inflammation of the skin or mucosae, flare-up phenomenon) and/or treatment-related procedures (ie, brachytherapy, uncomfortable positioning, or immobilization).1–3 Regardless of the clinical features (such as, tumor type and stage, treatment aim), RT-induced pain often results in further deterioration of patient quality of life and reduced treatment compliance, which may affect the dose intensity of systemic therapy or RT duration, potentially interfering with chances of tumor control.2,4,5
Independent studies have consistently shown that patient–health-care provider interactions may significantly influence patient satisfaction with received care, treatment adherence, and pain-related outcomes.6 For instance, placebo and nocebo effects arising from patients' positive or negative expectations about treatment and clinical outcomes are a case in point of how clinician–patient interaction can actually modulate patient pain perception.7,8
Being in daily contact with the patient during RT, radiation therapists (RTTs) are uniquely placed to explore and relieve patients' needs and concerns.9,10 Beyond and above the technical planning and delivery of the treatment, RTTs are key positioned to fine-tune information and provide reassurance to patients about treatment expectations and potential side effects.11,12 Indeed, evidence suggests that training RTTs on communication and interaction skills produces clear benefits in terms of patient understanding of RT and satisfaction with the treatment,13–16 leading to reduced feelings of distress, fear, and anxiety about RT.9,17,18
Whereas the current literature indicates increasingly focused attention on the need for managing RT-related pain, to the best of our knowledge no study has specifically investigated the role of RTT interaction and effective information communication on modulating patients' experiences of pain and discomfort during RT. In the present study, we address this issue by evaluating the association between patient satisfaction with the RTT relationship and information communication during RT, related experiences of pain and discomfort, and patient attitudes toward RT. Based on the literature on placebo and nocebo effects,7,8 we predicted that patients reporting high levels of satisfaction with RTT interaction and communication would experience lower pain and discomfort during RT and report a more positive attitude toward RT than less satisfied patients.
Methods
Eligible participants were cancer patients consecutively referred to the Radiotherapy Unit of Sant'Orsola - Malpighi Hospital from June 1, 2015 to October 31, 2016 and undergoing RT for the first time. A total of 91 patients were enrolled in the study. Approval was obtained from the local Ethical Committees on Human Research, and all patients provided signed informed consent.
Patient satisfaction with the quality of the relationship and information received by the RTTs was assessed by an adapted version section 2 of the Comunicazione Medico-Paziente Nella Sclerosi Multipla — shortened (COSM-S) scale.19 Specifically, section 2 of the COSM-S scale probes on different aspects of the communication process perceived by patients, including clarity, veridicity, and satisfaction with information received, trust in and patient satisfaction with the health-care provider, the latter's availability to listen to and address concerns of the patient, and the perceived respect and emotional support patients experienced during the interaction. For the purposes of this study, we slightly modified the instructions and scale items, asking patients to indicate their answer referring to the RTTs. Items were identical to the original scale, but specific reference was made to the RTT figure. For instance, where the original COSM-S item 1 would be “Was the health professional respectful toward you?” the modified version would read: “Was the radiation therapist respectful toward you?” We named the adapted version Communication with RTT — shortened (CORT-S; Supplementary Material 1). Each item of the CORT-S is graded on a 5-point Likert scale ranging from 0 (not at all) to 4 (extremely). The original COSM-S has been shown to have optimal internal consistency,19 and the very same could be stated for CORT-S, as confirmed by our internal-consistency analysis (Cronbach's α=0.91).
Attitudes toward RT were measured through a 7-item scale composed ad hoc asking patients to indicate whether they found RT useful, harmful, advantageous, dangerous, pleasant, healthy, or unpleasant, on a 1–10 response scale (not at all to extremely). Items were highly correlated, and given the small number of items, Cronbach's α was quite satisfactory (0.76 (Supplementary Material 1).
Pain and discomfort experienced during RT was assessed through two VASs where patients indicated their perceived pain and discomfort across a continuum ranging from none to an extreme. VASs are unidimensional measures often used in epidemiological and clinical research to assess the intensity or frequency of symptoms that are believed to range across a continuum of values and cannot easily be directly measured, such as pain and discomfort. Given the fact that from a patient’s perspective, pain or discomfort is perceived as continuous and does not take discrete jumps, as a categorization of none, mild, moderate, or severe would suggest, the VAS is the best instrument to capture this idea.
All measures were collected as patients were approximately halfway through the overall RT duration (on average at the end of the 12th session). In order to control for potential effects of administering questionnaires in a certain order on subjects’ responses, the order of questionnaires was counterbalanced between patients, and administration took approximately 20 minutes to be completed.
Statistical Analyses
Descriptive analyses were computed to provide a quantitative description of demographic and clinical characteristics of the sample. The relationship between number of RT sessions and perceived pain and discomfort during RT was assessed by means of correlation coefficients. A median split on participants’ CORT-S total scores was used to differentiate between low satisfaction (n=45, mean 40.15±7.25) and high satisfaction (n=46, mean 55.73±4.09). We initially subjected attitudes toward RT to one-way ANOVA, with group as a two-level between-subject factor (low vs high satisfaction) and number of RT sessions as covariates. Because a significant main effect of group was found, we included attitudes toward RT as a covariate in subsequent ANOVAs. Pain intensity and discomfort were submitted to two separate one-way ANCOVAs, with group (low vs high satisfaction), number of RT sessions, and attitude toward RT as covariates. Separate ANOVAs were performed to evaluate the role of cancer diagnoses and type of immobilization device used during RT on pain and discomfort experienced during treatment.
Results
Demographic and clinical characteristics of the patient population are summarized in Table 1. Separate ANOVAs performed on attitude toward RT and patient pain and discomfort experienced during RT revealed no significant effect for sex, concurrent chemotherapy, or previously received surgery. Instead, cancer diagnoses and type of immobilization device used during treatment significantly affected patient-reported levels of pain experienced during RT: patients with tumors in the head-and-neck area and undergoing RT through the use of a face-mask immobilization device reported the highest levels of pain (Fs1,89>7.57, ps<0.007; ηp2s>0.07).
Table 1.
Demographic And Clinical Characteristics Of The Sample (n=91)
| Mean (SD/ %) | |
|---|---|
| Age, years | 65.5 (13.8) |
| Sex | |
| Male | 37 (40.6) |
| Female | 54 (59.3) |
| Cancer diagnosis | |
| Prostate | 21 (23.1) |
| Rectal | 10 (11.1) |
| Head and neck | 10 (11.1) |
| Breast | 35 (38.4) |
| Gynecological | 6 (6.6) |
| Other | 9 (9.8) |
| Metastatic spread | |
| Yes | 55 (60.4) |
| No | 36 (39.5) |
| Concurrent chemotherapy treatment | |
| Yes | 29 (31.9) |
| No | 62 (68.1) |
| Surgical intervention within 6 months prior to radiotherapy | |
| Yes | 48 (52.7) |
| No | 43 (47.3) |
| Immobilization device used | |
| Head and neck area | 10 (11.1) |
| Trunk area | 90 (98.9) |
| Extremities | 6 (6.6) |
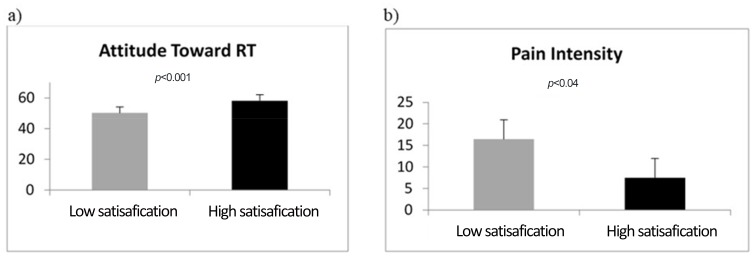
The number of RT sessions (at the time of data collection) significantly correlated with pain intensity and discomfort (r=0.23 and 0.25, respectively, ps<0.05), ie, the higher the number of RT sessions received by data collection, the higher the level of pain and discomfort experienced. Analyses strictly related to the object of the study showed a significant main effect of group on both attitude toward RT (F1,88=13.48, p<0.001, ηp2=0.54 [Figure 1A]) and pain intensity (F1,88=4.66, p=0.034, ηp2=0.05 [Figure 1B]). No significant differences between high and low satisfaction were found for discomfort (F1,88=1.66, ηp2=0.02).
Figure 1.
Attitude toward RT (A) and pain intensity (B) mean scores as a function of satisfaction with the quality of the relationship and information from the RTTs.
Discussion
Despite growing recognition of the importance of managing RT-induced pain to reduce the likelihood of treatment interruption and thus improve the chance of tumor control, evidence supporting the relationship between quality of patient–RTT communication and pain has remained unexplored. In accordance with the literature highlighting the role of an effective health-care provider–patient relationship and communication on patient outcomes,20,21 the results of the present study show that patient satisfaction with the RTT relationship and treatment-information communication was significantly related to RT-induced pain intensity and patient attitudes toward RT. Patients who were more satisfied with RTT interaction and communication reported more positive attitudes toward RT and lower pain intensity during treatment. As consistently suggested by the literature on nocebo-related effects, unwanted effects, and side effects, such as worsening of RT-related pain (as in our case), may occur, not only due to the impact of negative diagnoses on the patient but also when distrust toward medical personnel and therapies are present.22,23
Our results are also in line with studies profiling a key role of RTTs in alleviating treatment-related stress and anxiety in cancer patients.9,13 It is widely recognized that anxiety can induce hyperalgesia, and thus we may hypothesize that the mechanism at the basis of these findings could be related to nocebo effects. However, this is an issue warranting further examination. Nevertheless, various limitations must be acknowledged. First, the analyses were correlational in nature and the instrument we used for measuring attitudes toward RT was created ad hoc for the purposes of this study. Future research should validate the usefulness of this tool in capturing patient attitudes toward RT. Although a median split–based analysis illustrates potential causal relations among model variables, definitive conclusions about causation cannot be drawn. Furthermore, it should be taken into consideration that the study was conducted in a cohort of patients with different types of cancer and different immobilization devices used during RT. Therefore, subgroup comparisons based on cancer diagnosis should be the object of future studies in a wider sample.
Despite these limitations, our findings are relevant in terms of practical clinical implications in suggesting a need for RTTs to be mindful of both their technical and supportive role in delivery of patient care. For these reasons, RTTs may clearly benefit from training interventions and structured education sessions with a focus on patient-centered communication and care, as recommended by the European Society for Radiotherapy and Oncology core curriculum for RTTs.16 Particular attention should be given to building interpersonal skills and structuring information content in a way that is tuned to contrasting the effect of patients' negative expectations about RT and thus deactivating nocebo-like processes.
Acknowledgment
The authors would like to thank the patients participating in the study for their precious contribution, as well as the radiotherapists of the Radiotherapy Unit of Sant’Orsola-Malpighi Hospital (Professor Morganti) for their invaluable collaboration and support.
Disclosure
Professor Alessio Morganti reports financial support from Elekta and Beyer outside the submitted work. The other authors report no conflicts of interest in this work.
References
- 1.Mirabile A, Numico G, Russi EG, et al. Sepsis in head and neck cancer patients treated with chemotherapy and radiation: literature review and consensus. Crit Rev Oncol Hematol. 2015;95(2):191–213. doi: 10.1016/j.critrevonc.2015.03.003 [DOI] [PubMed] [Google Scholar]
- 2.Ripamonti CI, Bossi P, Santini, et al. Pain related to cancer treatments and diagnostic procedures: a no man’s land? Ann Oncol. 2014;25(6):1097–1106. doi: 10.1093/annonc/mdu011 [DOI] [PubMed] [Google Scholar]
- 3.Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253–262. doi: 10.1016/s0167-8140(02)00404-8 [DOI] [PubMed] [Google Scholar]
- 4.Blanchard D, Bollet M, Dreyer C, et al. Management of somatic pain induced by head and neck cancer treatment: pain following radiation therapy and chemotherapy — guidelines of the French Otorhinolaryngology Head and Neck Surgery Society (SFORL). Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(4):253–256. doi: 10.1016/j.anorl.2014.07.001 [DOI] [PubMed] [Google Scholar]
- 5.Russo G, Haddad R, Posner M, et al. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist. 2008;13(8):886–898. doi: 10.1634/theoncologist.2008-0024 [DOI] [PubMed] [Google Scholar]
- 6.Ruben BD. Communication theory and health communication practice: the more things change, the more they stay the same. Health Commun. 2015;3:1–11. [DOI] [PubMed] [Google Scholar]
- 7.Blasini M, Corsi N, Klinger R, et al. Nocebo and pain: an overview of the psychoneurobiological mechanisms. Pain Rep. 2017;2:e585. doi: 10.1097/PR9.0000000000000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Psychol Rev. 2013;93:1207–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsner K, Naehrig D, Halkett G, et al. Reduced patient anxiety as a result of radiation therapist-led psychosocial support: a systematic review. J Med Radiat Sci. 2017;64:220–231. doi: 10.1002/jmrs.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell DL, Lozano RG. Understanding patient psychosocial issues. Radiat Ther. 2012;21:96–99. [Google Scholar]
- 11.Halkett GK, Merchant S, Jiwa M, et al. Effective communication and information provision in radiotherapy—the role of radiation therapists. J Radiother Pract. 2010;9(1):3–16. doi: 10.1017/S1460396909990173 [DOI] [Google Scholar]
- 12.Douma KF, Koning CC, De Haes H, et al. Do radiation oncologists tailor information to patients needs? And, if so, does it affect patients? Acta Oncol (Madr). 2012;51(4):512–520. doi: 10.3109/0284186X.2012.665476 [DOI] [PubMed] [Google Scholar]
- 13.Heshmati Nabavi F, Behboudifar A, Pouresmail Z, et al. Effect of pre-treatment education programs on the anxiety of patients receiving radiotherapy: an integrative literature review. Evidence Based Care. 2016;6(1):49–62. [Google Scholar]
- 14.Famiglietti RM, Neal EC, Edwards TJ, et al. Determinants of patient satisfaction during receipt of radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87(1):148–152. doi: 10.1016/j.ijrobp.2013.05.020 [DOI] [PubMed] [Google Scholar]
- 15.Becker-Schiebe M, Pinkert U, Ahmad T, et al. Predictors of overall satisfaction of cancer patients undergoing radiation therapy. Patient Prefer Adherence. 2015;9:1381. doi: 10.2147/PPA.S82441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coffey MA, Mullaney L, Bojen A, et al. Recommended ESTRO core curriculum for RTTs (Radiation TherapisTs)–3 rd edition. Radiother Oncol. 2012;103:103–108. doi: 10.1016/j.radonc.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Halkett G, O’Connor M, Aranda, et al. Communication skills training for radiation therapists: preparing patients for radiation therapy. J Med Radiat Sci. 2016;63(4):232–241. doi: 10.1002/jmrs.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egestad H. How does the radiation therapist affect the cancer patients’ experience of the radiation treatment? Eur J Cancer Care (Engl). 2013;22(5):580–588. doi: 10.1111/ecc.12062 [DOI] [PubMed] [Google Scholar]
- 19.Solari A, Grzeda M, Giordano A, et al; SIMS-Trial, SIMS-Practice and Agorà studies. Use of Rasch analysis to refine a patient-reported questionnaire on satisfaction with communication of the multiple sclerosis diagnosis. Mult Scler J. 2014;20(9):1224–1233. doi: 10.1177/1352458513518261 [DOI] [PubMed] [Google Scholar]
- 20.Dimoska A, Butow PN, Dent E, et al. An examination of the initial cancer consultation of medical and radiation oncologists using the Cancode interaction analysis system. Br J Cancer. 2018;98(9):1508. doi: 10.1038/sj.bjc.6604348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong S, Butow PN, Costa DS, et al. The influence of patient-centered communication during radiotherapy education sessions on post-consultation patient outcomes. Patient Educ Couns. 2014;95(3):305–312. doi: 10.1016/j.pec.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Flaten MA, Simonsen T, Olsen H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom Med. 1999;61(2):250–255. doi: 10.1097/00006842-199903000-00018 [DOI] [PubMed] [Google Scholar]
- 23.Barsky AJ, Saintfort R, Rogers M, et al. Nonspecific medication side effects and the nocebo phenomenon. Jama. 2002;287(5):622–627. doi: 10.1001/jama.287.5.622 [DOI] [PubMed] [Google Scholar]